 Other information on identity and properties
Molecular Weight 505.2
Physical Form Technical material is a white crystalline
powder.
Purity >98.00% for technical grade material.
Melting Point 98 to 101°C
Vapour pressure 1.5 × 10-8mm Hg at 25°C
Solubility Insoluble in water (less than 0.002 mg/kg at
20°C); soluble in acetone, DMSO, DMF,
benzene, xylene, cyclohexanone, HMTP, ethyl
acetate, THF, dioxan; slightly sol. in ethanol,
isopropanol, acetonitrile (9g/100ml).
Stability Stable in acidic and neutral solution;
unstable in alkaline solution. No alteration of
the technical product was observed after
24-month storage in white glass vials at 40°C in
dark, in polyethylene flasks at 40°C in dark, in
aluminum bottles at 40°C, in metal tins with
liner at 40°C, in white glass vials at R.T.
exposed to light, in polyethylene flasks at
R.T. exposed to light. Under standardized
irradiation conditions with a Xenon lamp,
the half-life was >21 hours.
Formulations Available formulations are: emulsifiable
concentrate (10g/l-25g/l), ultra low volume
(1g/l-10g/l), wettable powders (2.5%-5%),
and dusts (0.05%-0.1%). In some countries, a
certain quantity of cotton seed oil has been
introduced into the EC or ULV formulations;
these are designated with the letters NH in
the trade mark.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Distribution, excretion and biotransformation
Male rats (1-3 animals) received a single oral dose of 0.90 mg/kg bw
14Cv-deltamethrin, 1.60 mg/kg bw 14C(alpha)-deltamethrin, 0.64 mg/kg
bw 14CN-deltamethrin, 3.74 mg/kg bw 14Cv-becisthemic acid or 0.94
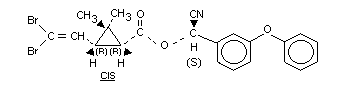
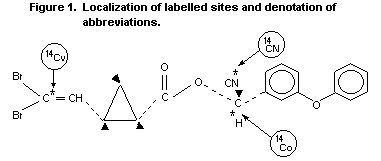
mg/kg bw 14Cv-[1R,3S,trans]deltamethrin (Figure 1).
Other information on identity and properties
Molecular Weight 505.2
Physical Form Technical material is a white crystalline
powder.
Purity >98.00% for technical grade material.
Melting Point 98 to 101°C
Vapour pressure 1.5 × 10-8mm Hg at 25°C
Solubility Insoluble in water (less than 0.002 mg/kg at
20°C); soluble in acetone, DMSO, DMF,
benzene, xylene, cyclohexanone, HMTP, ethyl
acetate, THF, dioxan; slightly sol. in ethanol,
isopropanol, acetonitrile (9g/100ml).
Stability Stable in acidic and neutral solution;
unstable in alkaline solution. No alteration of
the technical product was observed after
24-month storage in white glass vials at 40°C in
dark, in polyethylene flasks at 40°C in dark, in
aluminum bottles at 40°C, in metal tins with
liner at 40°C, in white glass vials at R.T.
exposed to light, in polyethylene flasks at
R.T. exposed to light. Under standardized
irradiation conditions with a Xenon lamp,
the half-life was >21 hours.
Formulations Available formulations are: emulsifiable
concentrate (10g/l-25g/l), ultra low volume
(1g/l-10g/l), wettable powders (2.5%-5%),
and dusts (0.05%-0.1%). In some countries, a
certain quantity of cotton seed oil has been
introduced into the EC or ULV formulations;
these are designated with the letters NH in
the trade mark.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Distribution, excretion and biotransformation
Male rats (1-3 animals) received a single oral dose of 0.90 mg/kg bw
14Cv-deltamethrin, 1.60 mg/kg bw 14C(alpha)-deltamethrin, 0.64 mg/kg
bw 14CN-deltamethrin, 3.74 mg/kg bw 14Cv-becisthemic acid or 0.94
mg/kg bw 14Cv-[1R,3S,trans]deltamethrin (Figure 1).
 The radiocarbon of all compounds except for 14CN was nearly fully
excreted within 8 days with the main portion within 1 day.
14C-becisthemic acid showed the highest excretion with 94% in the
urine and 6% in the faeces, followed by 14C(alpha)-deltamethrin and
14C-trans-isomere, both with 75% and 25% respectively and 14C-deltamethrin with
54% and 44% respectively. No 14CO2 was found in the exhaled air.
Residues in carcase at 8 days amounted 0.2% with 14Cv-becisthemic
acid and 1.1-1.5% with the 14Cv-labelled compounds. Tissues with a
relatively high radiocarbon content were different at each
14C-compound: 59 µg/kg from 14Cv-deltamethrin in the fat, 182 µg/kg
in the fat and 89 µg/kg in the blood from 14C and 27 µg/kg from
14Cv-becisthemic acid in the liver.
Excretion after 14CN administration was slow and amounted to not more
than 79% within 8 days with 43% in the urine and 36% in the faeces.
No CO2 was expired. Residues in carcase and tissues amounted 21%
with relatively high radiocarbon contents in the stomach (603 µg/kg)
and the skin (645 µg/kg). Oral and i.p. applications of 0.1 mg/kg
14CN showed only small differences in excretion and distribution
pattern after 3 days. A radiocarbon content of the stomach of 3%
after both applications was noticed. Compared with these patterns
oral and i.p. application respectively of 0.1 mg/kg potassium cyanide-
14C showed a 2-3 times higher excretion in urine at 3 days. The
excretion in the faeces and the residues in carcase were about the
same and only after i.p. administration of KCN a lower stomach residue
as with 14CN was noticed.
The parent compound (6-21% of the applied dose) and hydroxylated
deltamethrins (7-15%; 4'-hydroxy- and 5-hydroxy deltamethrin) were
present in faeces only. The other metabolites, primarily carboxylic
acids or their conjugates, derived from ester cleavage and oxidation,
appeared mainly in urine. The major urinary metabolite from 14CN was
thiocyanate; those from the other labelled compounds were
4'-hydroxy-phenoxybenzoic acid sulphate and becisthemic acid
glucuronide (Ruzo et al., 1977).
In a separate experiment with rats it was shown that the residual 14C
in skin and gastrointestinal tract from 14CN was 14C-thiocyanate.
This latter compound has a relatively high tissue affinity, accounting
probably for the delayed and incomplete excretion of
14CN-deltamethrin (Ruzo et al., 1977).
Incubation of 14C(alpha)- and 14Cv-deltamethrin with rat stomach
strips in pH 7.2 medium for 6-24 hours yields approximately 60%
metabolism, the major products being phenoxybenzoic acid and
becisthemic acid (Ruzo et al., 1977).
The metabolism of orally given deltamethrin in mice is essentially the
same as in rats. The faeces contain less parent compound and more
mono- and di-hydroxylated esters than in rats. Intraperitoneal
administration in mice yields the same metabolites as oral injection
but in different ratios (Ruzo et al., 1978b).
Deltamethrin incubated with mouse liver microsomal fraction yields in
an oxidase system more metabolised (59%) and hydroxylated parent
compound (8%) than in an oxidase plus esterase system (25% and 4%
respectively). In the latter system the total amount of derivatives
from the acid and alcohol moieties of the ester is more than doubled.
No racemization occurred since only alpha S epimers were recovered in
the alcohol moiety (Shono et al., 1978).
TOXICOLOGICAL STUDIES
Special studies on primary irritation
Cutaneous irritation
Male albino rabbits (12/group) weighing 2.5 to 3.5 kg were
administered 0.5 g deltamethrin to either shaven intact or abraded
skin. The occulsive patch was fixed on the skin for 23 hours.
Scoring for erythematous and oedematous lesions occurred 1 hour and 49
hours after removal of the patches. Technical deltamethrin, 98% ai
showed no irritant effect (Coquet, 1976a).
Ocular irritation
Deltamethrin (0.1 g/animal) was administered into the conjunctival sac
of the eye of 6 male albino rabbits, weighing 2.5 kg, with or without
rinsing 60 sec after instillation. Observations for conjunctival
lesions, chemosis, discharge, conjunctival enanthema, opacity and
affected cornea were made 1 hour, 24 hours, 2, 3, 4 and 7 days
following instillation. Deltamethrin showed both with and without
rinsing transient irritating effects (Coquet, 1976b).
Special studies on sensitization
Deltamethrin (0.5 g/animal ) was applied topically to the skin of
albino guinea pigs (10 male and female) 3 times per week, with a 2-day
interval for 3 weeks, and once at the start of the 4th week. The
preparation was covered with an occlusive patch for 48 hours. On day
1 and 10 the guinea pigs received an intradermal injection of 0.1 ml
of Freund's adjuvant. The animals were challenged 12 days after the
last application with 0.5 g deltamethrin. The macroscopic and
histological examination did not reveal evidence of sensitisation.
(Guillot and Guilaine, 1977).
Special studies on reproduction
Rat
Groups of 10 female and 10 male Charles River rats were fed
deltamethrin in the diet at 0, 2, 20 or 50 mg/kg and mated to begin a
three-generation, 2 litter (first generation, 3 litter) standard
reproduction study. Parental body weights and food consumption were
recorded during the study. After weaning of the second litter the
survival parental rats were sacrificed and necropsied.
Five male and 5 female pups of the F3b were necropsied. No changes
in general behaviour, or survival of parental rats or pups relevant to
tent material were observed. The body weight of F0-males of the 50
mg/kg group was decreased from week 11 onward; there were some slight
decreases in mean food consumption of the parental F1 male rats in
the 50 mg/kg feed group.
The basic reproduction indices (fertility, gestation, lactation,
viability and litter size) were not affected by the treatment.
However, the mean pup weight was in some litters, especially of the 50
mg/kg group, slightly decreased in comparison to the controls. Gross
external examination revealed no abnormalities. No gross or
microscopic lesions of treatment-related significance or significant
effects on the organ weights of the F3b generation were observed
(Wrenn et al, 1980).
Special studies on teratogenicity
Mouse
Mated female Swiss CDI.SPF mice (24/group) were given orally
deltamethrin dissolved in sesame oil at dose levels of 0, 0.1, 1 or 10
mg/kg bw/day during day 6-17 of pregnancy. The animals were
necropsied on day 18 of gestation. No teratogenic effects could be
detected. Total implantation sites, foetal losses, living foetuses
and examinations of skeletal tissue were normal. Minor embryotoxic
effects as dose-dependent decrease of average fetal weight and delayed
ossifications were observed at all dose levels tested (Glomot and
Vannier, 1977).
Rat
Mated female Sprague Dawley rats (24/group) were administered orally
0, 0.1, 1 or 10 mg/kg deltamethrin bw/day during day 6-18 of
pregnancy. Examination occurred on day 21 of gestation. Twelve
females in control and 10 mg/kg bw-group were allowed to deliver.
There were no effects on the reproduction or teratogenicity parameters
examined, with the exception of a slight delayed ossification in the
highest dose level (Glomot and Vannier, 1977).
Rabbit
Groups of fifteen mated females received deltamethrin dissolved in
sesame oil at levels of 0, 1, 4 or 16 mg/kg bw/day from day 6-19 of
pregnancy. Examination was carried out on day 28 of gestation. The
average foetal losses were not related to dose increase at all doses
tested. This effect was mainly caused by a higher rate of expelling
traces. The average foetal weight in the highest dose group was
decreased. Some malformations (one animal with hydrocephalia, and one
with exencephalia and thoracogastrochisis) were observed in 2 animals
of the highest dose level. A complementary study with 16 mg/kg bw/
day was performed. In this study one foetus with spina bifida and
shortened tail was detected among 69 apparently normal foetuses. It
was concluded that the malformations were within the normal limits of
the strain used and were not related to the treatment despite the
occurrence at the highest does level only (Glomot and Vannier, 1977
and 1978).
Special study on delayed neurotoxicity
Chicken
Adult hens (10/group) were gavaged with a single dose of O, 500, 1250
or 5000 mg/kg bw deltamethrin suspended in corn oil or 0 or 100 mg/kg
bw dissolved in sesame oil. Tri-o-cresylphosphate (TOCP) (500 mg/kg
bw) was used an positive control for delayed neurotoxicity. During 21
days observations were made on mortality, health, neurotoxic signs and
body weight.
In the TOCP group 8 out of 10 animals died whereas only 2 mortalities
were observed in the group dosed at 1000 mg deltamethrin/kg with
sesame oil as the vehicle. Deltamethrin induced no clinical,
macroscopic or histological signs of delayed neurotoxicity. TOCP-
treated hens showed severe ataxia and degenerative changes in spinal
cord and occasionally in sciatic nerve (Ross et al., 1978).
Special studies on potentiation
Mice
Deltamethrin is hydrolysed in vitro by esterases in blood and in
brain, kidney, liver and stomach preparations of mice. Pretreatment
of mice with oxidase inhibitor, piperonyl butoxide (PB), or esterase
inhibitor, S,S,S-tributylphosphorotrithicate (DEF), delayed
metabolism of i.p. administered deltamethrin. Using oxidase or
esterase inhibitor, different vehicular and different administration
routes, it was possible to induce similar toxic effects with a wide
range of deltamethrin doses (6-191 mg/kg bw). The different
treatments showed that PB or DEF made mice more sensitive to
deltamethrin (Ruzo et al., 1978a).
Special studies on mutagenicity
Bacterial growth tests
In a growth inhibition test with E. coli, deltamethrin at levels
of 1250, 2500, or µg/ml DMSO (0.1 ml per plate) had the same marginal
inhibitor effect on the mother strains (W3110 and WP2) as on their
mutants (p3478 and CM611). Chloramphenicol and N-methyl
N'-nitro-N-nitroguanidine (MNNG) were used as positive controls and
induced clear inhibition (Peyre et al., 1980).
Deltamethrin was compared with MMNG, 9-aminoacridine, 2-nitrofluorene
and 2-aminoanthracene for mutagenic activity in the Ames test with
Salmonella typhimurium his strains TA 1535, TA 100, TA 1537, TA
1528, and TA 98. The concentrations of deltamethrin used were 2, 10,
50, 200, 500, 1000 or 5000 µg/plate. Deltamethrin began to
precipitate at 200 µg/plate.
The mean number of revertants wan not influenced by any concentration
of deltamethrin in any strain with or without S9-mix(metabolic
activation), whereas the positive control mutagens produced an
increase of the number of spontaneous revertants (Peyre et al,
1980).
In a similar experiment, deltamethrin (0, 2, 20, 200 or 400 µg/plate
dissolved in DMSO) in the presence of activated microsomal enzymes did
not influence the number of revertants of Salmonella typhimurium
strains TA 1535, 1537, 1538, 98 and 100. 2-Aminoanthracene,
3-methylcholanthrene, benzo(alpha)pyrene and acridine orange showed
mutagenic activity, whereas thio-TEPA was negative (Fouillet, 1976).
Test with mammalian cells
Deltamethrin dissolved In Cremaphor oil (0, 0.04, 0.2, 1 or 5 mMol, 0,
0.08, 0.4, 1 or 10% v/v respectively) in the presence of a metabolic
activation system increased the incidence of chromosome and chromatid
aberrations and SCE's, after incubation with Chinese hamster ovary
cells at 1 mMol. In the absence of S9-mix (metabolic activation) no
higher rate of aberration was observed.
It was shown that this increased incidence was due to a sub-toxic
effect of some reaction product of Cremaphor oil and S9-mix.
Deltamethrin in Cremaphor oil without S9-mix and dissolved
in DMSO (1%) at levels of 0, 0.001, 0.01, 0.1 or 0.2 mMol with or
without metabolic activation had no effect on the number of
aberrations and SCE's. Due to insolubility no higher concentrations
were tested (Sobels et al., 1978).
Animal tests
Mice, 3 males and 3 females per dose, were gavaged for two consecutive
days with 5 or 10 mg deltamethrin (dissolved in sesame oil)/kg bw.
Control mice were gavaged with 0.3 ml sesame oil. The incidence of
chromatid aberrations in bone marrow cells or of micronuclei in
polychromic erythrocytes did not show any significant statistical
difference in treated and control groups (Sobels, et al., 1978).
A single oral administration of 15 mg/kg deltamethrin was given to
Swiss mice. Groups of 2 animals were sacrificed every 3 hours during
a 24-hour period. Several animals died after treatment. The
incidence of chromatid aberrations in the bone marrow of femora was
low. There was no consistent time-related trend in the distribution
of these aberrations (Sobels, et al., 1978).
Deltamethrin dissolved in sesame oil in groups of 9-13 male mice dosed
orally with 0 or 3 mg/kg bw for 7 days and 6 or 15 mg/kg bw in a
single dose showed after mating with 6-18 non-treated females, no
effect on the rate of pre- or post-implantation losses.
The highest dose tested was toxic to the males. 7 out of 20 animals
died shortly after treatment. Histological examination of the testes
of all animals revealed no abnormalities.
Triethylene thiophosphoramide (10 mg/kg bw) used as positive control
reduced considerably the rate of pregnancies in the second and third
week and increased the number of embryonal looses (Vannier and Glomot,
1977).
Special studies in man
Among plant workers dermally exposed to technical deltamethrin or its
formulations cutaneo-mucous manifestations were observed. Initial
lesions were tenacious and painful pruritus (pricking sensation),
especially observed after exposure to hot water or on perspiration,
followed by a blotchy local burning sensation with blotchy erythema
for about 2 days. Thereafter slight and regular desquamation,
restricted to the contaminated area, occurred. The cutaneous signs
are sometimes accompanied by itching of the face (mainly around the
mouth) and/or rhinorrhoea or lacrimation. No other symptoms related
to exposure were observed (Husson, 1978).
Acute toxicity
Mouse
Mice injected i.v. with deltamethrin showed intense tremors,
convulsions and taxis immediately after administration. Also
tachycardia and respiratory defects were observed at higher doses.
Surviving animals showed normal appearance after 4-5 hours.
Immediately after i.p. injection, jumping movements, slight
convulsions and prostration, ptosis, tail hypertonicity and cyanosis
were observed. Surviving animals showed normal appearance after 72
hours. Animals gavaged with deltamethrin showed 1 hour after dosing
muscular stiffening and convulsions. After 24 hours hypermotility,
stereotype movements of the head, tachycardia hypertonicity of the
tail and a few convulsion. Normal behaviour and appearance were
observed after 48 hours (Glomot and Chevalier, 1976a, b and c).
Rat
Rats injected i.v.with deltamethrin showed immediately following
treatment muscular contractions, piloerection, respiratory defects,
convulsions and paresis of the hind quarters. Death occurred within
10 min. After 25 hours only piloerection was visible, after 48 hours
surviving animals showed normal behaviour. After i.p. injection
immediate tremors, convulsions, prostration and cyanosis were
observed. After 48 hours surviving animals showed normal behaviour.
Gavage with deltamethrin shortly after dosing induced motor
incoordination, convulsions and respiratory defects. After 24 hours
and 48 hours, hypomotility and convulsions were still observed. After
3 days surviving animals showed normal behaviour (Glomot and
Chevalier, 1976a, b and c).
Rats (7 males and 7 females/group) were exposed (whole body) during 6
hours to aerosol concentrations of 0.049, 0.43, 0.54 or 0.72 g ai/m3.
The aerosol contained 66-86% of particles <5.5 µ. During exposure
hyperactivity and dose-dependent increase in grooming and irritation
was observed. The animals were hypersensitive to touch and noise and
showed uncoordinated movements. During the observation period of 14
days following exposure all animals except those from the lowest dose
group developed poor motor coordination and hypersensitivity. At the
end of the period all animals were recovered to normal. In these
groups the body weight gain and food intake was depressed during 3
days following exposure. In rats (4 of control and of highest dose
group) killed immediately after exposure stomach and small intestine
were gas-filled. In treated rats, as result of exposure, massive
haemorrhage and oedema in lungs was observed. Stomachs were filled
with gas, blood and mucus. In trachea white deposits were visible.
In animals killed after the observation period dose-dependent increase
of degenerations (coloured spots to congestion) in lungs was observed
(Coombs and Clark, 1978).
Rabbit
Rabbits (10 males and 10 females) were treated with 2 g deltamethrin
in 2 ml PEG 400/kg bw on 80 cm2 shaven akin for 24 hours on
occlusion. The animals were observed for 14 days.
One animal showed obvious erythema and another congested skin. No
weight changes or abnormal behaviour were observed. On histological
observation of liver, kidneys and skin small changes were observed
which were common for this strain of rabbits and not related to
treatment (Clair, 1977).
Birds
Oral administration of a.i. to hens, game duck, or partridge showed no
distinct symptoms except for a small initial weight loss occasionally.
In chickens, diarrhoea, convulsions and jerky movements of the head
were observed.
Mallard ducks, at lethal doses, exhibited signs of neurotoxic effects
which included ataxia, loss of equilibrium and of coordination. The
effect was dose related: at lower dose levels only some
hyperexcitibility and imbalance were observed (Beavers and Fink,
1977a).
Dog
Dogs showed, at non-lethal doses, transient hyperexcitibility,
akynesia, vomiting and stiffness of the hind legs (Glomot et al.,
1977).
Short-term studies
Quail and duck
Deltamethrin was given to 14-day old mallard ducks for 5 days in their
diet at doses of 0, 464, 1000, 2150, 4640 or 10,000 mg/kg feed. The
number of birds per group was 10. There was some mortality in the two
highest dose groups. Birds of the highest dose group showed ataxia
and loss of coordination. There was a dose-related decreased weight
gain and food consumption (Beavers and Fink, 1977b).
An identical experiment was performed with 14-day old Bobwhite quails
(10 birds/group). The effects were the same as in the mallard ducks,
except there was no mortality (Beavers and Fink, 1977c).
Rat
Male and female weanling Spargue Dawley rats (20/sex/group) were daily
dosed by oral gavage with 0, 0.1, 1.0, 2.5 or 10.0 mg deltamethrin in
PEG 200/kg bw/day for 13 weeks. No treatment related effects on food
and water consumption, mortality, urinalysis and haematology were
observed. Neurological examinations and ophthalmoscopy revealed no
abnormalities. In the 10 mg/kg bw group some hypersensitivity with
males was observed in week 6. Body weight gain among males receiving
deltamethrin was significantly lower at 2.5 and 10 mg/kg/day. The
body weight of the females was not affected by the treatment. The
male animals of the 1 mg/kg group showed a tendency to a reduced body
weight gain. In the females blood glucose and urea was significantly
increased in week 6 but no significant changes occurred in week 12 or
with other blood chemistry parameters. No clear effects were noted on
the weights of the organs. Gross and microscopic examination of a
variety of tissues and organs showed no treatment-related alterations.
Following the 13-week dosage period, 5 males and 5 females per group
were allowed to recover for 4 weeks. Autopsy was performed at the end
of this recovery period. The body weights of all previously treated
animals appeared not to be different from controls. Thyroid weights
were not dose-related increased in males. This increase was
significant in the 1.0 mg/kg and 10.0 mg/kg group. Marginal no effect
level was 1 mg/kg bw (Hunter et al., 1977).
Dog
Male and female beagle dogs (3-5/sex/group) at 25 weeks of age, were
daily dosed orally with O, 0.1, 1.0, 2.5 or 10.0 mg deltamethrin in
PEG 200/kg bw/day in gelatin capsules. Dosage was continued for 13
weeks, followed by a recovery period of 20 weeks for 2 dogs/sex, from
the groups receiving 1.0, 2.5 or 10.0 mg/kg bw/day.
Observations were made on behaviour, mortality, body weight, food and
water consumption. Haematology, blood chemistry, urinalysis and six
channel EEG-analysis were performed at week 0, 6 end 12;
ophthalmoscopy at week O, 5 and 12. Special attention was paid to the
muscular and nervous system.
Liquid faeces was associated with all groups of treated dogs
throughout the dosing period. All groups of animals receiving
deltamethrin gained less weight than the controls. The effects were
not strictly dose-related. The dogs from the control group were
leaving smaller quantities of the offered food than those of the
treated groups. Water consumption was not dose-related decreased in
any treated group.
Dilatation of the pupils was seen to occur in the dogs receiving 2.5
and 10.0 mg/kg/day. The sign was first seen 4-7 hours after dosing
and persisted throughout the day. They reacted normally prior to
dosing on the following day. The incidence of vomiting was
dose-related increased in all treated groups, except the 0.1 mg dose
level. The incidence decreased in all the animals affected as the
dosing period progressed.
In the highest dose group, unsteadiness, body tremors and jerking
movements were seen particularly in males in weeks 2, 3 and 4.
These effects were reduced during weeks 5 to 9 and were seen only in
one dog in week 13. Excessive salivation was seen initially and
diminished during the dosing period. After 5 and 12 weeks depression
of the gag reflex was noted in a proportion of animals in all treated
groups. Depression of the patellar reflex was observed in all treated
groups except the dogs administered 0.1 mg/kg. In the animals given 1
or 2.5 mg/kg/day exaggeration of the patellar reflex was noted only
after 5 weeks. Some animals of all treated groups showed variations
in the flexor reflex. A high proportion of the animals had depression
of the hind limb tactile placing reaction.
Dosage levels of 2.5 and 10 mg/kg/day deltamethrin caused modification
of the EEG pattern in some animals, following 12 weeks administration.
Histopathological evaluations of tissues and organs, including nervous
s stem and muscles did not reveal abnormalities that could be related
to dosage with the test compound. During recovery the gag reflex
continued to be depressed, whereas exaggeration of the patellar reflex
was still seen in some dogs that had previously received 1.0
mg/kg/day. One animal continued to show an abnormal EEG-pattern
(Chesterman et al., 1977).
TABLE 1. Acute toxicity of deltamethrin
LD50
Species sex route mg/kg bw references
mouse M + F intrav. 4 Glomot and Chevalier, 1976c
M intrap. 18 " " , 1976b
M intrap. 1711 " " , 1976b
M oral 21 " " , 1976a
M oral 331 " " , 1976a
F intrap. 12 " " , 1976b
F intrap. 1661 " " , 1976b
F oral 19 " " , 1976a
F oral 341 " " , 1976a
rat M + F intrav, 3 " " , 1976c
M intrap. 24 " " , 1976b
M intrap. 2091 " " , 1976b
M oral 67 " " , 1976a
M oral 1281 " " , 1976a
M + F inhal. 0.62 Coombs and Clark, 1978
M + F dermal >29403 Kynoch et al, 1979
F intrap. 25 Glomot and Chevalier, 1976b
F intrap. 1861 " " , 1976b
F oral 86 " " , 1976a
F oral 1391 " " , 1976a
rabbit M + F dermal >20001 Clair, 1977
chicken oral >10001 Anonymous, 1976a
adult hen F oral >25001 Ross et al, 1978
F oral >50001 " " , 1978
mallard duck oral >46401 Beavers and Fink, 1977a
game duck oral >40001 Anonymous, 1976b
grey partridge F + M oral >18006 " , 1976c
TABLE 1. Continued...
LD50
Species sex route mg/kg bw references
red partridge M + F oral >30006 " , 1976c
beagle dog M + F oral >300 Glomot et al, 1977
M + F oral >3006 1977
without index: suspended in polyethylene glycol 200
1 dissolved in sesame oil
2 expressed for LC50 in mg dust/m3 air
3 60% w/v suspension in aqueous methylcellulose on occulsion;
4 as paste in PEG 400 on occlusion;
5 dissolved in corn oil;
6 in capsules or cachets.
Long-term studies
Mouse
Male and female Charles River CD-1 mice (80/sex/group) were fed (in
the diet) at dosage levels of 0 (control), 1, 5, 25 or 100 mg
deltamethrin/kg for 24 months. In a second control group 60 mice/sex
were used. After 12 and 18 months 10 mice/sex/group except control
two were sacrificed.
There were no clear effects related to the administration of
deltamethrin on general behaviour, mortality, body weight and food
consumption. Blood chemistry, haematology and urine analysis
parameters were normal after 12, 18 and 24 months. Increases or
decreases in absolute and/or relative organ weights occurred in a few
organs at each dosage level at any time of sacrifice. Microscopic
examination of tissues did not reveal any lesions indicative of a
compound-related effect. The tumour incidence was unaffected by
deltamethrin administration. No-effect level was 100 mg/kg feed
(Goldenthal et al., 1980a).
Rat
Male and female Charles River CD rats (90/sex/group) were fed with 0
(control), 2, 20, or 50 mg decamethrin/kg in the diet for two years.
Sixty males and 60 females were used in a second control group. After
6, 12 and 18 months of compound administration 10 animals/sex/group
were sacrificed except for the second control group.
No changes in general behaviour and appearance in relation to compound
treatment were recorded. Survival was similar for control and treated
rats (50-67%). Rats at 50 mg/kg feed-group gained slightly less
weight than control rats, whereas the food consumption was essentially
the same. Ophthalmoscopic findings generally were similar for control
and treated rats. No haematological and biochemical parameters were
changed in a biologically significant way in relation to treatment at
any time, except for a decreased SGPT activity at 6 months, in the
mid- and high-dose groups.
No treatment-related effects were observed on organ weights. The
macroscopy and microscopy findings were common for the animals of
species and strain, except for a slightly increased incidence of
axonal degenerations in sciatic, tibial and/or plantar nerves at 18
months in the 20 and 50 mg/kg groups. Evaluation of incidence and/or
severity of these degenerations at termination of the study was
obscured by the age of the animals.
Seven interstitial cell adenomas were observed in the testes of the 50
mg/kg feed group, compared to 0 and 4 in the two control groups. Only
from some animals of the 2 and 20 mg/kg groups were some organs and
tissues, including the testes, studied histopathologically.
Evaluation of a possible dose-response effect on the testes is
therefore not possible. No-effect level is 2 mg/kg feed (Goldenthal
et al., 1980).
RESIDUES IN FOOD
USE PATTERN
Deltamethrin is a new synthetic pyrethroid insecticide manufactured
and marketed as a single diastereo isomer (>98%) out of eight
possible isomers. It is a contact and stomach insecticide with a very
large spectrum of action and considerable stability when exposed to
air and light.
Pre-harvest treatments
When applied on field crops, deltamethrin is active at the level of
only 0.01 lb./acre against very numerous species of insects. Current
recommendations for foliar applications on various crops in growth are
summarized in Table 2.
TABLE 2. Foliar dosage rates on various crops
Crop Rate, g ai/ha
Cotton 7.5-18.75
(usually 12.5 for medium
infestations most cotton
insects)
Vegetables
Artichokes 10
Eggplants 7.5-25
Cabbage 7-5-25
Strawberries 12.5-25
Beans 7.5-17.5
Lettuce 1.25-12.5
Melon 7.5-12.5
Tomatoes 7.5-25
Pimento 12.5-17.5
Leeks and onions 7.5-12.5
Peas 7.5-12.5
Fruit
Apricots 1.25-1.75 (g/hl)
Citrus fruit 1-1.5 (g/hl)
3 (g/ha, directed)
7.5 (g/ha, overall)
Cherries 0.75 (g/hl)
12.5
Figs 1.25 (g/hl)
Olives 0.625-1.75 (g/hl)
Bananas 1.25 (g/hl for 250 l/ha)
Pome fruit 0.75-1.25 (g/hl)
Peaches 0 75-1.75 (g/hl)
Plums 0.75-1.25 (g/hl)
Field crops
Sugarbeets 7.5-17.5
Coffee 7.5-12.5
Cereals(foliar treatments
during vegetative period) 7.5-12.5
Rapeseeds 5-7.5
Alfalfa 10-17.5
Maize 7.5-12.5
Potatoes 7.5-12.5
Soybeans 5-12.5
Grapes 7-5-17.5
Groundnuts(peanuts) 12.5
Sugarcane 17.5
Rice 7.5-25
Post-harvest treatment
Because of its extreme stability and persistence, deltamethrin (as
K-OTHRIN(R) is very effective against stored product pests, especially
in grain storage, including oil seeds and semi-finished products
deriving therefrom (flour, feed, etc.) It can be economically
synergized by piperonyl butoxide (PB), but to a lesser extent than
other pyrethroids. The recommended rates of application (which have
to be adapted according to local situations, local insects, and the
type of grain to be treated) are:
deltamethrin 0.75 to 1 g ai/ton.
deltamethrin/PB: 1/5 0.5 to 0.75 g ai/ton.
deltamethrin/PB: 1/10 0.25 to 0.5 g ai/ton.
Other uses
Special formulations for use on animals, as ectoparasiticides, for
household use, and for public health uses are still under development
and no data are available at this time.
RESIDUES RESULTING FROM SUPERVISED FIELD TRIALS
Extensive field trials have been conducted world-wide on a wide
variety of crops and the results are presented in Table 3. (Roussel
Uclaf, 1980).
Table 3. Delthamethrin residues in various crops.
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Root and Tuber
Vegetables
Carrots England 12.5g ai/ha 1 Root (76)
<0.01
France 12.5g ai/ha 1 Root 0.008 0.008 0.005
France 12.5g ai/ha 1 Root 0.015 0.008 0.007
Parsnips England 12.5g ai/ha 1 Root (76)
<0.01
Potatoes England 12.5g ai/ha 1 Tubers <0.01
F. R. Ger. 12.5g ai/ha 2 Whole n.d. n.d. n.d. n.d. n.d.
Tuber
2 Whole n.d. n.d. n.d. n.d. n.d.
Tuber
2 Whole n.d. n.d. n.d. n.d. n.d.
Tuber
Sugarbeets France 17.5g ai/ha 1 Root (11 wks)
<0.001
Beet (11 wks)
Top 0.002
25g ai/ha 1 Root (11 wks)
0.001
Beet (11 wks)
Top 0.001
17.5g ai/ha 1 Whole (17 wks)
Beet <0.001
25g ai/ha 1 Whole (17 wks)
Beet 0.001
17.5g ai/ha 1 Whole (21 weeks)
Beet <0.001
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
25g ai/ha 1 Whole (21 weeks)
Beet <0.001
17.5g ai/ha 2 Root (22 weeks)
0.004
Beet (22 weeks)
Top 0.003
25g ai/ha 2 Root (22 weeks)
0.002
Beet (22 weeks)
Top 0.003
England 12.5g ai/ha 1 Root (10 weeks)
<0.01
F. R. Ger. 25g ai/ha 3 Root n.d. (55) (109)
n.d. n.d.
25g ai/ha 3 Root n.d. n.d.
25g ai/ha 3 Root n.d. n.d.
25g ai/ha 4 Root n.d.(55) (66)
n.d. n.d.
Finland 15g ai/ha 2 Root (116)
<0.1
Bulb Vegetables
Leeks F. R. Ger. 13g ai/ha 2 Green 0.03 0.01 n.d. 0.02 n.d.
Part
13g ai/ha 2 White n.d. 0.03 0.02 n.d. n.d.
Part
13g ai/ha 2 Green 0.05 0.06 0.1 0.06 n.d.
Part
13g ai/ha 2 White n.d. n.d. n.d. n.d. 0.03
France 5g ai/ha 5 Leek 0.020
7.5g ai/ha 5 Leek 0.010
Table 3. Continued....
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Onions F. R. Ger. 13g ai/ha 2 Leaf 0.2 0.04 0.03 0.02 n.d.
13g ai/ha 2 Bulb 0.03 0.07 0.02 0.02 0.03
12g ai/ha 2 Peel n.d. n.d. n.d. n.d. n.d.
13g ai/ha 2 Leaf 0.05 0.01 0.02 0.03 0.03
13g ai/ha 2 Bulb 0.04 0.03 0.05 0.02 0.03
13g ai/ha 2 Peel n.d. n.d. n.d. n.d. n.d.
13g ai/ha 2 Leaf 0.4 0.1 n.d. 0.09 0.09
13g ai/ha 2 Bulb 0.04 0.05 0.1 n.d. 0.04
England 18.75g ai/ha 1 Bulb (52)
<0.01
Leafy Vegetables
Lettuce F. R. Ger. 25g ai/ha 2 Leaf 0.1 n.d. n.d. n.d.
25g ai/ha 2 Leaf 0.4 0.1 0.1 n.d. n.d.
25g ai/ha 2 Leaf 0.6 0.05 0.09 0.06 0.1
France 25g ai/ha 1 Leaf 0.25 0.25
25g ai/ha 1 Leaf 0.28 0.23
F.R.Ger. 12.5g ai/ha 2 Leaf 0.20 0.06 0.05 0.04 0.01
25g ai/ha 2 Leaf 0.30 0.20 0.10 0.08 0.01
12.5g ai/ha 2 Leaf 0.20 0.04 0.05 0.03 n.d.
25g ai/ha 2 Leaf 0.30 0.10 0.03 0.05 0.02
12.5g ai/ha 2 Leaf 0.10 0.04 0.01 n.d. 0.02
25g ai/ha 2 Leaf 0.20 0.10 0.02 0.01 n.d.
France 10g ai/ha 1 Leaf 0.23 0.067 0.004
17.5g ai/ha 1 Leaf 0.37 0.082 0.020
10g ai/ha 1 Leaf 0.22 0.044 0.006
17.5g ai/ha 1 Leaf 0.33 0.054 0.022
Spinach France 17.5g ai/ha 1 Crude Leaf 0.48 0.33 0.22
Cooked Leaf 0.27 0.21
Cooking Water 0.0001 0.00009
17.5g ai/ha 1 Crude Leaf 0.52 0.30 0.155
Cooked Leaf 0.28 0.12
Cooking Water 0.00015 0.00005
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
France 12.5g ai/ha 1 Crude Leaf 0.4 0.2 0.14 0.12
Cooked Leaf 0.12 0.10
Cooking Water 0.0001 n.d.
12.5g ai/ha 1 Crude Leaf 0.4 0.2 0.17 0.15
Cooked Leaf 0.15 0.12
Cooking Water 0.00015 0.0001
Brassica Leafy
Vegetables
Cabbages F. R. Ger. 25g ai/ha 2 Leaf 0.08 n.d. n.d. n.d. 0.01
25g ai/ha 2 Leaf 0.2 0.04 0.03 n.d. n.d.
25g ai/ha 2 Leaf 0.2 0.02 n.d. n.d. n.d.
25g ai/ha 2 Leaf 0.2 0.04 n.d. n.d. n.d.
S.Africa 5.0g ai/ha 1 Leaf <0.05 <0.05
7.5g ai/ha 1 <0.05 <0.05
10.Og ai/ha 1 <0.05 <0.05
Finland 3.8 mg/m 1 Leaf <0.1 <0.1
F.R.Ger. 12.5g ai/ha 2 Leaf 0.05 0.007 n.d. n.d. n.d.
Cabbage, Taiwan 50g ai/ha 8 Leaf 0.024 0.035 0.133 0.01 0.028 n.d.
Chinese
Cauliflower F. R. Ger. 12.5g ai/ha 2 n.d.
12.5g ai/ha 2 0.03 0.04 0.06 n.d. n.d.
12.5g ai/ha 2 0.09 0.03 0.02 0.02 0.01
Kohlrabi F.R.Ger. 12.5g ai/ha 2 0.006 n.d. n.d. n.d. n.d.
12.5g ai/ha 2 n.d. n.d. n.d. n.d. n.d.
12.5g ai/ha 2 n.d. n.d. n.d. n.d. n.d.
12.5g ai/ha 2 n.d. n.d. n.d. n.d. n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Stem Vegetables
Artichokes France 12.5g ai/ha 2 Whole 0.20 0.03 0.04 0.03
After 0.17 0.02 0.01 0.01
Cooking
Cooking 0.0005 0.0005 0.0001 0.0001
Water
12.5g ai/ha 2 Whole 0.23 0.06 0.05 0.04
After 0.17 0.06 0.05 0.04
Cooking
Cooking 0.0005 0.0005 0.0001 0.0001
Water
Legume Vegetables
Broad Beans France 12.5g ai/ha 1 Bean 0.01 0.02
Without
Pod 0.02 0.01
France 17.5g ai/ha 1 Whole Bean 0.001 0.0011 0.0008 0.0015
Whole 0.002 0.0009 0.0007 0.0015
Washed
France 15g ai/ha 1 Whole Bean 0.11 0.12 0.05 0.01
Whole 0.01 0.015 0.007 n.d.
Cooked
Cooking n.d. n.d. n.d. n.d.
Water
15g ai/ha 1 Whole Bean 0.1 0.06 0.05 n.d.
Whole 0.06 0.05 0.04 n.d.
Cooked
Cooking n.d. n.d. n.d. n.d.
Water
France 12.5g ai/ba 2 Peel 0.008
12.5g ai/ha 1 Grain n.d.
12.5g ai/ha 1 Peel 0.09 0.14 0.02 0.07
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
12.5g ai/ha 1 Whole Bean 0.04 0.07 0.01 0.03
12.5g ai/ha 1 Grain n.d. n.d. n.d. n.d.
12.5g ai/ha 1 Peel 0.10 0.02 0.01 0.03
12.5g ai/ha 1 Whole Bean 0.05 0.010 0.005 0.01
12.5g ai/ha 1 Grain n.d. n.d. n.d. n.d.
French Beans France 1.75g ai/ha 1 Whole Bean 0.001 0.0011 0.0008 0.0015
After Quick 0.002 0.0009 0.0012 0.0014
Washing
France 1.25g ai/hl 1 Whole Bean 0.11 0.12 0.05 0.01
Whole 0.01 0.015 0.007 n.d.
Cooked
Cooking n.d. n.d. n.d. n.d.
Water
France 1.25g ai/hl 1 Whole Bean 0.1 0.06 0.05 n.d.
Whole 0.06 0.05 0.04 n.d.
Cooked
Cooking n.d. n.d. n.d. n.d.
Soybeans France 12.5g ai/ha 1 Seed n.d. n.d. n.d. n.d. n.d.
Stem & 0.04 0.012
Leaves
Pod n.d. n.d.
12.5g ai/ha 2 Seed n.d. n.d. n.d. n.d.
Stem & 0.1
Leaves
17.5g ai/ha 1 Seed n.d. n.d. n.d. n.d. n.d.
Stem & 0.035 n.d.
Leaves
Pod 0.32 0.32
17.5g ai/ha 2 Seed n.d. n.d. n.d. n.d. n.d
Stem & n.d.
Leaves
Pod
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
France 12.5g ai/ha 2 Seed n.d.
12.5g ai/ha 2 Stem 0.13
12.5g ai/ha 2 Pod 0.45
Ivory Coast 25g ai/ha 7 Seed n.d.
Peas F. R. Ger. 12.5g ai/ha 2 Seed n.d. n.d. n.d. n.d. n.d.
12.5g ai/ha 2 Pod 0.04 0.03 0.04 0.04 0.04
12.5g ai/ha 2 Seed n.d. n.d. n.d. n.d. n.d.
12.5g ai/ha 2 Pod 0.05 0.01 0.02 n.d. n.d.
12.5g ai/ha 2 Seed n.d. n.d. n.d. n.d. n.d.
12.5g ai/ha 2 Pod 0.05 0.02 0.03 0.05 0.04
France 1.25g ai/hl 2 Seed 0.013 0.010
1.25g ai/hl 2 Pod 0.008 0.005
1.25g ai/hl 2 Whole Peas n.d. n.d.
England 12.5g ai/ha 1 Pod & Pea 0.02
Sugarpeas France 1.25g ai/hl 2 Whole Pea 0.030 0.015
Non-Washed
Whole Pea 0.030 0.005
Washed
France 1.25g ai/hl 1 Whole Pea 0.06 0.015 0.025 n.d.
Cooked Pea 0.02 0.007 0.008 n.d.
Cooking n.d. n.d. n.d. n.d.
Water
France 1.25g ai/hl 1 Whole Pea 0.10 0.025 n.d. n.d.
Cooked Pea 0.011 n.d. n.d. n.d.
Cooking n.d. n.d. n.d. n.d.
Water
Lentils Morocco 10g ai/ha 1 Grain n.d.
15g ai/ha 1 Grain n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Fruiting Vegetables
with Edible Peel
Cucumbers Finland 2.1 mg/l 1 Whole 0.02 0.03 0.01 n.d.
Fruit
France 12.5g ai/ha Whole 0.009 0.004 n.d. n.d.
Fruit
Pulp n.d. n.d. n.d. n.d.
Peel 0.04 0.005 n.d. n.d.
12.5g ai/ha Whole 0.0045 0.002 n.d. n.d.
Fruit
Pulp n.d. n.d. n.d. n.d.
Peel 0.026 0.012 n.d. n.d.
Eggplants France 1.25g ai/hl 1 Whole 0.025 0.010 0.006
Fruit
After 0.030 0.010 0.010
Washing
After 0.015 0.005 0.005
Cooking
Cooking 0.0001 0.00009 0.00007
Water
Gherkin Belgium 37.5g ai/ha 1 Whole (0.5)
Fruit 0.078 0.051 0.036 0.021
37.5g ai/ha 1 Whole 0.049 0.035 0.008 0.002
Fruit
37.5g ai/ha 1 Whole (0.5)
Fruit 0.009 0.013 0.007 0.004
37.5g ai/ha 1 Whole 0.019 0.004 0.001 0.004
Fruit
Jamaica France 12.5g ai/ha 1 Whole 0.10 0.03 0.03 0.04
Pepper Fruit
France 12.5g ai/ha 1 Whole 0.01 0.01 n.d. n.d.
Fruit
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Tomatoes France 12.5g ai/ha 1 Whole 0.023 0.023 0.027
Fruit
25g ai/ha 1 Whole 0.047 0.023 0.015
Fruit
12.5g ai/ha 1 Whole 0.008 0.009 0.024
Fruit
25g ai/ha 1 Whole 0.006 0.019 0.042
Fruit
France 25g ai/ha 2 Whole 0.017 0.014 0.016 0.011
Fruit
France 10g ai/ha 3 Whole 0.009 0.009
Fruit
10g ai/ha 3 Whole 0.010 0.007
Fruit
Spain 12.5g ai/ha 1 Whole <0.03 n.d. n.d.
Fruit
France 12.5g ai/ha 2 Whole 0.019 0.016 0.014 0.008 0.008 0.004 0.004
Fruit
Whole Washed 0.008 0.003
Whole Peeled n.d. n.d.
Peel 0.049 0.043
Juice 0.003 0.002
Washing Water 0.0019 0.001
Washing Water+48H 0.0001 n.d.
Finland 1 mg/l 1 Whole Fruit 0.04 0.03 0.01
Fruiting Vegetables
with Inedible Peel
Melons France 12.5g ai/ha 2 Peel 0.022 0.022 0.014
Pulp 0.0015 0.0016 0.0016
Whole Fruit 0.009 0.009 0.006
12.5g ai/ha 2 Peel 0.04 0.03 0.01
Pulp n.d. n.d. n.d.
Whole Fruit 0.018 0.015 0.004
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Citrus Fruits
Oranges Morocco 50g ai/ha 3 Peel (180) (180)
0.020 0.002
Pulp (180) (180)
0.003 n.d.
Whole Fruit (180) (180)
0.008 0.0006
75g ai/ha 3 Peel (180) (180)
0.035 0.005
Pulp (180) (180)
0.001 n.d.
Whole Fruit (180) (180)
0.011 0.0015
100g ai/ha 3 Peel (180) (180)
0.060 0.007
Pulp (180) (180)
n.d. n.d.
Whole (180) (180)
Fruit 0.017 0.0023
S. Africa 82.5g ai/ha 1 Peel 0.085 0.060 0.047 0.037 0.030 0.063 0.027 (35) (42)
0.044 0.023
Pulp 0.0003 0.0008 0.0004 0.0005 0.0008 0.0003 0.0008 (35) (45)
0.0009 0.0004
Whole 0.026 0.018 0.014 0.010 0.0095 0.018 0.0086 (35) (42)
Fruit 0.014 0.007
165g ai/ha 1 Peel 0.109 0.079 0.060 0.067 0.058 0.083 0.140 (35) (42)
0.100 0.042
Pulp 0.0009 0.0006 0.0007 0.0004 0.0006 0.0009 0.0002 (35) (42)
0.0009 0.0005
Whole 0.0355 0.0255 0.0215 0.020 0.022 0.026 0.043 (35) (42)
Fruit 0.032 0.013
Morocco 10g ai/ha 5 Peel (42)
0.05
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Pulp (42)
n.d.
Whole (42)
Fruit 0.01
Spain 12.75g ai/ha 1 Whole Fruit 0.1 n.d n.d
S.Africa 220g ai/ha 1 Peel (62)
0.10
Pulp (62)
n.d.
Whole (62)
Fruit 0.03
34.3g ai/ha 1 Peel (196)
0.005
Pulp (196)
0.0009
Whole (196)
Fruit 0.002
68.7g ai/ha 1 Peel (231)
n.d.
Pulp (231)
n.d.
Whole (231)
Fruit n.d.
S. Africa 110g ai/ha 1 Peel (86)
0.075
Pulp (86)
n.d.
Whole (86)
Fruit 0.025
220g ai/ha 1 Peel (86)
0.105
Pulp (86)
n.d.
Whole (86)
Fruit 0.035
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
396g ai/ha 1 Peel 0.27 0.28 0.24 0.15 0.22 0.25 0.16(135)(49)(63)
0.18 0.24 0.25
Pulp n.d. n.d. n.d. n.d. n.d. n.d. n.d.(35)(49)(63)
n.d.n.d.n.d.
Whole 0.10 0.09 0.09 0.06 0.08 0.06 (35)(49)(63)
Fruit 0.06 0.08 0.07
396g ai/ha 1 Peel 0.22 0.22 0.24 0.23 0.22 0.17 0.19 (35)(49)(63)
+132 l of oil 0.21 0.20 0.16
Pulp n.d. n.d. n.d. n.d. n.d. n.d. n.d. (35)(49)(63)
n.d.n.d.n.d.
Whole 0.10 0.10 0.095 0.08 0.10 0.08 0.08 (35)(49)(63)
Fruit 0.08 0.07 0.05
Clementines Morocco 10g ai/ha 5 Peel (42)
0.05
Pulp (42)
n.d.
Whole (42)
Fruit 0.01
Pome Fruits
Apples F. R. Ger. 50g ai/ha 4 Whole 0.05 0.03 0.03 0.02 0.03
Fruit
50g ai/ha 4 Whole 0.03 0.02 0.02 0.04 0.02
Fruit
50g ai/ha 4 Whole 0.2 0.1 0.08 0.1 0.06
Fruit
50g ai/ha 4 Whole Fruit 0.2 0.1 0.09 0.1
France 12.5g ai/ha 7 Whole (57)
Fruit 0.007
25g ai/ha 7 Whole 0.048 0.50 (57)
Fruit 0.032
25g ai/ha 6 Whole Fruit 0.061
25g ai/ha 9 Whole Fruit 0.194 0.264 0.144
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
France 7.5g ai/ha 8 Whole Fruit 0.005
12.5g ai/ha 8 Whole Fruit 0.007
7.5g ai/ha 10 Whole 0.003
12.5g ai/ha 10 Whole 0.004
12.5g ai/ha 5 Whole (34)
Fruit 0.002
12.5g ai/ha 5 Whole (34)
Fruit 0.005
7.5g ai/ha 8 Whole 0.008
Fruit
12.5g ai/ha 8 Whole 0.020 0.012 0.020
Fruit
F. R. Ger. 25g ai/ha 6 Whole 0.07 0.06 0.06 0.07 0.05
Fruit
50g ai/ha 6 Whole 0.06 0.06 n.d. 0.07 0.07
Fruit
25g ai/ha 6 Whole Fruit 0.05 0.05 0.04 0.05
50g ai/ha 6 Whole 0.05 0.04 0.04 0.05 0.04
Fruit
France 11.25g ai/ha 7 Whole Fruit 0.01
18.75g ai/ha 7 Whole Fruit 0.014
7.5g ai/ha 6 Whole Fruit 0.020 0.011
12.5g ai/ha 6 Whole Fruit 0.035 0.008
8.25g ai/ha 6 Whole Fruit 0.011
13.75g ai/ha 6 Whole Fruit 0.012
7.5g ai/ha 10 Whole Fruit 0.005
12.5g ai/ha 10 Whole Fruit 0.015
7.5g ai/ha 8 Whole Fruit 0.01
12.5g ai/ha 8 Whole Fruit 0.011
7.5g ai/ha 8 Whole Fruit 0.08
12.5g ai/ha 8 Whole Fruit 0.01 0.014 0.011
12.5g ai/ha 5 Whole (34)
Fruit 0.012
England 12.5g ai/ha 2 Whole 0.03
Fruit
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
F. R. Ger. 26g ai/ha 6 Whole 0.1 0.08 0.07 0.07
Fruit
26g ai/ha 6 Whole 0.08 0.05 0.04 0.05
Fruit
26g ai/ha 6 Whole 0.08 0.08 0.05 0.06
Fruit
France 0.75g ai/hl 8 Whole Fruit 0.035 0.030 0.032
1.25g ai/hl 8 Whole Fruit 0.068 0.040 0.050
0.75g ai/hl 5 Whole Fruit 0.017 0.008 0.006 0.012
1.25g ai/hl 5 Whole Fruit 0.025 0.011 0.010 0.006
0.75g ai/hl 5 Whole Fruit 0.013 0.010 0.008 0.016
1.25g ai/hl 5 Whole Fruit 0.013 0.012 0.013 0.012
0.75g ai/hl 5 Whole Fruit 0.025 0.020 0.011 0.013
1.25g ai/hl 5 Whole Fruit 0.0i8 0.034 0.030 0.019
0.75g ai/hl 5 Whole Fruit 0.016 0.015 0.017 0.018
1.25g ailhl 5 Whole Fruit 0.025 0.033 0.025 0.015
0.75g ai/hl 5 Whole Fruit 0.034 0.024 0.023 0.020
1.25g ai/hl 5 Whole Fruit 0.057 0.040 0.036 0.030
0.75g ai/hl 5 Whole Fruit 0.027 0.016 0.014 0.030
1.25g ai/hl 5 Whole Fruit 0.041 0.042 0.030 0.049
Sweden 22.5g ai/ha 3 Whole (63)
Fruit 0.010
S.Africa 0.31g ai/hl 2 Whole <0.05 <0.05 <0.05 <0.05
Fruit
0.75g ai/hl 4 Whole <0.05 <0.05 <0.05 <0.05
Fruit
France 7.5g ai/ha 6 Whole Fruit 0.045 0.035 0.02 0.01
7.5g ai/ha 4 Whole Fruit 0.03 0.03 0.01
Sweden 43.2 & 21.6g ai/ha 2 (58)
0.006
43.2 & 21.6g ai/ha 2 (58)
0.004
43.2 & 21.6g ai/ha 2 (58)
0.006
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Pears France 7.5g ai/ha 3 Whole (33)
Fruit 0.006
12.5g ai/ha 3 Whole (33)
Fruit 0.011
15g ai/ha 3 Whole (33)
Fruit 0.015
Italy 1.25g ai/hl 1 Whole Fruit 0.01 0.02 0.005
1.875g ai/hl 1 Whole Fruit 0.025 0.015 0.015
France 1.25g ai/hl 1 Whole Fruit 0.004
1.75g ailhi 1 Whole Fruit 0.004
1.25g ai/hl 1 Whole Fruit 0.006
1.75g ai/hl 1 Whole Fruit 0.006
0.75g ai/hl 4 Whole Fruit 0.018 0.021 0.015
1.25g ai/hl 4 Whole Fruit 0.017 0.014 0.004
France 7.5g ai/ha 5 Whole Fruit 0.125 0.015 0.010 0.010
7.5g ai/ha 5 Whole Fruit 0.09 0.02 0.02 0.02
Stone Fruits
Apricots France 12.5g ai/ha 2 Pulp 0.22 0.026 0.022 0.03
Whole Fruit 0.20 0.02 0.02 0.02
12.5g ai/ha 2 Pulp 0.077 0.026 0.026 0.011
Whole Fruit 0.07 0.02 0.02 0.01
France 12.5g ai/ha 2 Pulp 0.03 0.025 0.008 0.006
Whole Fruit 0.025 0.023 0.007 0.005
Cooked n.d. n.d.
12.5g ai/ha 2 Pulp 0.03 0.024 0.008 n.d.
Whole Fruit 0.027 0.020 0.007 n.d.
Cooked n.d. n.d.
Cherries France 15g ai/ha 2 Whole Fruit 0.055
21g ai/ha 2 Whole Fruit 0.095
27g ai/ha 2 Whole Fruit 0.045
F. R. Ger 25g ai/ha 3 Whole 0.04 0.04 0.02 0.04 0.03
Fruit
25g ai/ha 3 Whole 0.1 0.04 0.07 0.05 0.05
Fruit
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
25g ai/ha 3 Whole 0.08 0.06 0.06 0.05 0.04
Fruit
France 12.5g ai/ha 1 Pulp n.d. 0.0023 0.002
Whole Fruit n.d. 0.002 0.0018
Preserved 0.003 0.0017 0.0023
Pulp
Preserved 0.0025 0.0015 0.002
Pulp
Peaches France 12.5g ai/ha 1 Whole Fruit 0.05 0.04 0.02
12.5g ai/ha 1 Whole Fruit 0.03 0.04 0.02
25g ai/ha 1 Whole Fruit 0.09 0.07 0.08
25g ai/ha. 1 Whole Fruit 0.15 0.08 0.08
France 12.4g ai/ha 3 Whole Fruit 0.008 0.015
22.5g ai/ha 3 Whole Fruit 0.10 0.045
France 17.5g ai/ha 2 Pulp 0.03 0.047 0.043 0.029
Whole Fruit 0.03 0.043 0.040 0.026
Preserved n.d. n.d. n.d. 0.0015
Fruit
17.5g ai/ha 2 Pulp 0.027 0.045 0.040 0.024
Whole 0.025 0.04 0.037 0.022
Preserved n.d. n.d. n.d. n.d.
Fruit
F. R. Ger. 18.75g ai/ha 3 Whole 0.1 0.05 0.04 0.03
Fruit
18.75g ai/ha 3 Whole O.04 0.03 0.02 0.03
Fruit
18.75g ai/ha 3 Whole 0.06 0.02 0.04 0.02
Fruit
France 17.5g ai/ha 1 Whole 0.05 0.04 0.03 0.02
Fruit
Preserved 0.01.
Fruit
Juice n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Plum F. R. Ger. 25g ai/ha 5 Whole 0.07 0.08 0.08 0.07 0.05
Fruit
25g ai/ha 5 Whole 0.05 0.05 0.03 0.04 0.05
Fruit
25g ai/ha 5 Whole 0.02 0.04 0.05 0.05 0.04
Fruit
France 1.25g ai/hl 2 Whole Fruit 0.018 0.009 0.009 n.d.
Juice n.d.
Small Fruits
and Berries
Blackberries Finland 6.3 mg/bush 1 Berries 0.1 0.1
Grapes France 12.5g ai/ha 1 Whole Fruit 0.020 0.035 0.055 0.035 0.035
Fruit 12.5g ai/ha 1 Whole Fruit 0.055 0.020 0.020 0.020 0.025
25g ai/ha 1 Whole Fruit 0.025 0.045 0.033 0.015 0.010
25g ai/ha 1 Whole Fruit 0.040 0.015 0.015 0.015 0.005
France 25g ai/ha 1 Whole Fruit 0.006 0.075 0.040
25g ai/ha 1 Whole Fruit 0.015 0.010 0.030
F. R. Ger. 50g ai/ha 2 Whole 0.07 0.07 0.07 0.08 (35)
Fruit 0.06
50g ai/ha 2 Whole 0.05 0.08 0.07 0.05 (35)
Fruit 0.05
France 17.5g ai/ha 1 Whole Fruit 0.035 0.035
Juice Traces Traces
17.5g ai/ha 1 Whole Fruit 0.045 0.045
Juice Traces Traces
France 22.5g ai/ha 1 Whole Fruit 0.025 0.03 0.01 0.01
Juice 0.006
22.5g ai/ha 1 Whole Fruit 0.06 0.04 0.015 0.01
F. R. Ger. 25.Og ai/ha 2 Whole n.d. 0.04 0.07 0.05 0.02
Fruit
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Strawberries F. R. Ger. 50g ai/ha 1 Whole (5W)(6W)
Fruit n.d. n.d.
50g ai/ha 1 Whole (12W)(13W)
Fruit n.d. n.d.
50g ai/ha 1 Whole (6W)(7W)
Fruit n.d. n.d.
50g ai/ha 1 Whole (5W) (6W)
Fruit n.d.n.d.
France 12.5g ai/ha 1 Whole Fruit 0.015 0.004 0.008
Whole 0.014 n.d. n.d.
Washed
12.5g ai/ha 1 Whole Fruit 0.011 0.010 0.004
Whole 0.008 0.008 0.005
Washed
France 12.5g ai/ha 1 Whole Fruit 0.017 0.015 0.012 0.002
Jam n.d. n.d.
Assorted Fruits
with Edible Peel
Figs France 16g ai/ha 3 Whole Fruit <0.002
21g ai/ha 3 Whole Fruit <0.002
27g ai/ha 3 Whole Fruit 0.002
Olives France 15g ai/ha 3 Pulp <0.007
21g ai/ha 3 Pulp <0.007
27g ai/ha 3 Pulp <0.007
15g ai/ha 3 Whole Fruit <0.005
21g ai/ha 3 Whole Fruit <0.0O5
27g ai/ha 3 Whole Fruit <0.005
15g ai/ha 3 Pulp <0.007
21g ai/ha 3 Pulp <0.007
27g ai/ha 3 Pulp <0.007
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
15g ai/ha 3 Whole Fruit <0.005
21g ai/ha 3 Whole Fruit <0.005
27g ai/ha 3 Whole Fruit <0.005
17.5g ai/ha 4 Pulp 0.225 0.115 0.130 0.155 0.110 0.058
17.5g ai/ha 4 Whole Fruit 0.140 0.080 0.085 0.100 0.070 0.040
Oil 0.035 0.040 0.060 0.006
France 15g ai/ha 3 Whole Fruit 0.003
21g ai/ha 3 Whole Fruit 0.004
25g ai/ha 3 Whole Fruit 0.003
21g ai/ha 3 Whole Fruit 0.003
25g ai/ha 3 Whole Fruit 0.006
18.75g ai/ha 1 Whole (75)
Fruit 0.007
18.75g ai/ha 1 Whole (55)
Fruit 0.005
18.75g ai/ha 2 Whole (55)
Fruit 0.016
25g ai/ha 1 Whole (55)
Fruit 0.018
37.5g ai/ha 1 Whole (55)
Fruit 0.029
France 28g ai/ha 2 Pulp n.d. n.d. n.d.
Oil n.d. n.d. n.d.
Tunisia 12.5g ai/ha 1 Pulp (135)
n.d.
Assorted Fruits
with Inedible Peel
Bananas Phillippines 2.5g ai/ha 3-6 Pulp (77)
<0.01
Peel (77)
<0.01
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Whole (77)
Fruit <0.01
2.5g ai/ha 3-6 Pulp (77)
<0.01
Peel (77)
<0.01
Whole (77)
Fruit <0.01
2.5g ai/ha 3-6 Pulp (77)
<0.01
Peel (77)
<0.01
Whole (77)
Fruit <0.01
2.5g ai/ha 3-6 Pulp (77)
0.01
Peel (77)
1.0
Whole (77)
Fruit 0.39
2.5g ai/ha 3-6 Pulp (77)
<0.01
Peel (77)
0.10
Whole (77)
Fruit 0.04
Guadeloupe 22.5g ai/ha 1 Pulp (77)
n.d.
Peel (77)
0.07
Whole (77)
Fruit 0.016
46.8g al/ha 1 Pulp (77)
n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Peel (77)
0.03
Whole (77)
Fruit 0.01
93.7g ai/ha 1 Pulp (77)
0.003
Peel (77)
0.10
Whole (77)
Fruit 0.03
Pineapples 75g ai/ha 1 Juice (31)(61)
n.d. n.d.
Pulp (31)(61)
n.d. n.d.
Peel (31)(61)
0.025 0.018
Whole (31)(61)
Fruit 0.007 0.005
150g ai/ha 1 Juice (31)(61)
n.d. n.d.
Pulp (31)(61)
n.d. n.d.
Peel (31)(61)
0.2 0.025
Whole (31)(61)
Fruit 0.07 0.008
Cereal Grains
Wheat Brazil 7.5g ai/ha 1 Grain n.d.
10g ai/ha 1 Grain n.d.
12.5g ai/ha 1 Grain n.d.
15g ai/ha 1 Grain n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
France 10g ai/ha 1 Grain (53)
0.003
17.5g ai/ha 1 Grain (53)
0.003
10g ai/ha 1 Grain (53)
0.004
17.5g ai/ha 1 Grain (53)
0.0035
France 7.5g ai/ha 1 Grain (80)
0.002
Stem (80)
0.015
15g ai/ha 1 Grain (80)
0.04
Stem (80)
0.05
7.5g ai/ha 1 Grain (74)
0.001
Stem (74)
0.025
Flour (74)
n.d.
Bran (74)
n.d.
15g ai/ha 1 Grain (74)
0.003
Stem (74)
0.025
Flour (74)
n.d.
Bran (74)
n.d.
7.5g ai/ha 1 Grain (61)
0.0015
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Stem (61)
0.025
Flour (61)
n.d.
Bran (61)
n.d.
15g ai/ha 1 Grain (61)
0.002
Stem (61)
0.025
Flour (61)
n.d.
Bran (61)
n.d.
France 7.5g ai/ha 1 Grain (64)
n.d.
Stem (64)
n.d.
Flour (64)
n.d.
Bran (64)
n.d.
15g ai/ha 1 Grain (64)
n.d.
Stem (64)
n.d.
Flour (64)
n.d.
Bran (64)
n.d.
Maize F. R. Ger. 17.5g ai/ha 1 Corn/ (55)
Grain 0.006
Brazil 15g ai/ha 2 Corn/ n.d. n.d. n.d. n.d. n.d.
Grain
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
France 7.5g ai/ha 1 Corn/ (70)
Grain n.d.
12.5g ai/ha 1 Corn/ (70)
Grain n.d.
France 7.5g ai/ha 1 Corn/ (94)
Grain n.d.
12.5g ai/ha 1 Corn/ (94)
Grain n.d.
Rice Guyanne 12.5g ai/ha 1 Grain (50)
n.d.
12.5g ai/ha 1 Grain (50)
0.020
Suriname 6.25g ai/ha 1 Grain (43)(49)
0.001 n.d.
Straw (43)(49)
n.d. n.d.
12.5g ai/ha 1 Grain (43)(49)
0.005 n.d.
Straw (43)(49)
0.010 0.008
18.75g ai/ha 1 Grain (43)(49)
n.d. 0.005
Straw (43)(49)
0.010 0.010
25g ai/ha 1 Grain (43)(49)
0.015 0.015
Straw (43)(49)
0.080 0.050
Ivory Coast 18.75g ai/ha 3 Grain (39)
0.03
Philippines 17.5g ai/ha 5 Grain (37)
n.d.
Husk (37)
n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
25g ai/ha 5 Grain (37)
n.d.
Husk (37)
n.d.
Fodders & Straws
Grass New Zealand 5g ai/ha 1 0.52 0.42 0.4 0.2 0.13
(Pasture) 10g ai/ha 1 1.07 1.07 0.8 0.67 0.46 0.07
15g ai/ha 1 1.20 1.23 1.3 0.70 0.5
20g ai/ha 1 1.37 1.30 2.3 0.97 0.8 0.47
35g ai/ha 1 2.89 2.42 2.68 1.87 1.0 1.3
50g ai/ha 1 4.7 3.7 3.6 3.6 1.9 1.1
Legume Oilseed
Peanuts Ivory Coast 18.5g ai/ha 7 Grain (71)
n.d.
Pods (71)
0.005
Legume
Animal Feeds
Alfalfa France 12.5g ai/ha 2 Whole 1.5 l.0 0.45 0.11
Fodder Crop
(Lucerne)
12.5g ai/ha 2 Whole 1.6 0.75 0.48 0.10
Crop
New Zealand 10.Og ai/ha 1 Whole 0.25 0.25 0.23 0.09 n.d.
Crop
France 12.5g ai/ha 2 Whole 1.0 0.16 0.10
Green
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
12.5g ai/ha 2 Whole Dry 0.20
12.5g ai/ha 2 Whole 0.25 0.065 0.03
Green
12.5g ai/ha 2 Whole Dry 0.03
Oilseeds
Cottonseed USA 22.5g ai/ha 3 Cottonseed n.d.
22.5g ai/ha 8 Cottonseed n.d.
22.5g ai/ha 5 Cotton (43)
Seed <0.02
22.5g ai/ha 4 Cotton (45)
Seed <0.02
22.5g al/ha 5 Cotton (49)
Seed <0.02
22.5g ai/ha 14 Cotton (52)
Seed n.d.
22.5g ai/ha 7 Cotton (77)
Seed n.d.
USA 0.03 mg/kg 1 Cotton 0.03
(Fortified Seed
Cotton Delinted <0.02
Seed) Seed
Hulls 0.035
Linters 0.325
Solvent n.d.
Extracted
Meal
Refined <0.02
Oil
Alkaline <0.02
Soap
Stock
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
0.03 mg/kg 1 Total n.d.
Full
Fatty
Acids
0.34 mg/kg 1 Cotton 0.33
Seed
Delinted 0.05
Seed
Hulls 0.39
Linters 3.2
Solvent n.d.
Extracted
Meal
Refined n.d.
Oil
Alkaline n.d.
Soap
Stock
Total n.d.
Free
Fatty
Acids
Morocco 25g ai/ha 7 Cotton (62)
Seed <0.01
25g ai/ha 6 Cotton (121)
Seed 0.002
USA 22.5g ai/ha 6 Cottonseed n.d.
22.5g ai/ha 6 Cottonseed n.d.
22.5g ai/ha 7 Cottonseed <0.02
22.5g ai/ha 8 Cotton (40)
Seed 0.03
22.5g ai/ha 8 Cottonseed n.d.
22.5g ai/ha 10 Cotton (40)
Seed <0.02
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
22.5g ai/ha 14 Cottonseed 0.08
22.5g ai/ha 15 Cottonseed <0.02
22.5g ai/ha 15 Cottonseed <0.02
22.5g ai/ha 15 Cottonseed <0.02
22.5g ai/ha 15 Cottonseed 0.01
22.5g ai/ha 15 Cottonseed n.d.
USA 22.5g ai/ha 15 Cottonseed <0.02
Delinted n.d.
Seed
Linters <0.05
Hulls n.d.
Solvent n.d.
Extracted
Meal
Refined <0.02
Oil
Alkaline n.d.
Soap
Stock
Morocco 12.5g ai/ha 7 Cottonseed n.d.
12.5g ai/ha 7 Cottonseed n.d.
+ NH
Ivory Coast 15g ai/ha 6 Fibers 0.007
India 10g ai/ha 8 Cotton n.d.
Seed
Fiber n.d.
Oil n.d.
Cake n.d.
20g ai/ha 8 Cottonseed n.d.
Fiber n.d.
Oil n.d.
Cake n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Rapeseed F. R. Ger. 12.5g ai/ha 1 Seed (73)
n.d.
Cake (73)
n.d.
Oil (73)
n.d.
Seed (51)(80)
0.01 0.03
Cake (51)(80)
n.d. n.d.
Oil (51)(80)
n.d. n.d.
Sweden 100g ai/ha 1 Seed (84)
n.d.
Finland 15g ai/ha 1 Seed (87)
<0.05
France 5g ai/ha 1 Seed (38)
n.d.
5g ai/ha 1 Seed (48)
n.d.
Tropical Seeds
Cocoa Ivory 6.25g ai/ha 8 Cocoa (38)
Beans Coast Bean 0.006
8.75g ai/ha 8 Cocoa (38)
Bean 0.025
8.75g ai/ha 8 Cocoa (38)
Bean 0.007
Ivory 18.75g ai/ha 3 Cocoa (72)
Coast Bean n.d.
Coffee Brazil 6.25g ai/ha 1 Grain n.d. n.d. n.d.
Beans 6.25g ai/ha 2 Grain n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
12.5g ai/ha 1 Grain n.d. n.d. n.d.
12.5g ai/ha 2 Grain 0.02
Ivory 12.5g ai/ha 2 Grain (198)
Coast n.d.
18.75g ai/ha 2 Grain (198)
n.d.
Brazil 10g ai/ha 2 Grain (46)
n.d.
Ivory 18.75g ai/ha 2 Grain (178)
Coast n.d.
Spices
Pepper Cameroun 12.5g ai/ha 1 Black (56)
Grains n.d.
12.5g ai/ha 1 Grey (56)
Grains n.d.
12.5g ai/ha 1 Green (35)
Grains n.d.
Tea
Tea China 6.6g ai/ha 1 Leaf 3.1 3.0 1.1 0.75 0.65
Water 0.001 0.005 0.0006 n.d. n.d.
10.0g ai/ha 1 Leaf 4.3 1.6 1.4 1.5 0.85
Water 0.002 0.0005 0.0009 n.d. n.d.
13.3g ai/ha 1 Leaf 3.2 3.2 2.5 1.9 1.2
Water 0.002 0.0013 0.0009 n.d. n.d.
20.0g ai/ha 1 Leaf 2.5 4.8 3.4 2.4 1.5
Water 0.005 0.001 0.0008 n.d. n.d.
India 10.0g ai/ha 1 Leaf 5.5 2.1 1.7 1.0
Water n.d. n.d. n.d. n.d.
15.0g ai/ha 1 Leaf 7.8 5.1 2.9 2.3
Water n.d. 0.0005 n.d. n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Miscellaneous
Hops England 12.5g ai/ha 7 Cone 0.02
Mushrooms Netherlands 6.25g ai/ha 2 Whole 0.0033 0.0017 0.0010
6.25g ai/ha 2 Whole 0.0014 0.0012
25g ai/ha 2 Whole 0.0088 0.0036 0.0029
25g ai/ha 2 Whole 0.0041 0.0027
Sugarcane Brazil 25g ai/ha 2 Juice n.d.
Martinique 7.5g ai/ha 1 Juice (98)
n.d.
25g ai/ha 1 Juice (98)
n.d.
7.5g ai/ha 1 Juice n.d.
25g ai/ha 1 Juice n.d.
Trials on various root and tuber vegetables resulted in residues
ranging from n.d. (<0.001 mg/kg) to 0.015 mg/kg at 2-3 days after
last application.
Trials on bulb vegetables (leeks and onions) resulted in residues
ranging from <0.01 to 0.1 mg/kg on the bulb and 0.1 mg/kg on the leaf
at 2-5 days after last application.
Trials on leafy vegetables (lettuce and spinach) resulted in residues
ranging from 0.52 mg/kg (highest value) at 2-3 days after last
application to 0.15 mg/kg (highest value) at 13-16 days.
Trials on Brassica leafy vegetables (cabbages, cauliflower, and
kohlrabi) ranged from 0.13 mg/kg (highest value) at 2-3 days to 0.03
mg/kg (highest value) at 13-16 days after last application.
Trials on artichokes gave residues ranging from 0.23 mg/kg at 2-3 days
to 0.04 mg/kg at 13-16 days after last application. Cooking did not
reduce the residue levels significantly.
Trials on legume vegetables gave residues ranging from 0.1 mg/kg (high
value) at 2-3 days to 0.07 mg/kg ( high value) at 13-16 days after
last application except for soybean pods which had residues up to 0.35
mg/kg at 13-16 days.
Trials on fruiting vegetables (edible peel) resulted in residues
ranging from 0.1 mg/kg (high value, jamaica pepper) at 2-3 days to
0.04 mg/kg (high value, tomato) at 13-16 days after last application.
Residues from trials on melons did not exceed 0.0016 mg/kg in the pulp
or 0.018 mg/kg in the whole fruit at any interval from 1-8 days after
last application.
Residues on citrus fruits resulting from the highest rate of use (396
g ai/ha + oil) declined from 0.1 mg/kg (whole fruit) on the day of
treatment to 0.08 mg/kg (whole fruit) at 35 days after last
application.
Trials on pome fruits resulted in residues on whole fruit that did not
vary significantly with the number of days after treatment but were
largely dose-dependent. A dosage rate of 50g ai/ha gave a highest
residue of 0.2 mg/kg on day of treatment. Dosage rates of 1.25 g
ai/hl gave residues that did not exceed 0.057 mg/kg.
Trials on stone fruits resulted in residues on whole fruit ranging
from 0.2 mg/kg (apricots) at 2-3 days to 0.08 mg/kg ( highest value,
peaches) at 13-16 days after last treatment.
Residues on small fruits and berries ranged from 0.1 mg/kg (high
value, blackberries) and 0.08 mg/kg (high value, grapes) to 0.017
mg/kg (high value, strawberries).
Trials on assorted fruits (edible peel) resulted in residues on figs
of <0.002 mg/kg at 13-16 days after last application. For olives the
results ranged from 0.225 mg/kg (pulp) to 0.003 mg/kg at 1 day after
treatment. By 55 days after treatment residues did not exceed 0.018
mg/kg (whole fruit).
Residues on assorted fruits (inedible peel) did not exceed 0.01 mg/kg
in the pulp of bananas at 77 days after last treatment even at the
highest dosage tested (93.7 ai/ha). Residues in pineapple pulp were
n.d. at 31 days post-treatment, but residues in whole fruit were 0.*07
mg/kg at the same interval. No residues were detected in pineapple
juice.
Trials on cereal grains resulted in maximum residues of 0.04 mg/kg in
wheat grain at 80 days after last treatment. Residues in wheat flour
and bran were n.d. after 61 days post-treatment at dosages up to 15 g
ai/ha.
A maximum residue of 0.006 mg/kg was found in maize grain at 55 days
post-treatment. A maximum residue of 0.03 mg/kg was found in rice
grain at 39 days post-treatment. There were no detectable residues in
rice husks.
Trials on pasture grass resulted in residues up to 4.7 mg/kg on day of
treatment with dosage of 50 g ai/ha. The residue had declined to 1.1
mg/kg by 9-12 days post-treatment.
A trial on peanuts resulted in no detectable residue in the grain and
0.005 mg/kg in the pod at 71 days after last treatment.
Trials on alfalfa animal fodder resulted in residues that ranged from
0.25 to 1.6 mg/kg at 2-3 days after last treatment and steadily
declined to values of n.d. to 0.2 mg/kg after 21-24 days. Trials on
soybean stem and leaves resulted in residues ranging from n.d. to 0.1
mg/kg at 13-16 days after last treatment.
Residues in cotton seed were 0.03 mg/kg or less at 40 or more days
after field treatment at 22.5 g ai/ha for 3-15 treatments. Cotton
seed fortified at 0.34 mg/kg gave residues after processing
distributed as follows: seed-0.33 mg/kg, delinted seed-0.05 mg/kg,
hulls-0.39 mg/kg, linters-3.2 mg/kg, solvent extracted meal-n.d.,
refined oil-n.d., alkaline soap stock-n.d., total free fatty
acids-n.d. Residues in processed products from cotton field-treated
15 times at 22.5 g ai/ha did not exceed 0.05 mg/kg (linters).
Residues in rapeseed, cake, and oil were less than 0.05 mg/kg at 51-87
days post-treatment.
Trials on cacao resulted in residues in cocoa beans that did not
exceed 0.025 mg/kg at 38 days after last treatment regardless of trial
dosage or number of applications. Residues in coffee beans were n.d.
at 13-198 days after last treatment at dosages of 6.25 to 18.75 g
ai/ha. However, analysis of a sample at 1 day after the last of 2
treatments at 12.5 g ai/ha found a residue of 0.02 mg/kg.
Residues on spices (pepper; black, grey and green grains) were n.d. at
35-56 days post-treatment with 12.5 g ai/ha.
Residues on tea (leaves) ranged from 2.5 to 7.8 mg/kg (not
dose-related) at 0-1 day post-treatment with single dosages from 6.6
to 20 g ai/ha. Residues declined thereafter to a range of 0.65 to 1.5
mg/kg at 13-16 days post-treatment. Analysis of tea water brewed from
leaves picked on day of treatment gave residues ranging from 0.001 to
0.005 mg/kg which became n.d. (<0.0005 mg/kg) by 6-8 days
post-treatment.
Trials on miscellaneous crops (hops, mushrooms, sugarcane) resulted in
residues on hops cones of 0.02 mg/kg at 9-12 days after the last of 7
treatments. Residues on mushrooms ranged from 0.0033 to 0.0088 mg/kg
(dose-dependent) at 1 day after last treatment with either 6.25 or 25
g ai/ha and declined to 0.0012 to 0.0029 mg/kg by 4-5 days
post-treatment. Residues in the juice of sugarcane were n.d. (<0.005
mg/kg) at 6-8 days after last treatment at any level tested.
FATE OF RESIDUES
General
On foliage in the field, deltamethrin degrades rapidly to a large
variety of hydrolytic, oxidative, and photolytic products and their
conjugates. The following extractable compounds have been identified:
trans-deltamethrin, trans-hydroxymethyl deltamethrin, 4'-hydroxy
deltamethrin, 4'-hydroxy-trans-hydroxymethyl deltamethrin,
3-(2,2-dibromovinyl)-2,2 dimethyl-cyclopropane-carboxylic acid
(Br2CA) and 3 conjugates, trans-hydroxymethyl Br2CA,
3-phenoxybenzaldehyde (pb ald) and the corresponding alcohol (pb alc)
and acid (pb acid), 4'-hydroxyphenoxy-benzoic acid, and 2 or 3
conjugates of each of phenoxybenzyl alcohol, phenoxybenzoic acid, and
alpha-cyanophenoxybenzyl alcohol. Considerable amounts of
unextractable residues of unknown composition (up to 35% of the
applied dose) are also formed. Metabolism in animals is generally
similar to that in plants, differing in the nature of the conjugates.
Rapid excretion of deltamethrin and its metabolites occurs in animals
with negligible tendency to bioaccumulate.
In animals
Metabolism in a lactating cow
Studies were carried out in two phases on a single lactating Jersey
cow, weighing 350 kg, giving milk of approx. 6.3% butterfat, using
C14-deltamethrin labelled in the alpha-methine group of the alcohol
moiety. In phase 1, 0.27 g of labelled deltamethrin was injected
intrarumenally as a solution in a sesame oil/alcohol mixture.
Radioactivity was rapidly excreted, mainly in urine and faeces
(85.3%). Only 0.4% was found in whole milk, corresponding to peak
residue levels after only one day of 0.045 and 0.92 mg/kg deltamethrin
equivalents in whole milk and rendered butterfat respectively. The
residue found in the peak butter sample was mainly (89%) unchanged
deltamethrin. The half-life in milk and butter was 0.8 days. Omental
fat and leg muscle biopsy samples, removed two days after treatment,
contained 0.088 and 0.008 mg/kg deltamethrin equivalents respectively.
In phase 2, conducted 49 days after phase 1, 0.21 g of labelled
deltamethrin in the form of a miscible oil formulation was applied
externally to the cow (except on the udder) by brushing. Although
well restrained by a neck halter, the sow succeeded in licking herself
to a nominal degree. Peak residue levels were attained after 2-5 days
(0.0057 mg/kg) in whole milk and 2 days (0.1 mg/kg) in butterfat.
Half-lives for depletion of activity from whole milk, butterfat, and
body hair were in the range of 4 to 4 1/2 days. There was no apparent
degradation of deltamethrin on body hair during the first 20 days.
Since good control of flies in stables is obtained by spraying 7.5 mg
ai/m2 on the walls, an estimate of residue potential in milk and
butter from this source was made on the assumption that a restrained
dairy cow cannot lick more than 1 m2 of treated wall. Calculated
residues of deltamethrin would thus be 0.00125 mg/kg and 0.0255 mg/kg
in milk and butter respectively (WELL 79 04 HIBH/A).
In plants
Cotton was treated topically on the leaves with each of
C14-deltamethrin samples labelled in the dibromovinyl, benzylic, and
cyano carbons in field and greenhouse studies in California. Initial
deposits were 0.04-0.33 g/cm2 of leaf surface (3-15 mg/kg based on
fresh leaf weight). Leaves were harvested at 2 and 6 weeks after
treatment and immediately cut into approx. 5 mm2 pieces. The 14C
products were extracted by soaking in acetonitrile-chloroform (2:1)
and decantation. The concentrated extracts were analysed for total
14C content by liquid scintillation counting of an aliquot and for
individual 14C compounds by TLC. Unextractable residue was
determined by combustion analysis.
Metabolism in cotton and bean leaf discs from leaves freshly removed
from greenhouse-grown plants was also studied. Discs of 10-mm diameter
were punched out under water and incubated for 5 h at 30°C under
artificial illumination in 25-ml Erlenmeyer flasks containing 25
discs, 2 ml distilled water, and 1-5 g of 14C substrate in 30 l
ethanol.
Under the greenhouse conditions the half-life of deltamethrin is 1.1
weeks with a 90% loss in 4.6 weeks. Under field conditions there is a
more rapid loss of parent compound, a higher proportion of trans- to
cis-deltamethrin and larger amounts of unextractable products. The
primary compounds found (1% of applied dose) after six weeks were
deltamethrin (1.7-6.1%), trans-deltamethrin (0.7-2.7%), Br2CA
(0.3-3%), Br CA-conj. (7.7-4.2%), pb ald (1.2-1.1%), pb acid (0-2%),
pb alc-conj. (1.9-1.2%), pb acid glyc. (0.9-1.7%), pb acid conj.
(5.9-1.5%), pb cy-conj. (8.8-3.2%), and unextractables (35-20%).
In the leaf disc experiments, bean leaf discs converted [14C]- and
[14C(alpha)]-deltamethrin in small yield (approx. 6%) to
[14C]Br2CA-glyc and [14C]alc-glyc respectively, whereas cotton leaf
discs did not. Cleavage products 14CBr2CA and [14C(alpha)]pb cy as
substrates undergo more extensive metabolism than deltamethrin in both
plants (Ruzo et al., 1979).
Uptake - translocation studies in cotton plant growth chambers
following hydroponic, foliar, and soil treatments were carried out
using C14 labelled (alpha-methrin position) deltamethrin. For the
hydroponic application, a 750 ml volume of nutrient solution/jar was
fortified with an ethanol solution of 14C-deltamethrin. For the soil
application 2.5% EC formulation prepared by adding 14C-deltamethrin
to an EC blank was applied evenly by pipette to the soil surface 3 cm
around 2-week old potted plants. Foliar application was made as a
droplet of ethanol solution of 14C-deltamethrin from a micropipette
to the midvein of the first leaf of the cotton plant. Plants were
harvested at 1, 3 and 7 days after treatment for autoradiography and
extraction. Analyses were by liquid scintillation, combustion of
post-extraction solids, and TLC. Recovery of radioactivity was 96.5%.
Results of the foliar and hydroponic experiments indicated virtually
no systemic behaviour for deltamethrin with observed transport being
extremely limited (0.5-0.8%) after 7 days in both cases. For soil
treatment, radioactivity detected in the shoots and roots was only 1.6
and 0.35% of applied dose respectively after 7 days. Most of the
radioactivity found in the shoot was concentrated in the polar and
bound residue fractions (PRO 77 1309/A).
In soil
No data.
In water
No data.
In storage and processing
Wheat
Trials were conducted on the persistence of deltamethrin residues on
stored wheat and processed wheat products in Morocco, Belgium, Greece,
and the U.K. following treatment of the grains with various
formulations at low, but effective, dosages as shown in Table 4. In
Morocco, dusts and EC formulations of equal strength (0.025 g ai/ql)
resulted in lower initial (8-11 weeks) residues (approx. 0.01 mg/kg)
for EC than for dust (approx. 0.05 mg/kg). However, after 40 weeks,
residues on grain were approximately equal (approx. 0.1 mg/kg) with no
measurable losses, taking account of the analytical variability.
Washing the grain after treatment had no effect on residue levels.
Bran and flour produced from the wheat after 40 weeks storage had mean
residues of 0.15 mg/kg and 0.005 mg/kg respectively (FP. 78.27.04/A).
In Belgium, low volume sprays of Decis EC 25 at 0.7, 0.85, or 1.0
mg/kg gave initial (1 week) residues proportional to dosage of 0.56,
0.69, or 0.78 mg/kg respectively. These had fallen to 0.27, 0.47, or
0.69 by the end of 35 weeks. The corresponding residues in flour and
bran were 0.1, 0.25, and 0.4 mg/kg and 1.2, 1.3, and 1.7 mg/kg
respectively (FP-78.07.12/A).
In Greece low volume sprays of K-OTHRINE EC 25 at 1 or 0.75 mg/kg on
wheat grain gave residues of 0.31 and 0.29 mg/kg respectively after 24
and 17 weeks (FP-80.05.02/A).
In the U.K. two liquid spray formulations containing piperonyl
butoxide were applied to wheat, at calculated dosages of 1 or 2 mg/kg.
Analysis of the wheat on the day of treatment however showed actual
levels of 0.44 and 0.80 mg/kg respectively. Residue analysis on
samples taken at monthly intervals for three months showed no
degradation of the deltamethrin. There was no detectable degradation
of deltamethrin by the process of milling and backing of either
freshly treated wheat or wheat that had been stored for three months
(WELL/80.21.05/A1).
Table 4. Deltamethrin residues in stored products
Number of Part Residues (mg/kg) at intervals (days) after last application1
Crop Country Dosage rate applications analyzed 0 1 2-3 4-7 8-11 12-19 20-29 30-39 40-49 50-59
Cereal Grains
Wheat Morocco 0.025g ai/ql 1 Non-washed 0.050 0.067 0.050 0.100 0.120
Dust
0.025g ai/ql 1 Washed 0.052 0.042 0.060 0.080 0.120
Dust
0.025g ai/ql 1 Non-washed 0.010 0.010 n.d. 0.110 0.080
EC 25 + PB2
0.025g ai/ql 1 Washed 0.015 0.012 n.d. 0.060 0.100
EC 25 + PB
0.025g ai/ql 1 Non-Washed 0.145
Dust Wheat bran
0.025g ai/ql 1 Washed 0.145
Dust Wheat bran
0.025g ai/ql 1 Non-Washed 0.16
EC 25 + PB Wheat bran
0.025g ai/ql 1 Washed 0.14
EC 25 + PB Wheat bran
0.025g ai/ql 1 Non-Washed 0.007
Dust Wheat flour
0.025g ai/ql 1 Washed 0.004
Dust Wheat flour
0.025g ai/ql 1 Non-Washed 0.004
EC 25 + PB Wheat flour
0.025g ai/ql 1 Washed 0.004
EC 25 + PB Wheat flour
Belgium 0.7 mg/kg 1 Grain 0.56 0.70 0.35 0.27
Low vol. spray Wheat flour 0.065 0.1
Low vol. spray Wheat bran 0.65 1.2
0.85 mg/kg 1 Grain 0.69 1.00 0.50 0.47
Wheat flour 0.07 0.25
Wheat bran 1.00 1.3
Table 4. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application1
Crop Country Dosage rate applications analyzed 0 1 2-3 4-7 8-11 12-19 20-29 30-39 40-49 50-59
1.0 mg/kg 1 Grain 0.78 1.10 0.70 0.69
Low vol. spray Wheat flour 0.09 0.4
Low vol. spray Wheat bran 1.20 1.7
Greece 1.0 mg/kg 1 Grain 0.31
Low vol. spray
0.75 1 Grain 0.29
U.K. 1.0 mg/kg + PB 1 Wheat 0.44 0.53 0.48 0.50
2. 0 mg/ 1 kg + PB 1 Wheat 0.80 1.25 1.33 1.46
Dil. ag. spray
1.0 mg/kg + PB 1 Wheat 0.44 0.50
Wholemeal 0.42 0.43
flour
Wholemeal 0.26 0.27
bread
Bran 3.0 1.8
Fine n.d. 0.5
Offal
First n.d. n.d.
reduction
flour
Total 0.09 0.05
white
flour
White 0.10 n.d.
bread
2.0 mg/kg + PB 1 Wheat 0.80 1.46
Whole 0.73 0.80
meal
flour
Whole 0.63 0.57
meal
bread
Bran 5.4 4.7
Fine n.d. 0.75
Offal
Table 4. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application1
Crop Country Dosage rate applications analyzed 0 1 2-3 4-7 8-11 12-19 20-29 30-39 40-49 50-59
First n.d. n.d.
reduction
flour
Total 0.20 0.20
white
flour
White 0.29 0.15
bread
Maize Brazil 0.5 mg/kg dust 1 Grain 0.6
1. 0 mg/kg dust 1 Grain 0.7
2.0 mg/kg dust 1 Grain 1.0
Brazil 1.0 mg/kg dust 1 Whole Fruit n.d.
1. 0 mg/kg dust 1 Grain n.d.
Cameroon 5 mg/kg 1 Straw/ 1.25
EC spray Stem
Cameroon 2.5 mg/kg 1 Grain 0.007
EC spray
Cameroon 2. 5 mg/kg 1 Grain 0.47
EC spray
Peanuts Cameroon 5 mg/kg 1 Straw/ 1.25
Stem
Coffee Brazil 1.0 mg/kg dust 1 Grain 0.95
2.0 mg/kg dust 1 Grain 1.65
Brazil 25g/350m2 1 Unroasted 0.01
Spray on bags Grain
25g/350m2 1 Roasted n.d.
Spray on bags Grain
Brazil 0.0040 m3 1 Grain n.d.
ULV fog
1 Residue data on wheat grain in Morocco at 127, 187, and 244 days is omitted for table size
and convenience since the values do not differ significantly from those occurring before and after.
2 PB = Piperonyl butoxide.
Maize
Trials were conducted in Brazil and Cameroon on stored maize treated
at various low levels with either dusts or low volume sprays as shown
in Table 4. Analysis of grain samples after 20-50 weeks showed no
measurable degradation for treatments at 0.5 mg/kg. However, for
treatments of 1 mg/kg and above, residues had decreased by 50 to 99.7%
(FP-78.30.09/A, FP-79.16.07/A, FP-79.18.07/A, FP-78.30.05/A, and
FP-78.02.10/A).
Peanuts
Table 4 also presents the results of one trial in Cameroon on stored
peanuts (hay?) treated once with DECIS EC 25 at a rate of 0.5 g ai.
for 100 kg crop. The residue of deltamethrin in straw/stem after 23
weeks was 1.25 mg/kg (FP-79.07.06/A).
Coffee
Trials in Brazil were conducted on 2 stored coffee beans using dust at
1 and 2 mg/kg, aqueous EC spray on bags at 25 g ai/350 m2, and ULV
fog at 4 g ai/100 m3. Dust treatments resulted in negligible loss of
residue after 8 months of storage. The EC spray resulted in a residue
of 0.01 mg/kg on the day of treatment; roasting lowered this to n.d.
(<0.005 mg/kg). A sample of coffee fortified at 0.05 mg/kg and
roasted resulted in a residue of 0.012 mg/kg. ULV fog resulted in
n.d. (0.005 mg/kg) residues 1 day after treatment (FP-78.29.09/A,
FP-79.18.10/A, and FP-79.19.10/A).
Photodecomposition
The photochemistry of deltamethrin is quite complex, as might be
expected. Exposure to UV radiation (lambda > 290 nm) in various
organic solvents results in cis-trans isomerization, eater cleavage
reactions, and loss of bromine. Films of deltamethrin on glass or
silica gel exposed to sunlight irradiation results in cis-trans
isomerization as the major reaction plus smaller amounts of various
cleavage products and polar materials. Twenty five photoproducts have
been identified from irradiation of deltamethrin or its initial
photolytic derivatives. The mixtures of deltamethrin photoproducts
from solution and solid-phase reactions are less toxic than
deltamethrin to mice treated intraperitoneally (Ruzo et al., 1977).
RESIDUES IN COMMERCE OR AT CONSUMPTION
Due to the short period of development and use of deltamethrin there
are no data yet available on residues in food in commerce or at
consumption. Because of the very low dosage rates usually recommended
for most field crops it seems quite unlikely that measurable residues
will be found on food ready for consumption. The exception might be
for processed foods (flour, bread, corn meal cakes, etc.) prepared
from stored grains treated in storage, as data in the preceding
section showed that storage, processing and cooking had little effect
on residue levels.
METHODS OF RESIDUE ANALYSIS
A sensitive general purpose method for the analysis of deltamethrin
residues in plant tissues has been developed, which is the fusion of
various preceding analytical methods used until now. The method is
based on extraction, purification by liquid-liquid partition, liquid
chromatography clean-up, and gas chromatography quantitation by
electron-capture detection. Recoveries range from 80 to 95% and the
sensitivity of the method is between 1-10 µg/kg.
A 20 g sample of vegetable material is extracted by blending with
solvent, sodium sulphate and Celite, filtered through a Buchner
funnel, and evaporated to about 30 ml. A choice of 3 extraction
solvents: acetonitrile (or hexane), petroleum ether-diethyl ether
(50/50; v/v), or acetone-toluene is made according to the nature of
the sample and its lipid content. For some crops these extracts are
clean enough to proceed directly to liquid chromatography clean-up.
If not, then one of two available liquid-liquid partition procedures
is used, according to whether the sample is an oily or non-oily crop.
For non-oily crops, the extract is washed with acetonitrile-saturated
petroleum ether and the acetonitrile phase then partitioned between
aqueous sodium chloride and petroleum ether-ethyl ether (1/1; v/v),
the organic phase filtered, dried, and evaporated to dryness. For
oily crops two methods can be used involving either (a) partition
between pet. ether and DMSO, followed by partition between DMSO,
aqueous sodium chloride, and ethyl acetate, or (b) partition between
hexane and acetonitrile, followed by back partition between
acetonitrile, aqueous sodium chloride, and hexane.
For liquid chromatography clean-up, two elution procedures may be
carried out; a dry-packed, 5 g Florisil (deactivated by 5% water)
column is eluted either with a) ethyl ether-petroleum ether (20/80;
v/v) or b) ethyl ether-hexane (10/90; v/v), depending on which
partition step was used.
Two gas chromatographic determination procedures are available,
depending on whether a 63Ni or 3H electron capture detector is
available. If a 63Ni detector is to be used then the eluate from the
clean-up step can be concentrated, adjusted to volume and aliquots
injected directly into a GC using a 1.5 m × 2 mm i.d. glass column
packed with either 2% DC-200, OV-1 or OV-101 on chromasorb AW-DMCS
(80-100 mesh). A column temperature of 245°C and detector temperature
of 300°C are used.
If only a 3H electron capture detector is available then the
concentrate from the clean-up step must be derivatised to
2,2-dimethyl-3-(2,2-dibromovinyl) cyclopropane carboxylic acid methyl
ester by means of transesterification. This procedure is carried out
by heating an aliquot of the clean-up concentrate in a mixture of
hexane, toluene, and 0.1 ml of 0.1 N KOH in methanol at 50°C for 15
minutes. After cooling the solution is neutralized with 0.1 ml
sulphuric acid (0.5% in methanol) and adjusted to volume (10 ml) with
hexane. The GLC analysis uses a 2 m glass column packed with 5% SE-30
on Gas-Chrom Q (100-120 mesh) at a temperature of 170°C. The detector
temperature is held at 200°C. Carrier gas flows of about 35 mg/min
are used in either procedure. If an internal standard in desired, the
use of o-nitroaniline has been found to be acceptable at this stage of
development, but improvements are being sought. Quantitation by peak
area rather than peak height is to be preferred for maximum precision
and reproducibility at the lowest residue levels (RU-78.11.07/A.;
RU-76.18.12/A; Mestres, 1978; Mestres et al., 1978).
For the analysis of deltamethrin residues in animal tissues (muscle,
kidney, and liver) a suitable gas chromatographic method is available
which is sensitive to about 1 µg/kg with a recovery factor of 90% at a
1O µg/kg level.
A 20 g tissue sample is homogenised in a blender with Celite and
petroleum ether-ethyl ether (50/50; v/v). Sodium sulphate is also
added for kidney or liver tissues. The solution is filtered through a
sintered glass Buchner funnel and evaporated to 3 ml under dry
nitrogen.
The extract is then partitioned between acetonitrile and petroleum
ether and the acetonitrile phase evaporated to dryness.
Clean-up is by gel permeation chromatography on a calibrated column (1
cm i.d., 120 cm long) of 30 ml of Styragel (porosity = 100 A, <37µ
particle diameter) swollen in isopropyl ether and eluted with
isopropyl ether at 2 ml/min. The elution volume of deltamethrin
standard (approx. 10 µg) is first determined by use of a U.V. detector
at 278 nm. The residue from the partition step is taken up in 250 µl
isopropyl ether and 200 µl are injected by a means of a sample loop.
Eluate corresponding to the elution volume of deltamethrin (approx. 10
ml) is collected and evaporated to dryness under nitrogen.
The residue is dissolved in 500 µl of a solution of internal standard
(0.25 µl/ml of an analogue of deltamethrin provided by the
manufacturer) in cyclohexane and analysed by G.C. using a 1.9 m × 4 mm
i.d. glass column packed with SE-30 on Gas Chrom P. and held at 245°C.
A 63Ni detector is required, which is operated at 300°C. Carrier gas
is argonmethane (90-10) at a flowrate of 40 mg/min (RU-80.01.26/A).
In all of these analytical methods it is only the parent deltamethrin
that is being determined although the derivatisation step in the
vegetable method might also pick up Br2CA if it were present in
sufficient quantity (unlikely).
NATIONAL TOLERANCES REPORTED TO THE MEETING
No national tolerances were reported to the Meeting at this time.
Formulations of deltamethrin are officially registered for use on one
or more crops in the following countries:
Europe
Bulgaria - Cyprus - Spain - France - Greece - Hungary - Holland
(ornamentals) - Ireland - Luxembourg - Poland - Portugal - German
Democratic Republic - Federal Republic of Germany - United Kingdom -
Switzerland - Czechoslovakia - Yugoslavia.
Africa
Algeria - Egypt - Mauritius - Mozambique - Morocco - Senegal - South
African Republic - Tunisia - Zimbabwe.
Americas
Argentina - Barbados - Brazil - British Guyana - Bolivia - Chile -
Colombia - Costa Rica - Dominican Republic - Ecuador - Guatemala -
Haiti - Honduras - Mexico - Nicaragua - Panama - Paraguay - Peru -
Suriname - Trinidad - Uruguay - Venezuela.
Asia and Oceania
Australia - Indonesia - India - Iran - Malaysia - New Zealand -
Pakistan - Philippines - South Korea - Syria - Taiwan Province of
China - Thailand - Turkey.
In the following countries deltamethrin is officially admitted since
no registration system exists.
West and Central African Countries
Benin - Cameroon - Chad - Central African Republic - Congo - Gabon -
Upper Volta - Mali - Niger - Nigeria - Togo - Zaire - Ivory Coast
(Registration system just underway - mid-1980).
Other African Countries
Malagasy Republic - Kenya - Ethiopia - Zambia.
Near East
Lebanon.
EVALUATION
COMMENT AND APPRAISAL
Deltamethrin is a synthetic pyrethroid produced as a single diastereo
isomer of 98% purity or better, which is active against a wide range
of insects that attack crops, animals and man. It is a powerful
contact and stomach insecticide that exhibits little or no systemic
activity, but has considerable stability when exposed to air and
light.
Deltamethrin is rapidly absorbed and metabolised by oxidation and
ester cleavage. Several metabolites have been identified. The
majority of these metabolites do not contain the intact pyrethrin
structure and on which there are no toxicological data.
Deltamethrin is rapidly and almost completely excreted, primarily in
urine and also in faeces. Only one metabolite, thiocyanate, has
accumulating properties caused by high tissue affinity in skin and
gastrointestinal tract. Deltamethrin is neurotoxic in several
mammalian species, and has a moderate acute oral toxicity in rats.
Plant workers exposed to deltamethrin experienced a pruritis followed
by a burning sensation and slight desquamation. No other
exposure-related symptoms are recorded. Quantitative information
about these effects is necessary to evaluate the sensitivity of man to
deltamethrin. It does not produce a delayed neurotoxic response in
hens following exposure to lethal or sublethal dosages. Neurological
changes, and modification of the EEG-pattern, were observed in a
90-day study with dogs. These animals also showed dilation of the
pupils, a dose-related increase of vomiting, body tremors and jerking
movements. Loose faeces, a reduced body weight gain, decreased
appetite and water consumption were associated features with all
groups of treated dogs.
Deltamethrin has some minor effects on mean body weight of rat pups
but did not effect reproduction in a three-generation study with rats.
Studies on teratogenic effects in mice, rats and rabbits revealed
possible embryotoxic effects in mice and rabbits, but no teratogenic
effects. The embryotoxic effects are decreased average foetal weight
and delayed ossification in mice and increased foetal losses in
rabbits.
The mutagenic properties of deltamethrin were studied with bacteria,
mammalian cell systems, the micronuclei test and dominant lethal test.
Deltamethrin showed no mutagenic activity in any of these tests. In a
90-day study with rats the effect on the body weight gain of the males
appeared to be the most sensitive criterion.
Two 2-year feeding studies with deltamethrin in mice and rats revealed
no clear treatment-related increase of tumour incidence. It was
concluded that the no-effect level in the mouse study was 100 mg/kg
feed. In the long-term study with rats a dose-dependent effect,
manifested as a slightly increased incidence of axonal degenerations
in sciatic, tibial and/or plantor nerves, was observed at 18 months in
the 20 and 50 mg/kg feed groups. Evaluation of the incidence and/or
severity of these degenerations at termination of the study was
obscured by the age of the animals. A suggested no-effect level was 2
mg/kg feed.
Owing to the absence of a no-effect level in dogs, and a possible
embryotoxic effect in mice at low dose levels, it was not possible to
allocate an ADI. This compound should be re-evaluated as soon as the
study on dogs, said to be in progress, is made available.
When applied to field crops, deltamethrin is active at only 9 g
a.i./ha with usual dosages of around 12.5 g a.i./ha. Because of its
stability and persistence, deltamethrin is very effective against
stored product pests at rates of 0.25 to 1.0 g a.i./ton depending on
local situations.
The residue data from supervised trials on field crops were evaluated
with regard to the commodity groups elaborated in Annex I, Alinorm
81/24, 1980. Because of the chemical nature of deltamethrin, the low
dosage rates normally applied, and the consequent low (generally)
residues even at short (2-4 days) intervals between last application
and harvest, it was considered appropriate to list maximum residue
levels suitable for MRL's on the basis of commodity groupings where
sufficient data existed on an adequate number of commodities in the
group and/or where the commodities in the group are known to have
uniform residue retention properties.
Residues on fruits and vegetables are found mainly on the peel, hull,
or outer leaves. Degradation in field crops occurs mainly by
hydrolysis and photolysis, followed by enzymatic oxidations leading to
a wide variety of products and conjugates, among which are
transdeltamethrin, trans-hydrooxymethyl deltamethrin, 4'-hydroxy
deltamethrin, 4'-hydroxy-transhydroxymethyl deltamethrin,
3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane carboxylic acid (Br2CA)
and its 3 conjugates, 3-phenoxybenzaldehyde and corresponding alcohol
and acid and their conjugates, and alpha-cyanophenoxylbenzyl alcohol.
Metabolism in animals is analogous to that in plants, differing mainly
in the nature of the conjugates. Rapid excretion of deltamethrin and
its metabolites occurs in animals with very little tendency to
bioaccumulate. Since deltamethrin comprises the major residue
component, it is the only entity sought in residue analysis.
When 0.27 g of C14-deltamethrin (labelled in the alpha-methine group)
was injected intrarumenally into a dairy cow, radioactivity was
rapidly excreted in urine and faeces (85.3%). Peak residues in whole
milk and butter were 0.045 and 0.92 mg/kg respectively with a
half-life of 0.8 days. Fat and muscle samples contained 0.088 and
0.008 mg/kg deltamethrin equivalents at two days after treatment. A
miscible oil formulation containing 0.21 g of labelled deltamethrin
was applied externally (except udder) to the same cow 49 days later.
Peak residues in whole milk and butter were 0.0057 and 0.1 mg/kg
respectively by two days after treatment with half-lives of four to
four and one half days. On the basis of these ratios it can be
calculated that residues arising from the consumption of forage or
fodder having residues up to 2 mg/kg would not be likely to exceed
0.01 mg/kg in whole milk or 0.02 mg/kg in butter or fat of meat.
Cereal grains treated with dust, EC, or low volume formulation
yielding initial deposits of up to 1 mg/kg deltamethrin exhibited
little or no losses after storage of up to 50 weeks. The majority of
the residue in wheat was found in the bran (2 to 10 times that in
whole grain) while white flour contained approximately one fourth of
the residue in whole grain. Baking of flour into bread had no
significant effect on residue levels leading to possible residues of
up to 0.3 mg/kg in baked goods from recommended storage treatment
dosages. Stored coffee beans treated at 1 or 2 mg/kg (dust, EC, and
ULV treatments) had negligible loss of residue after 8 months of
storage. Roasting of coffee beans fortified at 0.05 mg/kg resulted in
a residue of 0.012 mg/kg. At this level of residue in roasted beans
it can be calculated that less than 0.00003 mg/kg would be found in
brewed coffee water on the basis of the results of experiments with
tea in which the residues in brewed tea water did not exceed 1/500th
of those on tea leaves.
A sensitive general purpose method for the analysis of deltamethrin
residues in plant tissues has been developed. The method is based on
extraction, liquid-liquid partition (if needed), liquid chromatography
clean-up on a Florisil column and gas chromatographic quantitation
using electron capture detection. The use of a 63Ni electron capture
detector at a working temperature of 300°C can measure deltamethrin
directly and obviates the need for derivitisation. If only a tritium
electron capture detector is available then column temperature
considerations require the use of a transesterification reaction to
produce 2,2-dimethyl-3-(2,2-dibromovinyl) cyclopropane carboxylic acid
methyl ester. The use of an internal standard is to be preferred
(either o-nitroaniline or a deltamethrin analogue) and quantitation
should be by peak area rather than peak height for maximum precision
and accuracy. Recoveries range from 80 to 95% with a sensitivity
(overall) of 1-10 µg/kg.
For the analysis of deltamethrin residues in animal tissues a suitable
method is available that is sensitive to about 1 µg/kg, with a
recovery factor of 90% at the 10 µg/kg level. The method is similar
to that for plant tissue, differing mainly in the choice of solvents
in the extraction and partition stages and in the use of gel
permeation chromatography for clean-up. A 63Ni detector operating at
300°C is required.
The meeting examined residue data from supervised trials reflecting
established and proposed good agricultural practice on a number of
crops and commodities. From these data the meeting was able to
estimate the maximum residue levels that were likely to occur when
deltamethrin was used in practice and when reported intervals between
last application and harvest were observed.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concluded that the residue levels listed below need not be
exceeded if recommended use patterns are adhered to. In the absence
of an ADI the meeting recommended that these are suitable as
guidelines only. These levels refer to parent compound only.
Interval between last
application and harvest,
Guideline residue on which guideline levels
Commodity levels, mg/kg are based, days1
Tea 10 none
Coffee beans 2 -
Legume animal feeds 0.5 (dry weight basis) 21
Wheat bran (unprocessed) 2 -
Cereal grains 1 -
Wheat flour (white) 0.5 -
Leafy vegetables 0.2 7
Bulb vegetables 0.1 7
Legume vegetables 0.1 7
Pome fruits 0.1 21
Assorted fruits-edible peel 0.1 14
Oilseeds 0.1 14
Brassica leafy vegetables 0.05 7
Artichokes 0.05 7
Fruiting vegetables-edible peel 0.05 3
Oranges, clementines 0.05 35
Stone fruits 0.05 14
Grapes, strawberries 0.05 7
Bananas 0.05 77
Cocoa beans 0.05 35
Root and tuber vegetables 0.01 7
Melons 0.01 7
Pineapples 0.01 31
Legume oilseeds 0.01 70
Mushrooms 0.01 1
1 No numerical entry in given where the levels are based on post-harvest
treatments.
FURTHER WORK OR INFORMATION
Required (by 1982 and before an acceptable daily intake can be
allocated)
1. Results of the two-year feeding study in dogs currently in
progress.
2. Clarification of possible embryotoxic effects in animals.
3. Further observations on reported effects in man.
4. Studies into the significance of neurological effects observed in
several animal species.
5. Results of supervised trials on residues in meat, milk, and eggs
arising from the use of deltamethrin for ectoparasite control, in
feeding studies and in stable treatments.
Desirable
1. Additional residue data from supervised trials on citrus,
especially grapefruit, lemons, tangerines, etc., if uses on these
commodities are developed.
2. Data on residues in food in commerce or at consumption.
3. Information on national tolerances.
REFERENCES
Anonymous. Test to determine the toxicity in the chicken by oral
route. (1976a) Unpublished report RU-76.05.05/A, submitted by Roussel
Uclaf to WHO.
Anonymous. Toxicity of deltamethrin or DECIS in single ingestion in
game duck, Anas platyrhynchos L. (1976b) Unpublished report
INRA-76.21.12/A, Institut National de la Recherche Agronomique,
Jony-en-Josas, France, submitted by Roussel Uclaf to WHO.
Anonymous. Toxicity of deltamethrin or DECIS by single ingestion in
grey partridge, Perdix Perdix L. and red partridge, Alectous
rufa L. (1976c) Unpublished report INRA-76.28.09/A, Institut
National de la Recherche Agronomique, Jony-en-Josas, France, submitted
by Roussel Uclaf to WHO.
Anonymous. DECIS. Photostability to air and light. (1977b)
Unpublished report RU-22.08.77/A, submitted by Roussel Uclaf to WHO.
Anonymous. Deltamethrin. (1978a) Unpublished report RI-78.09.11/A,
submitted by Roussel Uclaf to WHO.
Anonymous. Deltamethrin/stability. (1978b) Unpublished report
RU-78.23.11/A, submitted by Roussel Uclaf to WHO.
Beavers, J.B. and Fink, R. Acute oral LD50 - Mallard duck Technical
DECIS. Final report. (1977a) Unpublished report WI-77.06.06/A,
Wildlife International, USA, submitted by Roussel Uclaf to WHO.
Beavers, J.B. and Fink, R. Eight-day dietary LD50 - Mallard duck.
Technical DECIS. Final report. (1977b) Unpublished report
VO-77.18.05/A. Wildlife International, USA, submitted by Roussel Uclaf
to WHO.
Beavers, J.B. and Fink, R. Eight-day dietary LD50 - Bobwhite quail.
Technical DECIS. Final report. (1977c) Unpublished report
WI-77.19.05/A, Wildlife International, USA, submitted by Roussel Uclaf
to WHO.
Chesterman, H., Heywood, R., Perkin, C.J., Beard, D., Street, E. and
Prentice, D.E. RU 22974. Oral toxicity study in Beagle dogs. (1977)
Unpublished report RSL 253/7751/A3, Huntingdon Research Centre,
Huntingdon, England, submitted by Roussel Uclaf to WHO.
Clair, M. RU 22974 - DECIS. Acute toxicity in the rabbit by
percutaneous administration. Unpublished report IFREB-R 770257.1/A,
Institut Français de Recherches et Essais Biologiques,
Joinville-le-Pont, France, submitted by Roussel Uclaf to WHO.
Coombs, D.W. and Clark, G.C. RU 22974. Acute inhalation toxicity in
rats. 6 Hour LC50. (1978) Unpublished report RSL 310/78453/A,
Huntingdon Research Centre, Huntingdon, England, submitted by Roussel
Uclaf to WHO.
Coquet B. RU 22974. Test to determine primary cutaneous irritation in
the rabbit. (1976a) Unpublished report no. IFREB-R 761157/A, Institut
Français de Recherches et Essais Biologiques, Joinville-le-Pont,
France, submitted by Roussel Uclaf to WHO.
Coquet B. RU 22974. Test to evaluate ocular irritation in the rabbit.
(1976b) Unpublished report no. IFREB 761158/A, Institut Français de
Recherches et Essais Biologiques, Joinville-le-Pont, France, submitted
by Roussel Uclaf to WHO.
Elliot, M. et al. Synthetic insecticide with a new order of activity.
Nature 248, 710-11.
Fouillet, X. RU 22974. Mutagenicity study of various preparations.
Salmonella-microsome test. (1976) Unpublished report no. IFREB-R
761153/A, Institut Français de Recherches et Essais Biologiques,
Joinville-le-Pont, France, submitted by Roussel Uclaf to WHO.
FP-78.27.04/A. Residues determination in plants. Stored wheat. (1978)
Research Report. Université de Montpellier.
FP-78.07.12/A. Residues determination in plants. Wheat (stored).
(1978) Research Report. Université de Montpellier.
FP-80.05.02/A. Residues determination in plants. Wheat (stored).
(1980) Research Report. Université de Montpellier.
FP-78.30.09/A. Residues determination in plants. Corn-stored grains.
(1978) Research Report. Université de Montpellier.
FP-79.16.07/A. Residues determination in plants. Maize (stored).
(1979) Research Report. Université de Montpellier.
FP-79.18.07/A. Residues determination in plants. Maize (stored).
(1979) Research Report. Université de Montpellier.
FP-78.30.05/A. Residues determination in plants. Corn (stored). (1978)
Research Report. Université de Montpellier.
FP-78.02.10/A. Residues determination in plants. Corn (stored). (1978)
Research Report. Université de Montpellier.
FP-79.07.06/A. Residues determination in plants. Peanut (stored).
(1979) Research Report. Université de Montpellier.
FP-78.29.09/A Residues determination in plants. Coffee. (1978)
Research Report. Université de Montpellier.
FP-79.18.10/A. Residues determination in plants. Coffee (stored).
(1979) Research Report. Université de Montpellier.
FP-79.19.10/A. Residues determination in plants. Coffee (stored).
(1979) Research Report. Université de Montpellier.
Glomot R. and Chevalier, B. RU 22974. Acute toxicity study mouse and
rat by oral route. (1976a) Unpublished report Tox 76810/A, submitted
by Roussel Uclaf to WHO.
Glomot, R. and Chevalier, B. RU 22974. Acute toxicity study mouse and
rat by intraperitoneal route. (1976b) Unpublished report Tox 76811/A,
submitted by Roussel Uclaf to WHO.
Glomot, R. and Chevalier, B. RU 22974. Acute toxicity study mouse and
rat by intravenous route. (1976c) Unpublished report Tox 76812/A,
submitted by Roussel Uclaf to WHO.
Glomot, R., Chevalier, B., Collas, E. and Audegond, L. RU 22974.
Acute toxicity study by oral route in male and female beagle dogs.
(1977) Unpublished report Toxico 77804/JL-5, submitted by Roussel
Uclaf to WHO.
Glomot, R. and Vannier, B. RU 22974. Teratological study in mouse,
rat and rabbit. (1977) Unpublished report no. Tox. 76534-76536/2/A,
submitted by Roussel Uclaf to WHO.
Glomot, R. and Vannier, B. RU 22974. Teratological study in rabbit.
Complementary information. (1978) Unpublished report no. Tox.
76534-76536/A3, submitted by Roussel Uclaf to WHO.
Goldenthal, E.I., Blair, M., Jefferson, N.D., Spicer, E.J.F., Arceo,
R.J. and Clark Kahn III, A. RU 22974. Two year toxicity and
carcinogenicity study in mice. (1980a) Unpublished report IRDC
406-001 A/4, International Research and Development Corporation,
Mattawan (Mich.) USA, submitted by Roussel Uclaf to WHO.
Goldenthal, E.I., Jefferson, N.D., Blair, M., Thorstenson, J.H.,
Spicer, E.J.F., Arceo, R.J. and Kahn III, A. CRU 22974. Two year oral
toxicity and carcinogenicity study in rats. (1980b) Unpublished report
IRDC 406-002/A4, International Research and Development Corporation,
Mattawan (Mich.), USA, submitted by Roussel Uclaf to WHO.
Guillot, J.P. and Guilaine, J. RU 22974-Decamethrine. Decis technical
Roussel Uclaf. Sensitization test in the guinea pig. (1977)
Unpublished report no. IFREB-R 709241/A, Institut Français de
Recherches et Essais Biologiques, Joinville-le-Pont, France, submitted
by Roussel Uclaf to WHO.
Herve, J.J., Smolikowski, S., Pastre, P., Piedallu, C. and Roa, L.
NRDC 161 (RU 22974): A New Pyrethroid Insecticide for Use in
Agricultural Crops. Proceedings 1977 British Crop Protection
Conference - Pests and Diseases.
Husson, J.M. Medical observations made on people working on the
manufacture and formulation of the pyrethroid insecticide decamethrin.
(1978) Unpublished report no. RU 78.25.08/A, submitted by Roussel
Uclaf to WHO.
Hunter, B., Jordan, J., Heywood, R., Hepworth, P., Street, A.E. and
Prentice, D.E. RU 22974. Assessment of toxicity to rats by oral
administration for 13 weeks, (followed by a 4-week withdrawal period).
(1977) Unpublished report by Roussel Uclaf to WHO.
Kynoch, S.R., Lloyd, G.K. and Andrews, C.D. Acute percutaneous
toxicity to rats of decamethrin. (1979) Unpublished report RSL-10098/D
147/79/A, Huntingdon Research Centre, Huntingdon, England, submitted
by Roussel Uclaf to WHO.
Mestres, R. Les Residus de Decamethrine dans lea Vegetaux
Consommables. Phytiatrie - Phytopharmboie 27, 81-84.
Mestres, R., Chevallier, C., Espinoza, C. and Cornet, R. Dosage des
Residus de Decamethrine dans les Produits Vegetaux. Travaux de la
Societe de Pharmacie de Montpellier 38, (2) 183-92.
Peyre, M., Chantot, J.F., Glomot, R. and Penasse, L. Detection of a
mutagenic potency of decamethrin (RU 22974). Bacterial tests. (1980)
Unpublished report no. RU/TOX/80.21.01/A, submitted by Roussel Uclaf
to WHO.
PRO 77.13.09/A. Preliminary Uptake-Trans-location Studies of RU 22974
on Cotton. (1977) Research Report. Procida, Groupe Roussel Uclaf.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E., Cooke, L.
and Gibson, W.A. RU 22974, (Decamethrine) LD50 determination and
assessment of neurotoxicity in the domestic hen. (1978) Unpublished
report no. RSL 293-NT/7830/A, Huntingdon Research Centre, Huntingdon,
England submitted by Roussel Uclaf to WHO.
Roussel Uclaf. Information on the general chemistry of the active
ingredient, the technical material, and some formulations. (1980a).
Roussel Uclaf. List of main world crops on which decamethrin is
applied at indicated rates. (1980b).
Roussel Uclaf. Information on residues of decamethrin resulting from
supervised trials. Assembled Research Reports. (1980c).
Roussel Uclaf. List of countries where DECIS is officially registered
for agricultural uses plus countries where DECIS is officially
admitted since no registration system exists. (1980d).
RU-78.II.07/A. Quantitative determination of Decamethrin residues in
plant tissues. (1978) Research Report. Procida, Groupe Roussel Uclaf.
RU-76.18.12/A. Gas Chromatographic Determination of DECIS. Development
of a Method for the Estimation of DECIS. Residues in Plants. (1976)
Research Report. Roussel Uclaf.
RU-80.01.26/A. Determination of Decamethrin Residues in Animal
Tissues. (1980) Research Report. Roussel Uclaf.
Ruzo L.O., Unai, T. and Casida, J.E. Decamethrin metabolism in rats.
(1977) Unpublished report UC-77.06.12/A, submitted by Roussel Uclaf to
WHO.
Ruzo, L.O., Unai, T. and Casida, J.E. Decamethrin metabolism in rats.
J. Agric. Food Chem. 26: 918.
Ruzo, L.O., Engel, J.L., and Casida, J.E. Oxidative, hydrolytic and
conjugative reactions in the metabolism of decamethrin in mice.
(1978b) Unpublished report UC.78.10.11/A, submitted by Roussel Uclaf
to WHO.
Ruzo, L.O. and Casida, J.E. Degradation of Decamethrin on Cotton
Plants. J. Agric. Food Chem. 27, (3) 572-75.
Ruzo, Luis O., Holmstead, R.L. and Casida, J.E. Pyrethroid
Photochemistry: Decamethrin. J. Agric. Food Chem. 25, (6) 1385-94.
Shono T., Ohsawa, K. and Casida, J.E. Metabolism of trans- and
cis-permethrin, trans- and cis-cypermethrin and decamethrin by
microsomal enzymes. Unpublished report UC 78.18.05/A, submitted by
Roussel Uclaf to WHO.
Sobels, F.H., Tates, A.D. and Vannier, B. Cytogenetic study with RU
22974. Detection of a mutagenic potency in mammalian cells. (1978)
Unpublished report no. ULN-782211/A, State University of Leiden, The
Netherlands, submitted by Roussel Uclaf to WHO.
Vannier, B. and Glomot, R. RU 22974. Mutagenic study. Dominant lethal
assay in the male mouse. (1977) Unpublished report no. Toxico
76533/DB9/A, submitted by Roussel Uclaf to WHO.
WELL-79.04HIBH/A. Residues in a cow's milk resulting from
intrarumenal, and later, dermal administration of carbon-14 labelled
decamethrin. Group Research and Development, The Wellcome Foundation,
Ltd. Research Report. (1979).
WELL/80.21.05/AL. The Fate of Decamethrin Residues on Wheat during
Storage and after Milling and Baking. An Interim Report after 3 Months
Storage. Group Research and Development, The Wellcome Foundation. Ltd.
(1980).
Wrenn, J.M., Rodwell, D.E., Goldenthal, E.I., Spider, E.J.C. and
Rajasekaran, D. Three generation reproduction study in rats. (1980)
Unpublished report no. IRDC-406.003/A4, International Research and
Development Corporation, Michigan, USA, submitted by Roussel Uclaf to
WHO.
The radiocarbon of all compounds except for 14CN was nearly fully
excreted within 8 days with the main portion within 1 day.
14C-becisthemic acid showed the highest excretion with 94% in the
urine and 6% in the faeces, followed by 14C(alpha)-deltamethrin and
14C-trans-isomere, both with 75% and 25% respectively and 14C-deltamethrin with
54% and 44% respectively. No 14CO2 was found in the exhaled air.
Residues in carcase at 8 days amounted 0.2% with 14Cv-becisthemic
acid and 1.1-1.5% with the 14Cv-labelled compounds. Tissues with a
relatively high radiocarbon content were different at each
14C-compound: 59 µg/kg from 14Cv-deltamethrin in the fat, 182 µg/kg
in the fat and 89 µg/kg in the blood from 14C and 27 µg/kg from
14Cv-becisthemic acid in the liver.
Excretion after 14CN administration was slow and amounted to not more
than 79% within 8 days with 43% in the urine and 36% in the faeces.
No CO2 was expired. Residues in carcase and tissues amounted 21%
with relatively high radiocarbon contents in the stomach (603 µg/kg)
and the skin (645 µg/kg). Oral and i.p. applications of 0.1 mg/kg
14CN showed only small differences in excretion and distribution
pattern after 3 days. A radiocarbon content of the stomach of 3%
after both applications was noticed. Compared with these patterns
oral and i.p. application respectively of 0.1 mg/kg potassium cyanide-
14C showed a 2-3 times higher excretion in urine at 3 days. The
excretion in the faeces and the residues in carcase were about the
same and only after i.p. administration of KCN a lower stomach residue
as with 14CN was noticed.
The parent compound (6-21% of the applied dose) and hydroxylated
deltamethrins (7-15%; 4'-hydroxy- and 5-hydroxy deltamethrin) were
present in faeces only. The other metabolites, primarily carboxylic
acids or their conjugates, derived from ester cleavage and oxidation,
appeared mainly in urine. The major urinary metabolite from 14CN was
thiocyanate; those from the other labelled compounds were
4'-hydroxy-phenoxybenzoic acid sulphate and becisthemic acid
glucuronide (Ruzo et al., 1977).
In a separate experiment with rats it was shown that the residual 14C
in skin and gastrointestinal tract from 14CN was 14C-thiocyanate.
This latter compound has a relatively high tissue affinity, accounting
probably for the delayed and incomplete excretion of
14CN-deltamethrin (Ruzo et al., 1977).
Incubation of 14C(alpha)- and 14Cv-deltamethrin with rat stomach
strips in pH 7.2 medium for 6-24 hours yields approximately 60%
metabolism, the major products being phenoxybenzoic acid and
becisthemic acid (Ruzo et al., 1977).
The metabolism of orally given deltamethrin in mice is essentially the
same as in rats. The faeces contain less parent compound and more
mono- and di-hydroxylated esters than in rats. Intraperitoneal
administration in mice yields the same metabolites as oral injection
but in different ratios (Ruzo et al., 1978b).
Deltamethrin incubated with mouse liver microsomal fraction yields in
an oxidase system more metabolised (59%) and hydroxylated parent
compound (8%) than in an oxidase plus esterase system (25% and 4%
respectively). In the latter system the total amount of derivatives
from the acid and alcohol moieties of the ester is more than doubled.
No racemization occurred since only alpha S epimers were recovered in
the alcohol moiety (Shono et al., 1978).
TOXICOLOGICAL STUDIES
Special studies on primary irritation
Cutaneous irritation
Male albino rabbits (12/group) weighing 2.5 to 3.5 kg were
administered 0.5 g deltamethrin to either shaven intact or abraded
skin. The occulsive patch was fixed on the skin for 23 hours.
Scoring for erythematous and oedematous lesions occurred 1 hour and 49
hours after removal of the patches. Technical deltamethrin, 98% ai
showed no irritant effect (Coquet, 1976a).
Ocular irritation
Deltamethrin (0.1 g/animal) was administered into the conjunctival sac
of the eye of 6 male albino rabbits, weighing 2.5 kg, with or without
rinsing 60 sec after instillation. Observations for conjunctival
lesions, chemosis, discharge, conjunctival enanthema, opacity and
affected cornea were made 1 hour, 24 hours, 2, 3, 4 and 7 days
following instillation. Deltamethrin showed both with and without
rinsing transient irritating effects (Coquet, 1976b).
Special studies on sensitization
Deltamethrin (0.5 g/animal ) was applied topically to the skin of
albino guinea pigs (10 male and female) 3 times per week, with a 2-day
interval for 3 weeks, and once at the start of the 4th week. The
preparation was covered with an occlusive patch for 48 hours. On day
1 and 10 the guinea pigs received an intradermal injection of 0.1 ml
of Freund's adjuvant. The animals were challenged 12 days after the
last application with 0.5 g deltamethrin. The macroscopic and
histological examination did not reveal evidence of sensitisation.
(Guillot and Guilaine, 1977).
Special studies on reproduction
Rat
Groups of 10 female and 10 male Charles River rats were fed
deltamethrin in the diet at 0, 2, 20 or 50 mg/kg and mated to begin a
three-generation, 2 litter (first generation, 3 litter) standard
reproduction study. Parental body weights and food consumption were
recorded during the study. After weaning of the second litter the
survival parental rats were sacrificed and necropsied.
Five male and 5 female pups of the F3b were necropsied. No changes
in general behaviour, or survival of parental rats or pups relevant to
tent material were observed. The body weight of F0-males of the 50
mg/kg group was decreased from week 11 onward; there were some slight
decreases in mean food consumption of the parental F1 male rats in
the 50 mg/kg feed group.
The basic reproduction indices (fertility, gestation, lactation,
viability and litter size) were not affected by the treatment.
However, the mean pup weight was in some litters, especially of the 50
mg/kg group, slightly decreased in comparison to the controls. Gross
external examination revealed no abnormalities. No gross or
microscopic lesions of treatment-related significance or significant
effects on the organ weights of the F3b generation were observed
(Wrenn et al, 1980).
Special studies on teratogenicity
Mouse
Mated female Swiss CDI.SPF mice (24/group) were given orally
deltamethrin dissolved in sesame oil at dose levels of 0, 0.1, 1 or 10
mg/kg bw/day during day 6-17 of pregnancy. The animals were
necropsied on day 18 of gestation. No teratogenic effects could be
detected. Total implantation sites, foetal losses, living foetuses
and examinations of skeletal tissue were normal. Minor embryotoxic
effects as dose-dependent decrease of average fetal weight and delayed
ossifications were observed at all dose levels tested (Glomot and
Vannier, 1977).
Rat
Mated female Sprague Dawley rats (24/group) were administered orally
0, 0.1, 1 or 10 mg/kg deltamethrin bw/day during day 6-18 of
pregnancy. Examination occurred on day 21 of gestation. Twelve
females in control and 10 mg/kg bw-group were allowed to deliver.
There were no effects on the reproduction or teratogenicity parameters
examined, with the exception of a slight delayed ossification in the
highest dose level (Glomot and Vannier, 1977).
Rabbit
Groups of fifteen mated females received deltamethrin dissolved in
sesame oil at levels of 0, 1, 4 or 16 mg/kg bw/day from day 6-19 of
pregnancy. Examination was carried out on day 28 of gestation. The
average foetal losses were not related to dose increase at all doses
tested. This effect was mainly caused by a higher rate of expelling
traces. The average foetal weight in the highest dose group was
decreased. Some malformations (one animal with hydrocephalia, and one
with exencephalia and thoracogastrochisis) were observed in 2 animals
of the highest dose level. A complementary study with 16 mg/kg bw/
day was performed. In this study one foetus with spina bifida and
shortened tail was detected among 69 apparently normal foetuses. It
was concluded that the malformations were within the normal limits of
the strain used and were not related to the treatment despite the
occurrence at the highest does level only (Glomot and Vannier, 1977
and 1978).
Special study on delayed neurotoxicity
Chicken
Adult hens (10/group) were gavaged with a single dose of O, 500, 1250
or 5000 mg/kg bw deltamethrin suspended in corn oil or 0 or 100 mg/kg
bw dissolved in sesame oil. Tri-o-cresylphosphate (TOCP) (500 mg/kg
bw) was used an positive control for delayed neurotoxicity. During 21
days observations were made on mortality, health, neurotoxic signs and
body weight.
In the TOCP group 8 out of 10 animals died whereas only 2 mortalities
were observed in the group dosed at 1000 mg deltamethrin/kg with
sesame oil as the vehicle. Deltamethrin induced no clinical,
macroscopic or histological signs of delayed neurotoxicity. TOCP-
treated hens showed severe ataxia and degenerative changes in spinal
cord and occasionally in sciatic nerve (Ross et al., 1978).
Special studies on potentiation
Mice
Deltamethrin is hydrolysed in vitro by esterases in blood and in
brain, kidney, liver and stomach preparations of mice. Pretreatment
of mice with oxidase inhibitor, piperonyl butoxide (PB), or esterase
inhibitor, S,S,S-tributylphosphorotrithicate (DEF), delayed
metabolism of i.p. administered deltamethrin. Using oxidase or
esterase inhibitor, different vehicular and different administration
routes, it was possible to induce similar toxic effects with a wide
range of deltamethrin doses (6-191 mg/kg bw). The different
treatments showed that PB or DEF made mice more sensitive to
deltamethrin (Ruzo et al., 1978a).
Special studies on mutagenicity
Bacterial growth tests
In a growth inhibition test with E. coli, deltamethrin at levels
of 1250, 2500, or µg/ml DMSO (0.1 ml per plate) had the same marginal
inhibitor effect on the mother strains (W3110 and WP2) as on their
mutants (p3478 and CM611). Chloramphenicol and N-methyl
N'-nitro-N-nitroguanidine (MNNG) were used as positive controls and
induced clear inhibition (Peyre et al., 1980).
Deltamethrin was compared with MMNG, 9-aminoacridine, 2-nitrofluorene
and 2-aminoanthracene for mutagenic activity in the Ames test with
Salmonella typhimurium his strains TA 1535, TA 100, TA 1537, TA
1528, and TA 98. The concentrations of deltamethrin used were 2, 10,
50, 200, 500, 1000 or 5000 µg/plate. Deltamethrin began to
precipitate at 200 µg/plate.
The mean number of revertants wan not influenced by any concentration
of deltamethrin in any strain with or without S9-mix(metabolic
activation), whereas the positive control mutagens produced an
increase of the number of spontaneous revertants (Peyre et al,
1980).
In a similar experiment, deltamethrin (0, 2, 20, 200 or 400 µg/plate
dissolved in DMSO) in the presence of activated microsomal enzymes did
not influence the number of revertants of Salmonella typhimurium
strains TA 1535, 1537, 1538, 98 and 100. 2-Aminoanthracene,
3-methylcholanthrene, benzo(alpha)pyrene and acridine orange showed
mutagenic activity, whereas thio-TEPA was negative (Fouillet, 1976).
Test with mammalian cells
Deltamethrin dissolved In Cremaphor oil (0, 0.04, 0.2, 1 or 5 mMol, 0,
0.08, 0.4, 1 or 10% v/v respectively) in the presence of a metabolic
activation system increased the incidence of chromosome and chromatid
aberrations and SCE's, after incubation with Chinese hamster ovary
cells at 1 mMol. In the absence of S9-mix (metabolic activation) no
higher rate of aberration was observed.
It was shown that this increased incidence was due to a sub-toxic
effect of some reaction product of Cremaphor oil and S9-mix.
Deltamethrin in Cremaphor oil without S9-mix and dissolved
in DMSO (1%) at levels of 0, 0.001, 0.01, 0.1 or 0.2 mMol with or
without metabolic activation had no effect on the number of
aberrations and SCE's. Due to insolubility no higher concentrations
were tested (Sobels et al., 1978).
Animal tests
Mice, 3 males and 3 females per dose, were gavaged for two consecutive
days with 5 or 10 mg deltamethrin (dissolved in sesame oil)/kg bw.
Control mice were gavaged with 0.3 ml sesame oil. The incidence of
chromatid aberrations in bone marrow cells or of micronuclei in
polychromic erythrocytes did not show any significant statistical
difference in treated and control groups (Sobels, et al., 1978).
A single oral administration of 15 mg/kg deltamethrin was given to
Swiss mice. Groups of 2 animals were sacrificed every 3 hours during
a 24-hour period. Several animals died after treatment. The
incidence of chromatid aberrations in the bone marrow of femora was
low. There was no consistent time-related trend in the distribution
of these aberrations (Sobels, et al., 1978).
Deltamethrin dissolved in sesame oil in groups of 9-13 male mice dosed
orally with 0 or 3 mg/kg bw for 7 days and 6 or 15 mg/kg bw in a
single dose showed after mating with 6-18 non-treated females, no
effect on the rate of pre- or post-implantation losses.
The highest dose tested was toxic to the males. 7 out of 20 animals
died shortly after treatment. Histological examination of the testes
of all animals revealed no abnormalities.
Triethylene thiophosphoramide (10 mg/kg bw) used as positive control
reduced considerably the rate of pregnancies in the second and third
week and increased the number of embryonal looses (Vannier and Glomot,
1977).
Special studies in man
Among plant workers dermally exposed to technical deltamethrin or its
formulations cutaneo-mucous manifestations were observed. Initial
lesions were tenacious and painful pruritus (pricking sensation),
especially observed after exposure to hot water or on perspiration,
followed by a blotchy local burning sensation with blotchy erythema
for about 2 days. Thereafter slight and regular desquamation,
restricted to the contaminated area, occurred. The cutaneous signs
are sometimes accompanied by itching of the face (mainly around the
mouth) and/or rhinorrhoea or lacrimation. No other symptoms related
to exposure were observed (Husson, 1978).
Acute toxicity
Mouse
Mice injected i.v. with deltamethrin showed intense tremors,
convulsions and taxis immediately after administration. Also
tachycardia and respiratory defects were observed at higher doses.
Surviving animals showed normal appearance after 4-5 hours.
Immediately after i.p. injection, jumping movements, slight
convulsions and prostration, ptosis, tail hypertonicity and cyanosis
were observed. Surviving animals showed normal appearance after 72
hours. Animals gavaged with deltamethrin showed 1 hour after dosing
muscular stiffening and convulsions. After 24 hours hypermotility,
stereotype movements of the head, tachycardia hypertonicity of the
tail and a few convulsion. Normal behaviour and appearance were
observed after 48 hours (Glomot and Chevalier, 1976a, b and c).
Rat
Rats injected i.v.with deltamethrin showed immediately following
treatment muscular contractions, piloerection, respiratory defects,
convulsions and paresis of the hind quarters. Death occurred within
10 min. After 25 hours only piloerection was visible, after 48 hours
surviving animals showed normal behaviour. After i.p. injection
immediate tremors, convulsions, prostration and cyanosis were
observed. After 48 hours surviving animals showed normal behaviour.
Gavage with deltamethrin shortly after dosing induced motor
incoordination, convulsions and respiratory defects. After 24 hours
and 48 hours, hypomotility and convulsions were still observed. After
3 days surviving animals showed normal behaviour (Glomot and
Chevalier, 1976a, b and c).
Rats (7 males and 7 females/group) were exposed (whole body) during 6
hours to aerosol concentrations of 0.049, 0.43, 0.54 or 0.72 g ai/m3.
The aerosol contained 66-86% of particles <5.5 µ. During exposure
hyperactivity and dose-dependent increase in grooming and irritation
was observed. The animals were hypersensitive to touch and noise and
showed uncoordinated movements. During the observation period of 14
days following exposure all animals except those from the lowest dose
group developed poor motor coordination and hypersensitivity. At the
end of the period all animals were recovered to normal. In these
groups the body weight gain and food intake was depressed during 3
days following exposure. In rats (4 of control and of highest dose
group) killed immediately after exposure stomach and small intestine
were gas-filled. In treated rats, as result of exposure, massive
haemorrhage and oedema in lungs was observed. Stomachs were filled
with gas, blood and mucus. In trachea white deposits were visible.
In animals killed after the observation period dose-dependent increase
of degenerations (coloured spots to congestion) in lungs was observed
(Coombs and Clark, 1978).
Rabbit
Rabbits (10 males and 10 females) were treated with 2 g deltamethrin
in 2 ml PEG 400/kg bw on 80 cm2 shaven akin for 24 hours on
occlusion. The animals were observed for 14 days.
One animal showed obvious erythema and another congested skin. No
weight changes or abnormal behaviour were observed. On histological
observation of liver, kidneys and skin small changes were observed
which were common for this strain of rabbits and not related to
treatment (Clair, 1977).
Birds
Oral administration of a.i. to hens, game duck, or partridge showed no
distinct symptoms except for a small initial weight loss occasionally.
In chickens, diarrhoea, convulsions and jerky movements of the head
were observed.
Mallard ducks, at lethal doses, exhibited signs of neurotoxic effects
which included ataxia, loss of equilibrium and of coordination. The
effect was dose related: at lower dose levels only some
hyperexcitibility and imbalance were observed (Beavers and Fink,
1977a).
Dog
Dogs showed, at non-lethal doses, transient hyperexcitibility,
akynesia, vomiting and stiffness of the hind legs (Glomot et al.,
1977).
Short-term studies
Quail and duck
Deltamethrin was given to 14-day old mallard ducks for 5 days in their
diet at doses of 0, 464, 1000, 2150, 4640 or 10,000 mg/kg feed. The
number of birds per group was 10. There was some mortality in the two
highest dose groups. Birds of the highest dose group showed ataxia
and loss of coordination. There was a dose-related decreased weight
gain and food consumption (Beavers and Fink, 1977b).
An identical experiment was performed with 14-day old Bobwhite quails
(10 birds/group). The effects were the same as in the mallard ducks,
except there was no mortality (Beavers and Fink, 1977c).
Rat
Male and female weanling Spargue Dawley rats (20/sex/group) were daily
dosed by oral gavage with 0, 0.1, 1.0, 2.5 or 10.0 mg deltamethrin in
PEG 200/kg bw/day for 13 weeks. No treatment related effects on food
and water consumption, mortality, urinalysis and haematology were
observed. Neurological examinations and ophthalmoscopy revealed no
abnormalities. In the 10 mg/kg bw group some hypersensitivity with
males was observed in week 6. Body weight gain among males receiving
deltamethrin was significantly lower at 2.5 and 10 mg/kg/day. The
body weight of the females was not affected by the treatment. The
male animals of the 1 mg/kg group showed a tendency to a reduced body
weight gain. In the females blood glucose and urea was significantly
increased in week 6 but no significant changes occurred in week 12 or
with other blood chemistry parameters. No clear effects were noted on
the weights of the organs. Gross and microscopic examination of a
variety of tissues and organs showed no treatment-related alterations.
Following the 13-week dosage period, 5 males and 5 females per group
were allowed to recover for 4 weeks. Autopsy was performed at the end
of this recovery period. The body weights of all previously treated
animals appeared not to be different from controls. Thyroid weights
were not dose-related increased in males. This increase was
significant in the 1.0 mg/kg and 10.0 mg/kg group. Marginal no effect
level was 1 mg/kg bw (Hunter et al., 1977).
Dog
Male and female beagle dogs (3-5/sex/group) at 25 weeks of age, were
daily dosed orally with O, 0.1, 1.0, 2.5 or 10.0 mg deltamethrin in
PEG 200/kg bw/day in gelatin capsules. Dosage was continued for 13
weeks, followed by a recovery period of 20 weeks for 2 dogs/sex, from
the groups receiving 1.0, 2.5 or 10.0 mg/kg bw/day.
Observations were made on behaviour, mortality, body weight, food and
water consumption. Haematology, blood chemistry, urinalysis and six
channel EEG-analysis were performed at week 0, 6 end 12;
ophthalmoscopy at week O, 5 and 12. Special attention was paid to the
muscular and nervous system.
Liquid faeces was associated with all groups of treated dogs
throughout the dosing period. All groups of animals receiving
deltamethrin gained less weight than the controls. The effects were
not strictly dose-related. The dogs from the control group were
leaving smaller quantities of the offered food than those of the
treated groups. Water consumption was not dose-related decreased in
any treated group.
Dilatation of the pupils was seen to occur in the dogs receiving 2.5
and 10.0 mg/kg/day. The sign was first seen 4-7 hours after dosing
and persisted throughout the day. They reacted normally prior to
dosing on the following day. The incidence of vomiting was
dose-related increased in all treated groups, except the 0.1 mg dose
level. The incidence decreased in all the animals affected as the
dosing period progressed.
In the highest dose group, unsteadiness, body tremors and jerking
movements were seen particularly in males in weeks 2, 3 and 4.
These effects were reduced during weeks 5 to 9 and were seen only in
one dog in week 13. Excessive salivation was seen initially and
diminished during the dosing period. After 5 and 12 weeks depression
of the gag reflex was noted in a proportion of animals in all treated
groups. Depression of the patellar reflex was observed in all treated
groups except the dogs administered 0.1 mg/kg. In the animals given 1
or 2.5 mg/kg/day exaggeration of the patellar reflex was noted only
after 5 weeks. Some animals of all treated groups showed variations
in the flexor reflex. A high proportion of the animals had depression
of the hind limb tactile placing reaction.
Dosage levels of 2.5 and 10 mg/kg/day deltamethrin caused modification
of the EEG pattern in some animals, following 12 weeks administration.
Histopathological evaluations of tissues and organs, including nervous
s stem and muscles did not reveal abnormalities that could be related
to dosage with the test compound. During recovery the gag reflex
continued to be depressed, whereas exaggeration of the patellar reflex
was still seen in some dogs that had previously received 1.0
mg/kg/day. One animal continued to show an abnormal EEG-pattern
(Chesterman et al., 1977).
TABLE 1. Acute toxicity of deltamethrin
LD50
Species sex route mg/kg bw references
mouse M + F intrav. 4 Glomot and Chevalier, 1976c
M intrap. 18 " " , 1976b
M intrap. 1711 " " , 1976b
M oral 21 " " , 1976a
M oral 331 " " , 1976a
F intrap. 12 " " , 1976b
F intrap. 1661 " " , 1976b
F oral 19 " " , 1976a
F oral 341 " " , 1976a
rat M + F intrav, 3 " " , 1976c
M intrap. 24 " " , 1976b
M intrap. 2091 " " , 1976b
M oral 67 " " , 1976a
M oral 1281 " " , 1976a
M + F inhal. 0.62 Coombs and Clark, 1978
M + F dermal >29403 Kynoch et al, 1979
F intrap. 25 Glomot and Chevalier, 1976b
F intrap. 1861 " " , 1976b
F oral 86 " " , 1976a
F oral 1391 " " , 1976a
rabbit M + F dermal >20001 Clair, 1977
chicken oral >10001 Anonymous, 1976a
adult hen F oral >25001 Ross et al, 1978
F oral >50001 " " , 1978
mallard duck oral >46401 Beavers and Fink, 1977a
game duck oral >40001 Anonymous, 1976b
grey partridge F + M oral >18006 " , 1976c
TABLE 1. Continued...
LD50
Species sex route mg/kg bw references
red partridge M + F oral >30006 " , 1976c
beagle dog M + F oral >300 Glomot et al, 1977
M + F oral >3006 1977
without index: suspended in polyethylene glycol 200
1 dissolved in sesame oil
2 expressed for LC50 in mg dust/m3 air
3 60% w/v suspension in aqueous methylcellulose on occulsion;
4 as paste in PEG 400 on occlusion;
5 dissolved in corn oil;
6 in capsules or cachets.
Long-term studies
Mouse
Male and female Charles River CD-1 mice (80/sex/group) were fed (in
the diet) at dosage levels of 0 (control), 1, 5, 25 or 100 mg
deltamethrin/kg for 24 months. In a second control group 60 mice/sex
were used. After 12 and 18 months 10 mice/sex/group except control
two were sacrificed.
There were no clear effects related to the administration of
deltamethrin on general behaviour, mortality, body weight and food
consumption. Blood chemistry, haematology and urine analysis
parameters were normal after 12, 18 and 24 months. Increases or
decreases in absolute and/or relative organ weights occurred in a few
organs at each dosage level at any time of sacrifice. Microscopic
examination of tissues did not reveal any lesions indicative of a
compound-related effect. The tumour incidence was unaffected by
deltamethrin administration. No-effect level was 100 mg/kg feed
(Goldenthal et al., 1980a).
Rat
Male and female Charles River CD rats (90/sex/group) were fed with 0
(control), 2, 20, or 50 mg decamethrin/kg in the diet for two years.
Sixty males and 60 females were used in a second control group. After
6, 12 and 18 months of compound administration 10 animals/sex/group
were sacrificed except for the second control group.
No changes in general behaviour and appearance in relation to compound
treatment were recorded. Survival was similar for control and treated
rats (50-67%). Rats at 50 mg/kg feed-group gained slightly less
weight than control rats, whereas the food consumption was essentially
the same. Ophthalmoscopic findings generally were similar for control
and treated rats. No haematological and biochemical parameters were
changed in a biologically significant way in relation to treatment at
any time, except for a decreased SGPT activity at 6 months, in the
mid- and high-dose groups.
No treatment-related effects were observed on organ weights. The
macroscopy and microscopy findings were common for the animals of
species and strain, except for a slightly increased incidence of
axonal degenerations in sciatic, tibial and/or plantar nerves at 18
months in the 20 and 50 mg/kg groups. Evaluation of incidence and/or
severity of these degenerations at termination of the study was
obscured by the age of the animals.
Seven interstitial cell adenomas were observed in the testes of the 50
mg/kg feed group, compared to 0 and 4 in the two control groups. Only
from some animals of the 2 and 20 mg/kg groups were some organs and
tissues, including the testes, studied histopathologically.
Evaluation of a possible dose-response effect on the testes is
therefore not possible. No-effect level is 2 mg/kg feed (Goldenthal
et al., 1980).
RESIDUES IN FOOD
USE PATTERN
Deltamethrin is a new synthetic pyrethroid insecticide manufactured
and marketed as a single diastereo isomer (>98%) out of eight
possible isomers. It is a contact and stomach insecticide with a very
large spectrum of action and considerable stability when exposed to
air and light.
Pre-harvest treatments
When applied on field crops, deltamethrin is active at the level of
only 0.01 lb./acre against very numerous species of insects. Current
recommendations for foliar applications on various crops in growth are
summarized in Table 2.
TABLE 2. Foliar dosage rates on various crops
Crop Rate, g ai/ha
Cotton 7.5-18.75
(usually 12.5 for medium
infestations most cotton
insects)
Vegetables
Artichokes 10
Eggplants 7.5-25
Cabbage 7-5-25
Strawberries 12.5-25
Beans 7.5-17.5
Lettuce 1.25-12.5
Melon 7.5-12.5
Tomatoes 7.5-25
Pimento 12.5-17.5
Leeks and onions 7.5-12.5
Peas 7.5-12.5
Fruit
Apricots 1.25-1.75 (g/hl)
Citrus fruit 1-1.5 (g/hl)
3 (g/ha, directed)
7.5 (g/ha, overall)
Cherries 0.75 (g/hl)
12.5
Figs 1.25 (g/hl)
Olives 0.625-1.75 (g/hl)
Bananas 1.25 (g/hl for 250 l/ha)
Pome fruit 0.75-1.25 (g/hl)
Peaches 0 75-1.75 (g/hl)
Plums 0.75-1.25 (g/hl)
Field crops
Sugarbeets 7.5-17.5
Coffee 7.5-12.5
Cereals(foliar treatments
during vegetative period) 7.5-12.5
Rapeseeds 5-7.5
Alfalfa 10-17.5
Maize 7.5-12.5
Potatoes 7.5-12.5
Soybeans 5-12.5
Grapes 7-5-17.5
Groundnuts(peanuts) 12.5
Sugarcane 17.5
Rice 7.5-25
Post-harvest treatment
Because of its extreme stability and persistence, deltamethrin (as
K-OTHRIN(R) is very effective against stored product pests, especially
in grain storage, including oil seeds and semi-finished products
deriving therefrom (flour, feed, etc.) It can be economically
synergized by piperonyl butoxide (PB), but to a lesser extent than
other pyrethroids. The recommended rates of application (which have
to be adapted according to local situations, local insects, and the
type of grain to be treated) are:
deltamethrin 0.75 to 1 g ai/ton.
deltamethrin/PB: 1/5 0.5 to 0.75 g ai/ton.
deltamethrin/PB: 1/10 0.25 to 0.5 g ai/ton.
Other uses
Special formulations for use on animals, as ectoparasiticides, for
household use, and for public health uses are still under development
and no data are available at this time.
RESIDUES RESULTING FROM SUPERVISED FIELD TRIALS
Extensive field trials have been conducted world-wide on a wide
variety of crops and the results are presented in Table 3. (Roussel
Uclaf, 1980).
Table 3. Delthamethrin residues in various crops.
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Root and Tuber
Vegetables
Carrots England 12.5g ai/ha 1 Root (76)
<0.01
France 12.5g ai/ha 1 Root 0.008 0.008 0.005
France 12.5g ai/ha 1 Root 0.015 0.008 0.007
Parsnips England 12.5g ai/ha 1 Root (76)
<0.01
Potatoes England 12.5g ai/ha 1 Tubers <0.01
F. R. Ger. 12.5g ai/ha 2 Whole n.d. n.d. n.d. n.d. n.d.
Tuber
2 Whole n.d. n.d. n.d. n.d. n.d.
Tuber
2 Whole n.d. n.d. n.d. n.d. n.d.
Tuber
Sugarbeets France 17.5g ai/ha 1 Root (11 wks)
<0.001
Beet (11 wks)
Top 0.002
25g ai/ha 1 Root (11 wks)
0.001
Beet (11 wks)
Top 0.001
17.5g ai/ha 1 Whole (17 wks)
Beet <0.001
25g ai/ha 1 Whole (17 wks)
Beet 0.001
17.5g ai/ha 1 Whole (21 weeks)
Beet <0.001
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
25g ai/ha 1 Whole (21 weeks)
Beet <0.001
17.5g ai/ha 2 Root (22 weeks)
0.004
Beet (22 weeks)
Top 0.003
25g ai/ha 2 Root (22 weeks)
0.002
Beet (22 weeks)
Top 0.003
England 12.5g ai/ha 1 Root (10 weeks)
<0.01
F. R. Ger. 25g ai/ha 3 Root n.d. (55) (109)
n.d. n.d.
25g ai/ha 3 Root n.d. n.d.
25g ai/ha 3 Root n.d. n.d.
25g ai/ha 4 Root n.d.(55) (66)
n.d. n.d.
Finland 15g ai/ha 2 Root (116)
<0.1
Bulb Vegetables
Leeks F. R. Ger. 13g ai/ha 2 Green 0.03 0.01 n.d. 0.02 n.d.
Part
13g ai/ha 2 White n.d. 0.03 0.02 n.d. n.d.
Part
13g ai/ha 2 Green 0.05 0.06 0.1 0.06 n.d.
Part
13g ai/ha 2 White n.d. n.d. n.d. n.d. 0.03
France 5g ai/ha 5 Leek 0.020
7.5g ai/ha 5 Leek 0.010
Table 3. Continued....
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Onions F. R. Ger. 13g ai/ha 2 Leaf 0.2 0.04 0.03 0.02 n.d.
13g ai/ha 2 Bulb 0.03 0.07 0.02 0.02 0.03
12g ai/ha 2 Peel n.d. n.d. n.d. n.d. n.d.
13g ai/ha 2 Leaf 0.05 0.01 0.02 0.03 0.03
13g ai/ha 2 Bulb 0.04 0.03 0.05 0.02 0.03
13g ai/ha 2 Peel n.d. n.d. n.d. n.d. n.d.
13g ai/ha 2 Leaf 0.4 0.1 n.d. 0.09 0.09
13g ai/ha 2 Bulb 0.04 0.05 0.1 n.d. 0.04
England 18.75g ai/ha 1 Bulb (52)
<0.01
Leafy Vegetables
Lettuce F. R. Ger. 25g ai/ha 2 Leaf 0.1 n.d. n.d. n.d.
25g ai/ha 2 Leaf 0.4 0.1 0.1 n.d. n.d.
25g ai/ha 2 Leaf 0.6 0.05 0.09 0.06 0.1
France 25g ai/ha 1 Leaf 0.25 0.25
25g ai/ha 1 Leaf 0.28 0.23
F.R.Ger. 12.5g ai/ha 2 Leaf 0.20 0.06 0.05 0.04 0.01
25g ai/ha 2 Leaf 0.30 0.20 0.10 0.08 0.01
12.5g ai/ha 2 Leaf 0.20 0.04 0.05 0.03 n.d.
25g ai/ha 2 Leaf 0.30 0.10 0.03 0.05 0.02
12.5g ai/ha 2 Leaf 0.10 0.04 0.01 n.d. 0.02
25g ai/ha 2 Leaf 0.20 0.10 0.02 0.01 n.d.
France 10g ai/ha 1 Leaf 0.23 0.067 0.004
17.5g ai/ha 1 Leaf 0.37 0.082 0.020
10g ai/ha 1 Leaf 0.22 0.044 0.006
17.5g ai/ha 1 Leaf 0.33 0.054 0.022
Spinach France 17.5g ai/ha 1 Crude Leaf 0.48 0.33 0.22
Cooked Leaf 0.27 0.21
Cooking Water 0.0001 0.00009
17.5g ai/ha 1 Crude Leaf 0.52 0.30 0.155
Cooked Leaf 0.28 0.12
Cooking Water 0.00015 0.00005
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
France 12.5g ai/ha 1 Crude Leaf 0.4 0.2 0.14 0.12
Cooked Leaf 0.12 0.10
Cooking Water 0.0001 n.d.
12.5g ai/ha 1 Crude Leaf 0.4 0.2 0.17 0.15
Cooked Leaf 0.15 0.12
Cooking Water 0.00015 0.0001
Brassica Leafy
Vegetables
Cabbages F. R. Ger. 25g ai/ha 2 Leaf 0.08 n.d. n.d. n.d. 0.01
25g ai/ha 2 Leaf 0.2 0.04 0.03 n.d. n.d.
25g ai/ha 2 Leaf 0.2 0.02 n.d. n.d. n.d.
25g ai/ha 2 Leaf 0.2 0.04 n.d. n.d. n.d.
S.Africa 5.0g ai/ha 1 Leaf <0.05 <0.05
7.5g ai/ha 1 <0.05 <0.05
10.Og ai/ha 1 <0.05 <0.05
Finland 3.8 mg/m 1 Leaf <0.1 <0.1
F.R.Ger. 12.5g ai/ha 2 Leaf 0.05 0.007 n.d. n.d. n.d.
Cabbage, Taiwan 50g ai/ha 8 Leaf 0.024 0.035 0.133 0.01 0.028 n.d.
Chinese
Cauliflower F. R. Ger. 12.5g ai/ha 2 n.d.
12.5g ai/ha 2 0.03 0.04 0.06 n.d. n.d.
12.5g ai/ha 2 0.09 0.03 0.02 0.02 0.01
Kohlrabi F.R.Ger. 12.5g ai/ha 2 0.006 n.d. n.d. n.d. n.d.
12.5g ai/ha 2 n.d. n.d. n.d. n.d. n.d.
12.5g ai/ha 2 n.d. n.d. n.d. n.d. n.d.
12.5g ai/ha 2 n.d. n.d. n.d. n.d. n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Stem Vegetables
Artichokes France 12.5g ai/ha 2 Whole 0.20 0.03 0.04 0.03
After 0.17 0.02 0.01 0.01
Cooking
Cooking 0.0005 0.0005 0.0001 0.0001
Water
12.5g ai/ha 2 Whole 0.23 0.06 0.05 0.04
After 0.17 0.06 0.05 0.04
Cooking
Cooking 0.0005 0.0005 0.0001 0.0001
Water
Legume Vegetables
Broad Beans France 12.5g ai/ha 1 Bean 0.01 0.02
Without
Pod 0.02 0.01
France 17.5g ai/ha 1 Whole Bean 0.001 0.0011 0.0008 0.0015
Whole 0.002 0.0009 0.0007 0.0015
Washed
France 15g ai/ha 1 Whole Bean 0.11 0.12 0.05 0.01
Whole 0.01 0.015 0.007 n.d.
Cooked
Cooking n.d. n.d. n.d. n.d.
Water
15g ai/ha 1 Whole Bean 0.1 0.06 0.05 n.d.
Whole 0.06 0.05 0.04 n.d.
Cooked
Cooking n.d. n.d. n.d. n.d.
Water
France 12.5g ai/ba 2 Peel 0.008
12.5g ai/ha 1 Grain n.d.
12.5g ai/ha 1 Peel 0.09 0.14 0.02 0.07
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
12.5g ai/ha 1 Whole Bean 0.04 0.07 0.01 0.03
12.5g ai/ha 1 Grain n.d. n.d. n.d. n.d.
12.5g ai/ha 1 Peel 0.10 0.02 0.01 0.03
12.5g ai/ha 1 Whole Bean 0.05 0.010 0.005 0.01
12.5g ai/ha 1 Grain n.d. n.d. n.d. n.d.
French Beans France 1.75g ai/ha 1 Whole Bean 0.001 0.0011 0.0008 0.0015
After Quick 0.002 0.0009 0.0012 0.0014
Washing
France 1.25g ai/hl 1 Whole Bean 0.11 0.12 0.05 0.01
Whole 0.01 0.015 0.007 n.d.
Cooked
Cooking n.d. n.d. n.d. n.d.
Water
France 1.25g ai/hl 1 Whole Bean 0.1 0.06 0.05 n.d.
Whole 0.06 0.05 0.04 n.d.
Cooked
Cooking n.d. n.d. n.d. n.d.
Soybeans France 12.5g ai/ha 1 Seed n.d. n.d. n.d. n.d. n.d.
Stem & 0.04 0.012
Leaves
Pod n.d. n.d.
12.5g ai/ha 2 Seed n.d. n.d. n.d. n.d.
Stem & 0.1
Leaves
17.5g ai/ha 1 Seed n.d. n.d. n.d. n.d. n.d.
Stem & 0.035 n.d.
Leaves
Pod 0.32 0.32
17.5g ai/ha 2 Seed n.d. n.d. n.d. n.d. n.d
Stem & n.d.
Leaves
Pod
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
France 12.5g ai/ha 2 Seed n.d.
12.5g ai/ha 2 Stem 0.13
12.5g ai/ha 2 Pod 0.45
Ivory Coast 25g ai/ha 7 Seed n.d.
Peas F. R. Ger. 12.5g ai/ha 2 Seed n.d. n.d. n.d. n.d. n.d.
12.5g ai/ha 2 Pod 0.04 0.03 0.04 0.04 0.04
12.5g ai/ha 2 Seed n.d. n.d. n.d. n.d. n.d.
12.5g ai/ha 2 Pod 0.05 0.01 0.02 n.d. n.d.
12.5g ai/ha 2 Seed n.d. n.d. n.d. n.d. n.d.
12.5g ai/ha 2 Pod 0.05 0.02 0.03 0.05 0.04
France 1.25g ai/hl 2 Seed 0.013 0.010
1.25g ai/hl 2 Pod 0.008 0.005
1.25g ai/hl 2 Whole Peas n.d. n.d.
England 12.5g ai/ha 1 Pod & Pea 0.02
Sugarpeas France 1.25g ai/hl 2 Whole Pea 0.030 0.015
Non-Washed
Whole Pea 0.030 0.005
Washed
France 1.25g ai/hl 1 Whole Pea 0.06 0.015 0.025 n.d.
Cooked Pea 0.02 0.007 0.008 n.d.
Cooking n.d. n.d. n.d. n.d.
Water
France 1.25g ai/hl 1 Whole Pea 0.10 0.025 n.d. n.d.
Cooked Pea 0.011 n.d. n.d. n.d.
Cooking n.d. n.d. n.d. n.d.
Water
Lentils Morocco 10g ai/ha 1 Grain n.d.
15g ai/ha 1 Grain n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Fruiting Vegetables
with Edible Peel
Cucumbers Finland 2.1 mg/l 1 Whole 0.02 0.03 0.01 n.d.
Fruit
France 12.5g ai/ha Whole 0.009 0.004 n.d. n.d.
Fruit
Pulp n.d. n.d. n.d. n.d.
Peel 0.04 0.005 n.d. n.d.
12.5g ai/ha Whole 0.0045 0.002 n.d. n.d.
Fruit
Pulp n.d. n.d. n.d. n.d.
Peel 0.026 0.012 n.d. n.d.
Eggplants France 1.25g ai/hl 1 Whole 0.025 0.010 0.006
Fruit
After 0.030 0.010 0.010
Washing
After 0.015 0.005 0.005
Cooking
Cooking 0.0001 0.00009 0.00007
Water
Gherkin Belgium 37.5g ai/ha 1 Whole (0.5)
Fruit 0.078 0.051 0.036 0.021
37.5g ai/ha 1 Whole 0.049 0.035 0.008 0.002
Fruit
37.5g ai/ha 1 Whole (0.5)
Fruit 0.009 0.013 0.007 0.004
37.5g ai/ha 1 Whole 0.019 0.004 0.001 0.004
Fruit
Jamaica France 12.5g ai/ha 1 Whole 0.10 0.03 0.03 0.04
Pepper Fruit
France 12.5g ai/ha 1 Whole 0.01 0.01 n.d. n.d.
Fruit
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Tomatoes France 12.5g ai/ha 1 Whole 0.023 0.023 0.027
Fruit
25g ai/ha 1 Whole 0.047 0.023 0.015
Fruit
12.5g ai/ha 1 Whole 0.008 0.009 0.024
Fruit
25g ai/ha 1 Whole 0.006 0.019 0.042
Fruit
France 25g ai/ha 2 Whole 0.017 0.014 0.016 0.011
Fruit
France 10g ai/ha 3 Whole 0.009 0.009
Fruit
10g ai/ha 3 Whole 0.010 0.007
Fruit
Spain 12.5g ai/ha 1 Whole <0.03 n.d. n.d.
Fruit
France 12.5g ai/ha 2 Whole 0.019 0.016 0.014 0.008 0.008 0.004 0.004
Fruit
Whole Washed 0.008 0.003
Whole Peeled n.d. n.d.
Peel 0.049 0.043
Juice 0.003 0.002
Washing Water 0.0019 0.001
Washing Water+48H 0.0001 n.d.
Finland 1 mg/l 1 Whole Fruit 0.04 0.03 0.01
Fruiting Vegetables
with Inedible Peel
Melons France 12.5g ai/ha 2 Peel 0.022 0.022 0.014
Pulp 0.0015 0.0016 0.0016
Whole Fruit 0.009 0.009 0.006
12.5g ai/ha 2 Peel 0.04 0.03 0.01
Pulp n.d. n.d. n.d.
Whole Fruit 0.018 0.015 0.004
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Citrus Fruits
Oranges Morocco 50g ai/ha 3 Peel (180) (180)
0.020 0.002
Pulp (180) (180)
0.003 n.d.
Whole Fruit (180) (180)
0.008 0.0006
75g ai/ha 3 Peel (180) (180)
0.035 0.005
Pulp (180) (180)
0.001 n.d.
Whole Fruit (180) (180)
0.011 0.0015
100g ai/ha 3 Peel (180) (180)
0.060 0.007
Pulp (180) (180)
n.d. n.d.
Whole (180) (180)
Fruit 0.017 0.0023
S. Africa 82.5g ai/ha 1 Peel 0.085 0.060 0.047 0.037 0.030 0.063 0.027 (35) (42)
0.044 0.023
Pulp 0.0003 0.0008 0.0004 0.0005 0.0008 0.0003 0.0008 (35) (45)
0.0009 0.0004
Whole 0.026 0.018 0.014 0.010 0.0095 0.018 0.0086 (35) (42)
Fruit 0.014 0.007
165g ai/ha 1 Peel 0.109 0.079 0.060 0.067 0.058 0.083 0.140 (35) (42)
0.100 0.042
Pulp 0.0009 0.0006 0.0007 0.0004 0.0006 0.0009 0.0002 (35) (42)
0.0009 0.0005
Whole 0.0355 0.0255 0.0215 0.020 0.022 0.026 0.043 (35) (42)
Fruit 0.032 0.013
Morocco 10g ai/ha 5 Peel (42)
0.05
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Pulp (42)
n.d.
Whole (42)
Fruit 0.01
Spain 12.75g ai/ha 1 Whole Fruit 0.1 n.d n.d
S.Africa 220g ai/ha 1 Peel (62)
0.10
Pulp (62)
n.d.
Whole (62)
Fruit 0.03
34.3g ai/ha 1 Peel (196)
0.005
Pulp (196)
0.0009
Whole (196)
Fruit 0.002
68.7g ai/ha 1 Peel (231)
n.d.
Pulp (231)
n.d.
Whole (231)
Fruit n.d.
S. Africa 110g ai/ha 1 Peel (86)
0.075
Pulp (86)
n.d.
Whole (86)
Fruit 0.025
220g ai/ha 1 Peel (86)
0.105
Pulp (86)
n.d.
Whole (86)
Fruit 0.035
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
396g ai/ha 1 Peel 0.27 0.28 0.24 0.15 0.22 0.25 0.16(135)(49)(63)
0.18 0.24 0.25
Pulp n.d. n.d. n.d. n.d. n.d. n.d. n.d.(35)(49)(63)
n.d.n.d.n.d.
Whole 0.10 0.09 0.09 0.06 0.08 0.06 (35)(49)(63)
Fruit 0.06 0.08 0.07
396g ai/ha 1 Peel 0.22 0.22 0.24 0.23 0.22 0.17 0.19 (35)(49)(63)
+132 l of oil 0.21 0.20 0.16
Pulp n.d. n.d. n.d. n.d. n.d. n.d. n.d. (35)(49)(63)
n.d.n.d.n.d.
Whole 0.10 0.10 0.095 0.08 0.10 0.08 0.08 (35)(49)(63)
Fruit 0.08 0.07 0.05
Clementines Morocco 10g ai/ha 5 Peel (42)
0.05
Pulp (42)
n.d.
Whole (42)
Fruit 0.01
Pome Fruits
Apples F. R. Ger. 50g ai/ha 4 Whole 0.05 0.03 0.03 0.02 0.03
Fruit
50g ai/ha 4 Whole 0.03 0.02 0.02 0.04 0.02
Fruit
50g ai/ha 4 Whole 0.2 0.1 0.08 0.1 0.06
Fruit
50g ai/ha 4 Whole Fruit 0.2 0.1 0.09 0.1
France 12.5g ai/ha 7 Whole (57)
Fruit 0.007
25g ai/ha 7 Whole 0.048 0.50 (57)
Fruit 0.032
25g ai/ha 6 Whole Fruit 0.061
25g ai/ha 9 Whole Fruit 0.194 0.264 0.144
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
France 7.5g ai/ha 8 Whole Fruit 0.005
12.5g ai/ha 8 Whole Fruit 0.007
7.5g ai/ha 10 Whole 0.003
12.5g ai/ha 10 Whole 0.004
12.5g ai/ha 5 Whole (34)
Fruit 0.002
12.5g ai/ha 5 Whole (34)
Fruit 0.005
7.5g ai/ha 8 Whole 0.008
Fruit
12.5g ai/ha 8 Whole 0.020 0.012 0.020
Fruit
F. R. Ger. 25g ai/ha 6 Whole 0.07 0.06 0.06 0.07 0.05
Fruit
50g ai/ha 6 Whole 0.06 0.06 n.d. 0.07 0.07
Fruit
25g ai/ha 6 Whole Fruit 0.05 0.05 0.04 0.05
50g ai/ha 6 Whole 0.05 0.04 0.04 0.05 0.04
Fruit
France 11.25g ai/ha 7 Whole Fruit 0.01
18.75g ai/ha 7 Whole Fruit 0.014
7.5g ai/ha 6 Whole Fruit 0.020 0.011
12.5g ai/ha 6 Whole Fruit 0.035 0.008
8.25g ai/ha 6 Whole Fruit 0.011
13.75g ai/ha 6 Whole Fruit 0.012
7.5g ai/ha 10 Whole Fruit 0.005
12.5g ai/ha 10 Whole Fruit 0.015
7.5g ai/ha 8 Whole Fruit 0.01
12.5g ai/ha 8 Whole Fruit 0.011
7.5g ai/ha 8 Whole Fruit 0.08
12.5g ai/ha 8 Whole Fruit 0.01 0.014 0.011
12.5g ai/ha 5 Whole (34)
Fruit 0.012
England 12.5g ai/ha 2 Whole 0.03
Fruit
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
F. R. Ger. 26g ai/ha 6 Whole 0.1 0.08 0.07 0.07
Fruit
26g ai/ha 6 Whole 0.08 0.05 0.04 0.05
Fruit
26g ai/ha 6 Whole 0.08 0.08 0.05 0.06
Fruit
France 0.75g ai/hl 8 Whole Fruit 0.035 0.030 0.032
1.25g ai/hl 8 Whole Fruit 0.068 0.040 0.050
0.75g ai/hl 5 Whole Fruit 0.017 0.008 0.006 0.012
1.25g ai/hl 5 Whole Fruit 0.025 0.011 0.010 0.006
0.75g ai/hl 5 Whole Fruit 0.013 0.010 0.008 0.016
1.25g ai/hl 5 Whole Fruit 0.013 0.012 0.013 0.012
0.75g ai/hl 5 Whole Fruit 0.025 0.020 0.011 0.013
1.25g ai/hl 5 Whole Fruit 0.0i8 0.034 0.030 0.019
0.75g ai/hl 5 Whole Fruit 0.016 0.015 0.017 0.018
1.25g ailhl 5 Whole Fruit 0.025 0.033 0.025 0.015
0.75g ai/hl 5 Whole Fruit 0.034 0.024 0.023 0.020
1.25g ai/hl 5 Whole Fruit 0.057 0.040 0.036 0.030
0.75g ai/hl 5 Whole Fruit 0.027 0.016 0.014 0.030
1.25g ai/hl 5 Whole Fruit 0.041 0.042 0.030 0.049
Sweden 22.5g ai/ha 3 Whole (63)
Fruit 0.010
S.Africa 0.31g ai/hl 2 Whole <0.05 <0.05 <0.05 <0.05
Fruit
0.75g ai/hl 4 Whole <0.05 <0.05 <0.05 <0.05
Fruit
France 7.5g ai/ha 6 Whole Fruit 0.045 0.035 0.02 0.01
7.5g ai/ha 4 Whole Fruit 0.03 0.03 0.01
Sweden 43.2 & 21.6g ai/ha 2 (58)
0.006
43.2 & 21.6g ai/ha 2 (58)
0.004
43.2 & 21.6g ai/ha 2 (58)
0.006
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Pears France 7.5g ai/ha 3 Whole (33)
Fruit 0.006
12.5g ai/ha 3 Whole (33)
Fruit 0.011
15g ai/ha 3 Whole (33)
Fruit 0.015
Italy 1.25g ai/hl 1 Whole Fruit 0.01 0.02 0.005
1.875g ai/hl 1 Whole Fruit 0.025 0.015 0.015
France 1.25g ai/hl 1 Whole Fruit 0.004
1.75g ailhi 1 Whole Fruit 0.004
1.25g ai/hl 1 Whole Fruit 0.006
1.75g ai/hl 1 Whole Fruit 0.006
0.75g ai/hl 4 Whole Fruit 0.018 0.021 0.015
1.25g ai/hl 4 Whole Fruit 0.017 0.014 0.004
France 7.5g ai/ha 5 Whole Fruit 0.125 0.015 0.010 0.010
7.5g ai/ha 5 Whole Fruit 0.09 0.02 0.02 0.02
Stone Fruits
Apricots France 12.5g ai/ha 2 Pulp 0.22 0.026 0.022 0.03
Whole Fruit 0.20 0.02 0.02 0.02
12.5g ai/ha 2 Pulp 0.077 0.026 0.026 0.011
Whole Fruit 0.07 0.02 0.02 0.01
France 12.5g ai/ha 2 Pulp 0.03 0.025 0.008 0.006
Whole Fruit 0.025 0.023 0.007 0.005
Cooked n.d. n.d.
12.5g ai/ha 2 Pulp 0.03 0.024 0.008 n.d.
Whole Fruit 0.027 0.020 0.007 n.d.
Cooked n.d. n.d.
Cherries France 15g ai/ha 2 Whole Fruit 0.055
21g ai/ha 2 Whole Fruit 0.095
27g ai/ha 2 Whole Fruit 0.045
F. R. Ger 25g ai/ha 3 Whole 0.04 0.04 0.02 0.04 0.03
Fruit
25g ai/ha 3 Whole 0.1 0.04 0.07 0.05 0.05
Fruit
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
25g ai/ha 3 Whole 0.08 0.06 0.06 0.05 0.04
Fruit
France 12.5g ai/ha 1 Pulp n.d. 0.0023 0.002
Whole Fruit n.d. 0.002 0.0018
Preserved 0.003 0.0017 0.0023
Pulp
Preserved 0.0025 0.0015 0.002
Pulp
Peaches France 12.5g ai/ha 1 Whole Fruit 0.05 0.04 0.02
12.5g ai/ha 1 Whole Fruit 0.03 0.04 0.02
25g ai/ha 1 Whole Fruit 0.09 0.07 0.08
25g ai/ha. 1 Whole Fruit 0.15 0.08 0.08
France 12.4g ai/ha 3 Whole Fruit 0.008 0.015
22.5g ai/ha 3 Whole Fruit 0.10 0.045
France 17.5g ai/ha 2 Pulp 0.03 0.047 0.043 0.029
Whole Fruit 0.03 0.043 0.040 0.026
Preserved n.d. n.d. n.d. 0.0015
Fruit
17.5g ai/ha 2 Pulp 0.027 0.045 0.040 0.024
Whole 0.025 0.04 0.037 0.022
Preserved n.d. n.d. n.d. n.d.
Fruit
F. R. Ger. 18.75g ai/ha 3 Whole 0.1 0.05 0.04 0.03
Fruit
18.75g ai/ha 3 Whole O.04 0.03 0.02 0.03
Fruit
18.75g ai/ha 3 Whole 0.06 0.02 0.04 0.02
Fruit
France 17.5g ai/ha 1 Whole 0.05 0.04 0.03 0.02
Fruit
Preserved 0.01.
Fruit
Juice n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Plum F. R. Ger. 25g ai/ha 5 Whole 0.07 0.08 0.08 0.07 0.05
Fruit
25g ai/ha 5 Whole 0.05 0.05 0.03 0.04 0.05
Fruit
25g ai/ha 5 Whole 0.02 0.04 0.05 0.05 0.04
Fruit
France 1.25g ai/hl 2 Whole Fruit 0.018 0.009 0.009 n.d.
Juice n.d.
Small Fruits
and Berries
Blackberries Finland 6.3 mg/bush 1 Berries 0.1 0.1
Grapes France 12.5g ai/ha 1 Whole Fruit 0.020 0.035 0.055 0.035 0.035
Fruit 12.5g ai/ha 1 Whole Fruit 0.055 0.020 0.020 0.020 0.025
25g ai/ha 1 Whole Fruit 0.025 0.045 0.033 0.015 0.010
25g ai/ha 1 Whole Fruit 0.040 0.015 0.015 0.015 0.005
France 25g ai/ha 1 Whole Fruit 0.006 0.075 0.040
25g ai/ha 1 Whole Fruit 0.015 0.010 0.030
F. R. Ger. 50g ai/ha 2 Whole 0.07 0.07 0.07 0.08 (35)
Fruit 0.06
50g ai/ha 2 Whole 0.05 0.08 0.07 0.05 (35)
Fruit 0.05
France 17.5g ai/ha 1 Whole Fruit 0.035 0.035
Juice Traces Traces
17.5g ai/ha 1 Whole Fruit 0.045 0.045
Juice Traces Traces
France 22.5g ai/ha 1 Whole Fruit 0.025 0.03 0.01 0.01
Juice 0.006
22.5g ai/ha 1 Whole Fruit 0.06 0.04 0.015 0.01
F. R. Ger. 25.Og ai/ha 2 Whole n.d. 0.04 0.07 0.05 0.02
Fruit
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Strawberries F. R. Ger. 50g ai/ha 1 Whole (5W)(6W)
Fruit n.d. n.d.
50g ai/ha 1 Whole (12W)(13W)
Fruit n.d. n.d.
50g ai/ha 1 Whole (6W)(7W)
Fruit n.d. n.d.
50g ai/ha 1 Whole (5W) (6W)
Fruit n.d.n.d.
France 12.5g ai/ha 1 Whole Fruit 0.015 0.004 0.008
Whole 0.014 n.d. n.d.
Washed
12.5g ai/ha 1 Whole Fruit 0.011 0.010 0.004
Whole 0.008 0.008 0.005
Washed
France 12.5g ai/ha 1 Whole Fruit 0.017 0.015 0.012 0.002
Jam n.d. n.d.
Assorted Fruits
with Edible Peel
Figs France 16g ai/ha 3 Whole Fruit <0.002
21g ai/ha 3 Whole Fruit <0.002
27g ai/ha 3 Whole Fruit 0.002
Olives France 15g ai/ha 3 Pulp <0.007
21g ai/ha 3 Pulp <0.007
27g ai/ha 3 Pulp <0.007
15g ai/ha 3 Whole Fruit <0.005
21g ai/ha 3 Whole Fruit <0.0O5
27g ai/ha 3 Whole Fruit <0.005
15g ai/ha 3 Pulp <0.007
21g ai/ha 3 Pulp <0.007
27g ai/ha 3 Pulp <0.007
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
15g ai/ha 3 Whole Fruit <0.005
21g ai/ha 3 Whole Fruit <0.005
27g ai/ha 3 Whole Fruit <0.005
17.5g ai/ha 4 Pulp 0.225 0.115 0.130 0.155 0.110 0.058
17.5g ai/ha 4 Whole Fruit 0.140 0.080 0.085 0.100 0.070 0.040
Oil 0.035 0.040 0.060 0.006
France 15g ai/ha 3 Whole Fruit 0.003
21g ai/ha 3 Whole Fruit 0.004
25g ai/ha 3 Whole Fruit 0.003
21g ai/ha 3 Whole Fruit 0.003
25g ai/ha 3 Whole Fruit 0.006
18.75g ai/ha 1 Whole (75)
Fruit 0.007
18.75g ai/ha 1 Whole (55)
Fruit 0.005
18.75g ai/ha 2 Whole (55)
Fruit 0.016
25g ai/ha 1 Whole (55)
Fruit 0.018
37.5g ai/ha 1 Whole (55)
Fruit 0.029
France 28g ai/ha 2 Pulp n.d. n.d. n.d.
Oil n.d. n.d. n.d.
Tunisia 12.5g ai/ha 1 Pulp (135)
n.d.
Assorted Fruits
with Inedible Peel
Bananas Phillippines 2.5g ai/ha 3-6 Pulp (77)
<0.01
Peel (77)
<0.01
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Whole (77)
Fruit <0.01
2.5g ai/ha 3-6 Pulp (77)
<0.01
Peel (77)
<0.01
Whole (77)
Fruit <0.01
2.5g ai/ha 3-6 Pulp (77)
<0.01
Peel (77)
<0.01
Whole (77)
Fruit <0.01
2.5g ai/ha 3-6 Pulp (77)
0.01
Peel (77)
1.0
Whole (77)
Fruit 0.39
2.5g ai/ha 3-6 Pulp (77)
<0.01
Peel (77)
0.10
Whole (77)
Fruit 0.04
Guadeloupe 22.5g ai/ha 1 Pulp (77)
n.d.
Peel (77)
0.07
Whole (77)
Fruit 0.016
46.8g al/ha 1 Pulp (77)
n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Peel (77)
0.03
Whole (77)
Fruit 0.01
93.7g ai/ha 1 Pulp (77)
0.003
Peel (77)
0.10
Whole (77)
Fruit 0.03
Pineapples 75g ai/ha 1 Juice (31)(61)
n.d. n.d.
Pulp (31)(61)
n.d. n.d.
Peel (31)(61)
0.025 0.018
Whole (31)(61)
Fruit 0.007 0.005
150g ai/ha 1 Juice (31)(61)
n.d. n.d.
Pulp (31)(61)
n.d. n.d.
Peel (31)(61)
0.2 0.025
Whole (31)(61)
Fruit 0.07 0.008
Cereal Grains
Wheat Brazil 7.5g ai/ha 1 Grain n.d.
10g ai/ha 1 Grain n.d.
12.5g ai/ha 1 Grain n.d.
15g ai/ha 1 Grain n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
France 10g ai/ha 1 Grain (53)
0.003
17.5g ai/ha 1 Grain (53)
0.003
10g ai/ha 1 Grain (53)
0.004
17.5g ai/ha 1 Grain (53)
0.0035
France 7.5g ai/ha 1 Grain (80)
0.002
Stem (80)
0.015
15g ai/ha 1 Grain (80)
0.04
Stem (80)
0.05
7.5g ai/ha 1 Grain (74)
0.001
Stem (74)
0.025
Flour (74)
n.d.
Bran (74)
n.d.
15g ai/ha 1 Grain (74)
0.003
Stem (74)
0.025
Flour (74)
n.d.
Bran (74)
n.d.
7.5g ai/ha 1 Grain (61)
0.0015
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Stem (61)
0.025
Flour (61)
n.d.
Bran (61)
n.d.
15g ai/ha 1 Grain (61)
0.002
Stem (61)
0.025
Flour (61)
n.d.
Bran (61)
n.d.
France 7.5g ai/ha 1 Grain (64)
n.d.
Stem (64)
n.d.
Flour (64)
n.d.
Bran (64)
n.d.
15g ai/ha 1 Grain (64)
n.d.
Stem (64)
n.d.
Flour (64)
n.d.
Bran (64)
n.d.
Maize F. R. Ger. 17.5g ai/ha 1 Corn/ (55)
Grain 0.006
Brazil 15g ai/ha 2 Corn/ n.d. n.d. n.d. n.d. n.d.
Grain
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
France 7.5g ai/ha 1 Corn/ (70)
Grain n.d.
12.5g ai/ha 1 Corn/ (70)
Grain n.d.
France 7.5g ai/ha 1 Corn/ (94)
Grain n.d.
12.5g ai/ha 1 Corn/ (94)
Grain n.d.
Rice Guyanne 12.5g ai/ha 1 Grain (50)
n.d.
12.5g ai/ha 1 Grain (50)
0.020
Suriname 6.25g ai/ha 1 Grain (43)(49)
0.001 n.d.
Straw (43)(49)
n.d. n.d.
12.5g ai/ha 1 Grain (43)(49)
0.005 n.d.
Straw (43)(49)
0.010 0.008
18.75g ai/ha 1 Grain (43)(49)
n.d. 0.005
Straw (43)(49)
0.010 0.010
25g ai/ha 1 Grain (43)(49)
0.015 0.015
Straw (43)(49)
0.080 0.050
Ivory Coast 18.75g ai/ha 3 Grain (39)
0.03
Philippines 17.5g ai/ha 5 Grain (37)
n.d.
Husk (37)
n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
25g ai/ha 5 Grain (37)
n.d.
Husk (37)
n.d.
Fodders & Straws
Grass New Zealand 5g ai/ha 1 0.52 0.42 0.4 0.2 0.13
(Pasture) 10g ai/ha 1 1.07 1.07 0.8 0.67 0.46 0.07
15g ai/ha 1 1.20 1.23 1.3 0.70 0.5
20g ai/ha 1 1.37 1.30 2.3 0.97 0.8 0.47
35g ai/ha 1 2.89 2.42 2.68 1.87 1.0 1.3
50g ai/ha 1 4.7 3.7 3.6 3.6 1.9 1.1
Legume Oilseed
Peanuts Ivory Coast 18.5g ai/ha 7 Grain (71)
n.d.
Pods (71)
0.005
Legume
Animal Feeds
Alfalfa France 12.5g ai/ha 2 Whole 1.5 l.0 0.45 0.11
Fodder Crop
(Lucerne)
12.5g ai/ha 2 Whole 1.6 0.75 0.48 0.10
Crop
New Zealand 10.Og ai/ha 1 Whole 0.25 0.25 0.23 0.09 n.d.
Crop
France 12.5g ai/ha 2 Whole 1.0 0.16 0.10
Green
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
12.5g ai/ha 2 Whole Dry 0.20
12.5g ai/ha 2 Whole 0.25 0.065 0.03
Green
12.5g ai/ha 2 Whole Dry 0.03
Oilseeds
Cottonseed USA 22.5g ai/ha 3 Cottonseed n.d.
22.5g ai/ha 8 Cottonseed n.d.
22.5g ai/ha 5 Cotton (43)
Seed <0.02
22.5g ai/ha 4 Cotton (45)
Seed <0.02
22.5g al/ha 5 Cotton (49)
Seed <0.02
22.5g ai/ha 14 Cotton (52)
Seed n.d.
22.5g ai/ha 7 Cotton (77)
Seed n.d.
USA 0.03 mg/kg 1 Cotton 0.03
(Fortified Seed
Cotton Delinted <0.02
Seed) Seed
Hulls 0.035
Linters 0.325
Solvent n.d.
Extracted
Meal
Refined <0.02
Oil
Alkaline <0.02
Soap
Stock
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
0.03 mg/kg 1 Total n.d.
Full
Fatty
Acids
0.34 mg/kg 1 Cotton 0.33
Seed
Delinted 0.05
Seed
Hulls 0.39
Linters 3.2
Solvent n.d.
Extracted
Meal
Refined n.d.
Oil
Alkaline n.d.
Soap
Stock
Total n.d.
Free
Fatty
Acids
Morocco 25g ai/ha 7 Cotton (62)
Seed <0.01
25g ai/ha 6 Cotton (121)
Seed 0.002
USA 22.5g ai/ha 6 Cottonseed n.d.
22.5g ai/ha 6 Cottonseed n.d.
22.5g ai/ha 7 Cottonseed <0.02
22.5g ai/ha 8 Cotton (40)
Seed 0.03
22.5g ai/ha 8 Cottonseed n.d.
22.5g ai/ha 10 Cotton (40)
Seed <0.02
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
22.5g ai/ha 14 Cottonseed 0.08
22.5g ai/ha 15 Cottonseed <0.02
22.5g ai/ha 15 Cottonseed <0.02
22.5g ai/ha 15 Cottonseed <0.02
22.5g ai/ha 15 Cottonseed 0.01
22.5g ai/ha 15 Cottonseed n.d.
USA 22.5g ai/ha 15 Cottonseed <0.02
Delinted n.d.
Seed
Linters <0.05
Hulls n.d.
Solvent n.d.
Extracted
Meal
Refined <0.02
Oil
Alkaline n.d.
Soap
Stock
Morocco 12.5g ai/ha 7 Cottonseed n.d.
12.5g ai/ha 7 Cottonseed n.d.
+ NH
Ivory Coast 15g ai/ha 6 Fibers 0.007
India 10g ai/ha 8 Cotton n.d.
Seed
Fiber n.d.
Oil n.d.
Cake n.d.
20g ai/ha 8 Cottonseed n.d.
Fiber n.d.
Oil n.d.
Cake n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Rapeseed F. R. Ger. 12.5g ai/ha 1 Seed (73)
n.d.
Cake (73)
n.d.
Oil (73)
n.d.
Seed (51)(80)
0.01 0.03
Cake (51)(80)
n.d. n.d.
Oil (51)(80)
n.d. n.d.
Sweden 100g ai/ha 1 Seed (84)
n.d.
Finland 15g ai/ha 1 Seed (87)
<0.05
France 5g ai/ha 1 Seed (38)
n.d.
5g ai/ha 1 Seed (48)
n.d.
Tropical Seeds
Cocoa Ivory 6.25g ai/ha 8 Cocoa (38)
Beans Coast Bean 0.006
8.75g ai/ha 8 Cocoa (38)
Bean 0.025
8.75g ai/ha 8 Cocoa (38)
Bean 0.007
Ivory 18.75g ai/ha 3 Cocoa (72)
Coast Bean n.d.
Coffee Brazil 6.25g ai/ha 1 Grain n.d. n.d. n.d.
Beans 6.25g ai/ha 2 Grain n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
12.5g ai/ha 1 Grain n.d. n.d. n.d.
12.5g ai/ha 2 Grain 0.02
Ivory 12.5g ai/ha 2 Grain (198)
Coast n.d.
18.75g ai/ha 2 Grain (198)
n.d.
Brazil 10g ai/ha 2 Grain (46)
n.d.
Ivory 18.75g ai/ha 2 Grain (178)
Coast n.d.
Spices
Pepper Cameroun 12.5g ai/ha 1 Black (56)
Grains n.d.
12.5g ai/ha 1 Grey (56)
Grains n.d.
12.5g ai/ha 1 Green (35)
Grains n.d.
Tea
Tea China 6.6g ai/ha 1 Leaf 3.1 3.0 1.1 0.75 0.65
Water 0.001 0.005 0.0006 n.d. n.d.
10.0g ai/ha 1 Leaf 4.3 1.6 1.4 1.5 0.85
Water 0.002 0.0005 0.0009 n.d. n.d.
13.3g ai/ha 1 Leaf 3.2 3.2 2.5 1.9 1.2
Water 0.002 0.0013 0.0009 n.d. n.d.
20.0g ai/ha 1 Leaf 2.5 4.8 3.4 2.4 1.5
Water 0.005 0.001 0.0008 n.d. n.d.
India 10.0g ai/ha 1 Leaf 5.5 2.1 1.7 1.0
Water n.d. n.d. n.d. n.d.
15.0g ai/ha 1 Leaf 7.8 5.1 2.9 2.3
Water n.d. 0.0005 n.d. n.d.
Table 3. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application
Crop Country Dosage rate applications analyzed 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 >30
Miscellaneous
Hops England 12.5g ai/ha 7 Cone 0.02
Mushrooms Netherlands 6.25g ai/ha 2 Whole 0.0033 0.0017 0.0010
6.25g ai/ha 2 Whole 0.0014 0.0012
25g ai/ha 2 Whole 0.0088 0.0036 0.0029
25g ai/ha 2 Whole 0.0041 0.0027
Sugarcane Brazil 25g ai/ha 2 Juice n.d.
Martinique 7.5g ai/ha 1 Juice (98)
n.d.
25g ai/ha 1 Juice (98)
n.d.
7.5g ai/ha 1 Juice n.d.
25g ai/ha 1 Juice n.d.
Trials on various root and tuber vegetables resulted in residues
ranging from n.d. (<0.001 mg/kg) to 0.015 mg/kg at 2-3 days after
last application.
Trials on bulb vegetables (leeks and onions) resulted in residues
ranging from <0.01 to 0.1 mg/kg on the bulb and 0.1 mg/kg on the leaf
at 2-5 days after last application.
Trials on leafy vegetables (lettuce and spinach) resulted in residues
ranging from 0.52 mg/kg (highest value) at 2-3 days after last
application to 0.15 mg/kg (highest value) at 13-16 days.
Trials on Brassica leafy vegetables (cabbages, cauliflower, and
kohlrabi) ranged from 0.13 mg/kg (highest value) at 2-3 days to 0.03
mg/kg (highest value) at 13-16 days after last application.
Trials on artichokes gave residues ranging from 0.23 mg/kg at 2-3 days
to 0.04 mg/kg at 13-16 days after last application. Cooking did not
reduce the residue levels significantly.
Trials on legume vegetables gave residues ranging from 0.1 mg/kg (high
value) at 2-3 days to 0.07 mg/kg ( high value) at 13-16 days after
last application except for soybean pods which had residues up to 0.35
mg/kg at 13-16 days.
Trials on fruiting vegetables (edible peel) resulted in residues
ranging from 0.1 mg/kg (high value, jamaica pepper) at 2-3 days to
0.04 mg/kg (high value, tomato) at 13-16 days after last application.
Residues from trials on melons did not exceed 0.0016 mg/kg in the pulp
or 0.018 mg/kg in the whole fruit at any interval from 1-8 days after
last application.
Residues on citrus fruits resulting from the highest rate of use (396
g ai/ha + oil) declined from 0.1 mg/kg (whole fruit) on the day of
treatment to 0.08 mg/kg (whole fruit) at 35 days after last
application.
Trials on pome fruits resulted in residues on whole fruit that did not
vary significantly with the number of days after treatment but were
largely dose-dependent. A dosage rate of 50g ai/ha gave a highest
residue of 0.2 mg/kg on day of treatment. Dosage rates of 1.25 g
ai/hl gave residues that did not exceed 0.057 mg/kg.
Trials on stone fruits resulted in residues on whole fruit ranging
from 0.2 mg/kg (apricots) at 2-3 days to 0.08 mg/kg ( highest value,
peaches) at 13-16 days after last treatment.
Residues on small fruits and berries ranged from 0.1 mg/kg (high
value, blackberries) and 0.08 mg/kg (high value, grapes) to 0.017
mg/kg (high value, strawberries).
Trials on assorted fruits (edible peel) resulted in residues on figs
of <0.002 mg/kg at 13-16 days after last application. For olives the
results ranged from 0.225 mg/kg (pulp) to 0.003 mg/kg at 1 day after
treatment. By 55 days after treatment residues did not exceed 0.018
mg/kg (whole fruit).
Residues on assorted fruits (inedible peel) did not exceed 0.01 mg/kg
in the pulp of bananas at 77 days after last treatment even at the
highest dosage tested (93.7 ai/ha). Residues in pineapple pulp were
n.d. at 31 days post-treatment, but residues in whole fruit were 0.*07
mg/kg at the same interval. No residues were detected in pineapple
juice.
Trials on cereal grains resulted in maximum residues of 0.04 mg/kg in
wheat grain at 80 days after last treatment. Residues in wheat flour
and bran were n.d. after 61 days post-treatment at dosages up to 15 g
ai/ha.
A maximum residue of 0.006 mg/kg was found in maize grain at 55 days
post-treatment. A maximum residue of 0.03 mg/kg was found in rice
grain at 39 days post-treatment. There were no detectable residues in
rice husks.
Trials on pasture grass resulted in residues up to 4.7 mg/kg on day of
treatment with dosage of 50 g ai/ha. The residue had declined to 1.1
mg/kg by 9-12 days post-treatment.
A trial on peanuts resulted in no detectable residue in the grain and
0.005 mg/kg in the pod at 71 days after last treatment.
Trials on alfalfa animal fodder resulted in residues that ranged from
0.25 to 1.6 mg/kg at 2-3 days after last treatment and steadily
declined to values of n.d. to 0.2 mg/kg after 21-24 days. Trials on
soybean stem and leaves resulted in residues ranging from n.d. to 0.1
mg/kg at 13-16 days after last treatment.
Residues in cotton seed were 0.03 mg/kg or less at 40 or more days
after field treatment at 22.5 g ai/ha for 3-15 treatments. Cotton
seed fortified at 0.34 mg/kg gave residues after processing
distributed as follows: seed-0.33 mg/kg, delinted seed-0.05 mg/kg,
hulls-0.39 mg/kg, linters-3.2 mg/kg, solvent extracted meal-n.d.,
refined oil-n.d., alkaline soap stock-n.d., total free fatty
acids-n.d. Residues in processed products from cotton field-treated
15 times at 22.5 g ai/ha did not exceed 0.05 mg/kg (linters).
Residues in rapeseed, cake, and oil were less than 0.05 mg/kg at 51-87
days post-treatment.
Trials on cacao resulted in residues in cocoa beans that did not
exceed 0.025 mg/kg at 38 days after last treatment regardless of trial
dosage or number of applications. Residues in coffee beans were n.d.
at 13-198 days after last treatment at dosages of 6.25 to 18.75 g
ai/ha. However, analysis of a sample at 1 day after the last of 2
treatments at 12.5 g ai/ha found a residue of 0.02 mg/kg.
Residues on spices (pepper; black, grey and green grains) were n.d. at
35-56 days post-treatment with 12.5 g ai/ha.
Residues on tea (leaves) ranged from 2.5 to 7.8 mg/kg (not
dose-related) at 0-1 day post-treatment with single dosages from 6.6
to 20 g ai/ha. Residues declined thereafter to a range of 0.65 to 1.5
mg/kg at 13-16 days post-treatment. Analysis of tea water brewed from
leaves picked on day of treatment gave residues ranging from 0.001 to
0.005 mg/kg which became n.d. (<0.0005 mg/kg) by 6-8 days
post-treatment.
Trials on miscellaneous crops (hops, mushrooms, sugarcane) resulted in
residues on hops cones of 0.02 mg/kg at 9-12 days after the last of 7
treatments. Residues on mushrooms ranged from 0.0033 to 0.0088 mg/kg
(dose-dependent) at 1 day after last treatment with either 6.25 or 25
g ai/ha and declined to 0.0012 to 0.0029 mg/kg by 4-5 days
post-treatment. Residues in the juice of sugarcane were n.d. (<0.005
mg/kg) at 6-8 days after last treatment at any level tested.
FATE OF RESIDUES
General
On foliage in the field, deltamethrin degrades rapidly to a large
variety of hydrolytic, oxidative, and photolytic products and their
conjugates. The following extractable compounds have been identified:
trans-deltamethrin, trans-hydroxymethyl deltamethrin, 4'-hydroxy
deltamethrin, 4'-hydroxy-trans-hydroxymethyl deltamethrin,
3-(2,2-dibromovinyl)-2,2 dimethyl-cyclopropane-carboxylic acid
(Br2CA) and 3 conjugates, trans-hydroxymethyl Br2CA,
3-phenoxybenzaldehyde (pb ald) and the corresponding alcohol (pb alc)
and acid (pb acid), 4'-hydroxyphenoxy-benzoic acid, and 2 or 3
conjugates of each of phenoxybenzyl alcohol, phenoxybenzoic acid, and
alpha-cyanophenoxybenzyl alcohol. Considerable amounts of
unextractable residues of unknown composition (up to 35% of the
applied dose) are also formed. Metabolism in animals is generally
similar to that in plants, differing in the nature of the conjugates.
Rapid excretion of deltamethrin and its metabolites occurs in animals
with negligible tendency to bioaccumulate.
In animals
Metabolism in a lactating cow
Studies were carried out in two phases on a single lactating Jersey
cow, weighing 350 kg, giving milk of approx. 6.3% butterfat, using
C14-deltamethrin labelled in the alpha-methine group of the alcohol
moiety. In phase 1, 0.27 g of labelled deltamethrin was injected
intrarumenally as a solution in a sesame oil/alcohol mixture.
Radioactivity was rapidly excreted, mainly in urine and faeces
(85.3%). Only 0.4% was found in whole milk, corresponding to peak
residue levels after only one day of 0.045 and 0.92 mg/kg deltamethrin
equivalents in whole milk and rendered butterfat respectively. The
residue found in the peak butter sample was mainly (89%) unchanged
deltamethrin. The half-life in milk and butter was 0.8 days. Omental
fat and leg muscle biopsy samples, removed two days after treatment,
contained 0.088 and 0.008 mg/kg deltamethrin equivalents respectively.
In phase 2, conducted 49 days after phase 1, 0.21 g of labelled
deltamethrin in the form of a miscible oil formulation was applied
externally to the cow (except on the udder) by brushing. Although
well restrained by a neck halter, the sow succeeded in licking herself
to a nominal degree. Peak residue levels were attained after 2-5 days
(0.0057 mg/kg) in whole milk and 2 days (0.1 mg/kg) in butterfat.
Half-lives for depletion of activity from whole milk, butterfat, and
body hair were in the range of 4 to 4 1/2 days. There was no apparent
degradation of deltamethrin on body hair during the first 20 days.
Since good control of flies in stables is obtained by spraying 7.5 mg
ai/m2 on the walls, an estimate of residue potential in milk and
butter from this source was made on the assumption that a restrained
dairy cow cannot lick more than 1 m2 of treated wall. Calculated
residues of deltamethrin would thus be 0.00125 mg/kg and 0.0255 mg/kg
in milk and butter respectively (WELL 79 04 HIBH/A).
In plants
Cotton was treated topically on the leaves with each of
C14-deltamethrin samples labelled in the dibromovinyl, benzylic, and
cyano carbons in field and greenhouse studies in California. Initial
deposits were 0.04-0.33 g/cm2 of leaf surface (3-15 mg/kg based on
fresh leaf weight). Leaves were harvested at 2 and 6 weeks after
treatment and immediately cut into approx. 5 mm2 pieces. The 14C
products were extracted by soaking in acetonitrile-chloroform (2:1)
and decantation. The concentrated extracts were analysed for total
14C content by liquid scintillation counting of an aliquot and for
individual 14C compounds by TLC. Unextractable residue was
determined by combustion analysis.
Metabolism in cotton and bean leaf discs from leaves freshly removed
from greenhouse-grown plants was also studied. Discs of 10-mm diameter
were punched out under water and incubated for 5 h at 30°C under
artificial illumination in 25-ml Erlenmeyer flasks containing 25
discs, 2 ml distilled water, and 1-5 g of 14C substrate in 30 l
ethanol.
Under the greenhouse conditions the half-life of deltamethrin is 1.1
weeks with a 90% loss in 4.6 weeks. Under field conditions there is a
more rapid loss of parent compound, a higher proportion of trans- to
cis-deltamethrin and larger amounts of unextractable products. The
primary compounds found (1% of applied dose) after six weeks were
deltamethrin (1.7-6.1%), trans-deltamethrin (0.7-2.7%), Br2CA
(0.3-3%), Br CA-conj. (7.7-4.2%), pb ald (1.2-1.1%), pb acid (0-2%),
pb alc-conj. (1.9-1.2%), pb acid glyc. (0.9-1.7%), pb acid conj.
(5.9-1.5%), pb cy-conj. (8.8-3.2%), and unextractables (35-20%).
In the leaf disc experiments, bean leaf discs converted [14C]- and
[14C(alpha)]-deltamethrin in small yield (approx. 6%) to
[14C]Br2CA-glyc and [14C]alc-glyc respectively, whereas cotton leaf
discs did not. Cleavage products 14CBr2CA and [14C(alpha)]pb cy as
substrates undergo more extensive metabolism than deltamethrin in both
plants (Ruzo et al., 1979).
Uptake - translocation studies in cotton plant growth chambers
following hydroponic, foliar, and soil treatments were carried out
using C14 labelled (alpha-methrin position) deltamethrin. For the
hydroponic application, a 750 ml volume of nutrient solution/jar was
fortified with an ethanol solution of 14C-deltamethrin. For the soil
application 2.5% EC formulation prepared by adding 14C-deltamethrin
to an EC blank was applied evenly by pipette to the soil surface 3 cm
around 2-week old potted plants. Foliar application was made as a
droplet of ethanol solution of 14C-deltamethrin from a micropipette
to the midvein of the first leaf of the cotton plant. Plants were
harvested at 1, 3 and 7 days after treatment for autoradiography and
extraction. Analyses were by liquid scintillation, combustion of
post-extraction solids, and TLC. Recovery of radioactivity was 96.5%.
Results of the foliar and hydroponic experiments indicated virtually
no systemic behaviour for deltamethrin with observed transport being
extremely limited (0.5-0.8%) after 7 days in both cases. For soil
treatment, radioactivity detected in the shoots and roots was only 1.6
and 0.35% of applied dose respectively after 7 days. Most of the
radioactivity found in the shoot was concentrated in the polar and
bound residue fractions (PRO 77 1309/A).
In soil
No data.
In water
No data.
In storage and processing
Wheat
Trials were conducted on the persistence of deltamethrin residues on
stored wheat and processed wheat products in Morocco, Belgium, Greece,
and the U.K. following treatment of the grains with various
formulations at low, but effective, dosages as shown in Table 4. In
Morocco, dusts and EC formulations of equal strength (0.025 g ai/ql)
resulted in lower initial (8-11 weeks) residues (approx. 0.01 mg/kg)
for EC than for dust (approx. 0.05 mg/kg). However, after 40 weeks,
residues on grain were approximately equal (approx. 0.1 mg/kg) with no
measurable losses, taking account of the analytical variability.
Washing the grain after treatment had no effect on residue levels.
Bran and flour produced from the wheat after 40 weeks storage had mean
residues of 0.15 mg/kg and 0.005 mg/kg respectively (FP. 78.27.04/A).
In Belgium, low volume sprays of Decis EC 25 at 0.7, 0.85, or 1.0
mg/kg gave initial (1 week) residues proportional to dosage of 0.56,
0.69, or 0.78 mg/kg respectively. These had fallen to 0.27, 0.47, or
0.69 by the end of 35 weeks. The corresponding residues in flour and
bran were 0.1, 0.25, and 0.4 mg/kg and 1.2, 1.3, and 1.7 mg/kg
respectively (FP-78.07.12/A).
In Greece low volume sprays of K-OTHRINE EC 25 at 1 or 0.75 mg/kg on
wheat grain gave residues of 0.31 and 0.29 mg/kg respectively after 24
and 17 weeks (FP-80.05.02/A).
In the U.K. two liquid spray formulations containing piperonyl
butoxide were applied to wheat, at calculated dosages of 1 or 2 mg/kg.
Analysis of the wheat on the day of treatment however showed actual
levels of 0.44 and 0.80 mg/kg respectively. Residue analysis on
samples taken at monthly intervals for three months showed no
degradation of the deltamethrin. There was no detectable degradation
of deltamethrin by the process of milling and backing of either
freshly treated wheat or wheat that had been stored for three months
(WELL/80.21.05/A1).
Table 4. Deltamethrin residues in stored products
Number of Part Residues (mg/kg) at intervals (days) after last application1
Crop Country Dosage rate applications analyzed 0 1 2-3 4-7 8-11 12-19 20-29 30-39 40-49 50-59
Cereal Grains
Wheat Morocco 0.025g ai/ql 1 Non-washed 0.050 0.067 0.050 0.100 0.120
Dust
0.025g ai/ql 1 Washed 0.052 0.042 0.060 0.080 0.120
Dust
0.025g ai/ql 1 Non-washed 0.010 0.010 n.d. 0.110 0.080
EC 25 + PB2
0.025g ai/ql 1 Washed 0.015 0.012 n.d. 0.060 0.100
EC 25 + PB
0.025g ai/ql 1 Non-Washed 0.145
Dust Wheat bran
0.025g ai/ql 1 Washed 0.145
Dust Wheat bran
0.025g ai/ql 1 Non-Washed 0.16
EC 25 + PB Wheat bran
0.025g ai/ql 1 Washed 0.14
EC 25 + PB Wheat bran
0.025g ai/ql 1 Non-Washed 0.007
Dust Wheat flour
0.025g ai/ql 1 Washed 0.004
Dust Wheat flour
0.025g ai/ql 1 Non-Washed 0.004
EC 25 + PB Wheat flour
0.025g ai/ql 1 Washed 0.004
EC 25 + PB Wheat flour
Belgium 0.7 mg/kg 1 Grain 0.56 0.70 0.35 0.27
Low vol. spray Wheat flour 0.065 0.1
Low vol. spray Wheat bran 0.65 1.2
0.85 mg/kg 1 Grain 0.69 1.00 0.50 0.47
Wheat flour 0.07 0.25
Wheat bran 1.00 1.3
Table 4. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application1
Crop Country Dosage rate applications analyzed 0 1 2-3 4-7 8-11 12-19 20-29 30-39 40-49 50-59
1.0 mg/kg 1 Grain 0.78 1.10 0.70 0.69
Low vol. spray Wheat flour 0.09 0.4
Low vol. spray Wheat bran 1.20 1.7
Greece 1.0 mg/kg 1 Grain 0.31
Low vol. spray
0.75 1 Grain 0.29
U.K. 1.0 mg/kg + PB 1 Wheat 0.44 0.53 0.48 0.50
2. 0 mg/ 1 kg + PB 1 Wheat 0.80 1.25 1.33 1.46
Dil. ag. spray
1.0 mg/kg + PB 1 Wheat 0.44 0.50
Wholemeal 0.42 0.43
flour
Wholemeal 0.26 0.27
bread
Bran 3.0 1.8
Fine n.d. 0.5
Offal
First n.d. n.d.
reduction
flour
Total 0.09 0.05
white
flour
White 0.10 n.d.
bread
2.0 mg/kg + PB 1 Wheat 0.80 1.46
Whole 0.73 0.80
meal
flour
Whole 0.63 0.57
meal
bread
Bran 5.4 4.7
Fine n.d. 0.75
Offal
Table 4. Continued...
Number of Part Residues (mg/kg) at intervals (days) after last application1
Crop Country Dosage rate applications analyzed 0 1 2-3 4-7 8-11 12-19 20-29 30-39 40-49 50-59
First n.d. n.d.
reduction
flour
Total 0.20 0.20
white
flour
White 0.29 0.15
bread
Maize Brazil 0.5 mg/kg dust 1 Grain 0.6
1. 0 mg/kg dust 1 Grain 0.7
2.0 mg/kg dust 1 Grain 1.0
Brazil 1.0 mg/kg dust 1 Whole Fruit n.d.
1. 0 mg/kg dust 1 Grain n.d.
Cameroon 5 mg/kg 1 Straw/ 1.25
EC spray Stem
Cameroon 2.5 mg/kg 1 Grain 0.007
EC spray
Cameroon 2. 5 mg/kg 1 Grain 0.47
EC spray
Peanuts Cameroon 5 mg/kg 1 Straw/ 1.25
Stem
Coffee Brazil 1.0 mg/kg dust 1 Grain 0.95
2.0 mg/kg dust 1 Grain 1.65
Brazil 25g/350m2 1 Unroasted 0.01
Spray on bags Grain
25g/350m2 1 Roasted n.d.
Spray on bags Grain
Brazil 0.0040 m3 1 Grain n.d.
ULV fog
1 Residue data on wheat grain in Morocco at 127, 187, and 244 days is omitted for table size
and convenience since the values do not differ significantly from those occurring before and after.
2 PB = Piperonyl butoxide.
Maize
Trials were conducted in Brazil and Cameroon on stored maize treated
at various low levels with either dusts or low volume sprays as shown
in Table 4. Analysis of grain samples after 20-50 weeks showed no
measurable degradation for treatments at 0.5 mg/kg. However, for
treatments of 1 mg/kg and above, residues had decreased by 50 to 99.7%
(FP-78.30.09/A, FP-79.16.07/A, FP-79.18.07/A, FP-78.30.05/A, and
FP-78.02.10/A).
Peanuts
Table 4 also presents the results of one trial in Cameroon on stored
peanuts (hay?) treated once with DECIS EC 25 at a rate of 0.5 g ai.
for 100 kg crop. The residue of deltamethrin in straw/stem after 23
weeks was 1.25 mg/kg (FP-79.07.06/A).
Coffee
Trials in Brazil were conducted on 2 stored coffee beans using dust at
1 and 2 mg/kg, aqueous EC spray on bags at 25 g ai/350 m2, and ULV
fog at 4 g ai/100 m3. Dust treatments resulted in negligible loss of
residue after 8 months of storage. The EC spray resulted in a residue
of 0.01 mg/kg on the day of treatment; roasting lowered this to n.d.
(<0.005 mg/kg). A sample of coffee fortified at 0.05 mg/kg and
roasted resulted in a residue of 0.012 mg/kg. ULV fog resulted in
n.d. (0.005 mg/kg) residues 1 day after treatment (FP-78.29.09/A,
FP-79.18.10/A, and FP-79.19.10/A).
Photodecomposition
The photochemistry of deltamethrin is quite complex, as might be
expected. Exposure to UV radiation (lambda > 290 nm) in various
organic solvents results in cis-trans isomerization, eater cleavage
reactions, and loss of bromine. Films of deltamethrin on glass or
silica gel exposed to sunlight irradiation results in cis-trans
isomerization as the major reaction plus smaller amounts of various
cleavage products and polar materials. Twenty five photoproducts have
been identified from irradiation of deltamethrin or its initial
photolytic derivatives. The mixtures of deltamethrin photoproducts
from solution and solid-phase reactions are less toxic than
deltamethrin to mice treated intraperitoneally (Ruzo et al., 1977).
RESIDUES IN COMMERCE OR AT CONSUMPTION
Due to the short period of development and use of deltamethrin there
are no data yet available on residues in food in commerce or at
consumption. Because of the very low dosage rates usually recommended
for most field crops it seems quite unlikely that measurable residues
will be found on food ready for consumption. The exception might be
for processed foods (flour, bread, corn meal cakes, etc.) prepared
from stored grains treated in storage, as data in the preceding
section showed that storage, processing and cooking had little effect
on residue levels.
METHODS OF RESIDUE ANALYSIS
A sensitive general purpose method for the analysis of deltamethrin
residues in plant tissues has been developed, which is the fusion of
various preceding analytical methods used until now. The method is
based on extraction, purification by liquid-liquid partition, liquid
chromatography clean-up, and gas chromatography quantitation by
electron-capture detection. Recoveries range from 80 to 95% and the
sensitivity of the method is between 1-10 µg/kg.
A 20 g sample of vegetable material is extracted by blending with
solvent, sodium sulphate and Celite, filtered through a Buchner
funnel, and evaporated to about 30 ml. A choice of 3 extraction
solvents: acetonitrile (or hexane), petroleum ether-diethyl ether
(50/50; v/v), or acetone-toluene is made according to the nature of
the sample and its lipid content. For some crops these extracts are
clean enough to proceed directly to liquid chromatography clean-up.
If not, then one of two available liquid-liquid partition procedures
is used, according to whether the sample is an oily or non-oily crop.
For non-oily crops, the extract is washed with acetonitrile-saturated
petroleum ether and the acetonitrile phase then partitioned between
aqueous sodium chloride and petroleum ether-ethyl ether (1/1; v/v),
the organic phase filtered, dried, and evaporated to dryness. For
oily crops two methods can be used involving either (a) partition
between pet. ether and DMSO, followed by partition between DMSO,
aqueous sodium chloride, and ethyl acetate, or (b) partition between
hexane and acetonitrile, followed by back partition between
acetonitrile, aqueous sodium chloride, and hexane.
For liquid chromatography clean-up, two elution procedures may be
carried out; a dry-packed, 5 g Florisil (deactivated by 5% water)
column is eluted either with a) ethyl ether-petroleum ether (20/80;
v/v) or b) ethyl ether-hexane (10/90; v/v), depending on which
partition step was used.
Two gas chromatographic determination procedures are available,
depending on whether a 63Ni or 3H electron capture detector is
available. If a 63Ni detector is to be used then the eluate from the
clean-up step can be concentrated, adjusted to volume and aliquots
injected directly into a GC using a 1.5 m × 2 mm i.d. glass column
packed with either 2% DC-200, OV-1 or OV-101 on chromasorb AW-DMCS
(80-100 mesh). A column temperature of 245°C and detector temperature
of 300°C are used.
If only a 3H electron capture detector is available then the
concentrate from the clean-up step must be derivatised to
2,2-dimethyl-3-(2,2-dibromovinyl) cyclopropane carboxylic acid methyl
ester by means of transesterification. This procedure is carried out
by heating an aliquot of the clean-up concentrate in a mixture of
hexane, toluene, and 0.1 ml of 0.1 N KOH in methanol at 50°C for 15
minutes. After cooling the solution is neutralized with 0.1 ml
sulphuric acid (0.5% in methanol) and adjusted to volume (10 ml) with
hexane. The GLC analysis uses a 2 m glass column packed with 5% SE-30
on Gas-Chrom Q (100-120 mesh) at a temperature of 170°C. The detector
temperature is held at 200°C. Carrier gas flows of about 35 mg/min
are used in either procedure. If an internal standard in desired, the
use of o-nitroaniline has been found to be acceptable at this stage of
development, but improvements are being sought. Quantitation by peak
area rather than peak height is to be preferred for maximum precision
and reproducibility at the lowest residue levels (RU-78.11.07/A.;
RU-76.18.12/A; Mestres, 1978; Mestres et al., 1978).
For the analysis of deltamethrin residues in animal tissues (muscle,
kidney, and liver) a suitable gas chromatographic method is available
which is sensitive to about 1 µg/kg with a recovery factor of 90% at a
1O µg/kg level.
A 20 g tissue sample is homogenised in a blender with Celite and
petroleum ether-ethyl ether (50/50; v/v). Sodium sulphate is also
added for kidney or liver tissues. The solution is filtered through a
sintered glass Buchner funnel and evaporated to 3 ml under dry
nitrogen.
The extract is then partitioned between acetonitrile and petroleum
ether and the acetonitrile phase evaporated to dryness.
Clean-up is by gel permeation chromatography on a calibrated column (1
cm i.d., 120 cm long) of 30 ml of Styragel (porosity = 100 A, <37µ
particle diameter) swollen in isopropyl ether and eluted with
isopropyl ether at 2 ml/min. The elution volume of deltamethrin
standard (approx. 10 µg) is first determined by use of a U.V. detector
at 278 nm. The residue from the partition step is taken up in 250 µl
isopropyl ether and 200 µl are injected by a means of a sample loop.
Eluate corresponding to the elution volume of deltamethrin (approx. 10
ml) is collected and evaporated to dryness under nitrogen.
The residue is dissolved in 500 µl of a solution of internal standard
(0.25 µl/ml of an analogue of deltamethrin provided by the
manufacturer) in cyclohexane and analysed by G.C. using a 1.9 m × 4 mm
i.d. glass column packed with SE-30 on Gas Chrom P. and held at 245°C.
A 63Ni detector is required, which is operated at 300°C. Carrier gas
is argonmethane (90-10) at a flowrate of 40 mg/min (RU-80.01.26/A).
In all of these analytical methods it is only the parent deltamethrin
that is being determined although the derivatisation step in the
vegetable method might also pick up Br2CA if it were present in
sufficient quantity (unlikely).
NATIONAL TOLERANCES REPORTED TO THE MEETING
No national tolerances were reported to the Meeting at this time.
Formulations of deltamethrin are officially registered for use on one
or more crops in the following countries:
Europe
Bulgaria - Cyprus - Spain - France - Greece - Hungary - Holland
(ornamentals) - Ireland - Luxembourg - Poland - Portugal - German
Democratic Republic - Federal Republic of Germany - United Kingdom -
Switzerland - Czechoslovakia - Yugoslavia.
Africa
Algeria - Egypt - Mauritius - Mozambique - Morocco - Senegal - South
African Republic - Tunisia - Zimbabwe.
Americas
Argentina - Barbados - Brazil - British Guyana - Bolivia - Chile -
Colombia - Costa Rica - Dominican Republic - Ecuador - Guatemala -
Haiti - Honduras - Mexico - Nicaragua - Panama - Paraguay - Peru -
Suriname - Trinidad - Uruguay - Venezuela.
Asia and Oceania
Australia - Indonesia - India - Iran - Malaysia - New Zealand -
Pakistan - Philippines - South Korea - Syria - Taiwan Province of
China - Thailand - Turkey.
In the following countries deltamethrin is officially admitted since
no registration system exists.
West and Central African Countries
Benin - Cameroon - Chad - Central African Republic - Congo - Gabon -
Upper Volta - Mali - Niger - Nigeria - Togo - Zaire - Ivory Coast
(Registration system just underway - mid-1980).
Other African Countries
Malagasy Republic - Kenya - Ethiopia - Zambia.
Near East
Lebanon.
EVALUATION
COMMENT AND APPRAISAL
Deltamethrin is a synthetic pyrethroid produced as a single diastereo
isomer of 98% purity or better, which is active against a wide range
of insects that attack crops, animals and man. It is a powerful
contact and stomach insecticide that exhibits little or no systemic
activity, but has considerable stability when exposed to air and
light.
Deltamethrin is rapidly absorbed and metabolised by oxidation and
ester cleavage. Several metabolites have been identified. The
majority of these metabolites do not contain the intact pyrethrin
structure and on which there are no toxicological data.
Deltamethrin is rapidly and almost completely excreted, primarily in
urine and also in faeces. Only one metabolite, thiocyanate, has
accumulating properties caused by high tissue affinity in skin and
gastrointestinal tract. Deltamethrin is neurotoxic in several
mammalian species, and has a moderate acute oral toxicity in rats.
Plant workers exposed to deltamethrin experienced a pruritis followed
by a burning sensation and slight desquamation. No other
exposure-related symptoms are recorded. Quantitative information
about these effects is necessary to evaluate the sensitivity of man to
deltamethrin. It does not produce a delayed neurotoxic response in
hens following exposure to lethal or sublethal dosages. Neurological
changes, and modification of the EEG-pattern, were observed in a
90-day study with dogs. These animals also showed dilation of the
pupils, a dose-related increase of vomiting, body tremors and jerking
movements. Loose faeces, a reduced body weight gain, decreased
appetite and water consumption were associated features with all
groups of treated dogs.
Deltamethrin has some minor effects on mean body weight of rat pups
but did not effect reproduction in a three-generation study with rats.
Studies on teratogenic effects in mice, rats and rabbits revealed
possible embryotoxic effects in mice and rabbits, but no teratogenic
effects. The embryotoxic effects are decreased average foetal weight
and delayed ossification in mice and increased foetal losses in
rabbits.
The mutagenic properties of deltamethrin were studied with bacteria,
mammalian cell systems, the micronuclei test and dominant lethal test.
Deltamethrin showed no mutagenic activity in any of these tests. In a
90-day study with rats the effect on the body weight gain of the males
appeared to be the most sensitive criterion.
Two 2-year feeding studies with deltamethrin in mice and rats revealed
no clear treatment-related increase of tumour incidence. It was
concluded that the no-effect level in the mouse study was 100 mg/kg
feed. In the long-term study with rats a dose-dependent effect,
manifested as a slightly increased incidence of axonal degenerations
in sciatic, tibial and/or plantor nerves, was observed at 18 months in
the 20 and 50 mg/kg feed groups. Evaluation of the incidence and/or
severity of these degenerations at termination of the study was
obscured by the age of the animals. A suggested no-effect level was 2
mg/kg feed.
Owing to the absence of a no-effect level in dogs, and a possible
embryotoxic effect in mice at low dose levels, it was not possible to
allocate an ADI. This compound should be re-evaluated as soon as the
study on dogs, said to be in progress, is made available.
When applied to field crops, deltamethrin is active at only 9 g
a.i./ha with usual dosages of around 12.5 g a.i./ha. Because of its
stability and persistence, deltamethrin is very effective against
stored product pests at rates of 0.25 to 1.0 g a.i./ton depending on
local situations.
The residue data from supervised trials on field crops were evaluated
with regard to the commodity groups elaborated in Annex I, Alinorm
81/24, 1980. Because of the chemical nature of deltamethrin, the low
dosage rates normally applied, and the consequent low (generally)
residues even at short (2-4 days) intervals between last application
and harvest, it was considered appropriate to list maximum residue
levels suitable for MRL's on the basis of commodity groupings where
sufficient data existed on an adequate number of commodities in the
group and/or where the commodities in the group are known to have
uniform residue retention properties.
Residues on fruits and vegetables are found mainly on the peel, hull,
or outer leaves. Degradation in field crops occurs mainly by
hydrolysis and photolysis, followed by enzymatic oxidations leading to
a wide variety of products and conjugates, among which are
transdeltamethrin, trans-hydrooxymethyl deltamethrin, 4'-hydroxy
deltamethrin, 4'-hydroxy-transhydroxymethyl deltamethrin,
3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane carboxylic acid (Br2CA)
and its 3 conjugates, 3-phenoxybenzaldehyde and corresponding alcohol
and acid and their conjugates, and alpha-cyanophenoxylbenzyl alcohol.
Metabolism in animals is analogous to that in plants, differing mainly
in the nature of the conjugates. Rapid excretion of deltamethrin and
its metabolites occurs in animals with very little tendency to
bioaccumulate. Since deltamethrin comprises the major residue
component, it is the only entity sought in residue analysis.
When 0.27 g of C14-deltamethrin (labelled in the alpha-methine group)
was injected intrarumenally into a dairy cow, radioactivity was
rapidly excreted in urine and faeces (85.3%). Peak residues in whole
milk and butter were 0.045 and 0.92 mg/kg respectively with a
half-life of 0.8 days. Fat and muscle samples contained 0.088 and
0.008 mg/kg deltamethrin equivalents at two days after treatment. A
miscible oil formulation containing 0.21 g of labelled deltamethrin
was applied externally (except udder) to the same cow 49 days later.
Peak residues in whole milk and butter were 0.0057 and 0.1 mg/kg
respectively by two days after treatment with half-lives of four to
four and one half days. On the basis of these ratios it can be
calculated that residues arising from the consumption of forage or
fodder having residues up to 2 mg/kg would not be likely to exceed
0.01 mg/kg in whole milk or 0.02 mg/kg in butter or fat of meat.
Cereal grains treated with dust, EC, or low volume formulation
yielding initial deposits of up to 1 mg/kg deltamethrin exhibited
little or no losses after storage of up to 50 weeks. The majority of
the residue in wheat was found in the bran (2 to 10 times that in
whole grain) while white flour contained approximately one fourth of
the residue in whole grain. Baking of flour into bread had no
significant effect on residue levels leading to possible residues of
up to 0.3 mg/kg in baked goods from recommended storage treatment
dosages. Stored coffee beans treated at 1 or 2 mg/kg (dust, EC, and
ULV treatments) had negligible loss of residue after 8 months of
storage. Roasting of coffee beans fortified at 0.05 mg/kg resulted in
a residue of 0.012 mg/kg. At this level of residue in roasted beans
it can be calculated that less than 0.00003 mg/kg would be found in
brewed coffee water on the basis of the results of experiments with
tea in which the residues in brewed tea water did not exceed 1/500th
of those on tea leaves.
A sensitive general purpose method for the analysis of deltamethrin
residues in plant tissues has been developed. The method is based on
extraction, liquid-liquid partition (if needed), liquid chromatography
clean-up on a Florisil column and gas chromatographic quantitation
using electron capture detection. The use of a 63Ni electron capture
detector at a working temperature of 300°C can measure deltamethrin
directly and obviates the need for derivitisation. If only a tritium
electron capture detector is available then column temperature
considerations require the use of a transesterification reaction to
produce 2,2-dimethyl-3-(2,2-dibromovinyl) cyclopropane carboxylic acid
methyl ester. The use of an internal standard is to be preferred
(either o-nitroaniline or a deltamethrin analogue) and quantitation
should be by peak area rather than peak height for maximum precision
and accuracy. Recoveries range from 80 to 95% with a sensitivity
(overall) of 1-10 µg/kg.
For the analysis of deltamethrin residues in animal tissues a suitable
method is available that is sensitive to about 1 µg/kg, with a
recovery factor of 90% at the 10 µg/kg level. The method is similar
to that for plant tissue, differing mainly in the choice of solvents
in the extraction and partition stages and in the use of gel
permeation chromatography for clean-up. A 63Ni detector operating at
300°C is required.
The meeting examined residue data from supervised trials reflecting
established and proposed good agricultural practice on a number of
crops and commodities. From these data the meeting was able to
estimate the maximum residue levels that were likely to occur when
deltamethrin was used in practice and when reported intervals between
last application and harvest were observed.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concluded that the residue levels listed below need not be
exceeded if recommended use patterns are adhered to. In the absence
of an ADI the meeting recommended that these are suitable as
guidelines only. These levels refer to parent compound only.
Interval between last
application and harvest,
Guideline residue on which guideline levels
Commodity levels, mg/kg are based, days1
Tea 10 none
Coffee beans 2 -
Legume animal feeds 0.5 (dry weight basis) 21
Wheat bran (unprocessed) 2 -
Cereal grains 1 -
Wheat flour (white) 0.5 -
Leafy vegetables 0.2 7
Bulb vegetables 0.1 7
Legume vegetables 0.1 7
Pome fruits 0.1 21
Assorted fruits-edible peel 0.1 14
Oilseeds 0.1 14
Brassica leafy vegetables 0.05 7
Artichokes 0.05 7
Fruiting vegetables-edible peel 0.05 3
Oranges, clementines 0.05 35
Stone fruits 0.05 14
Grapes, strawberries 0.05 7
Bananas 0.05 77
Cocoa beans 0.05 35
Root and tuber vegetables 0.01 7
Melons 0.01 7
Pineapples 0.01 31
Legume oilseeds 0.01 70
Mushrooms 0.01 1
1 No numerical entry in given where the levels are based on post-harvest
treatments.
FURTHER WORK OR INFORMATION
Required (by 1982 and before an acceptable daily intake can be
allocated)
1. Results of the two-year feeding study in dogs currently in
progress.
2. Clarification of possible embryotoxic effects in animals.
3. Further observations on reported effects in man.
4. Studies into the significance of neurological effects observed in
several animal species.
5. Results of supervised trials on residues in meat, milk, and eggs
arising from the use of deltamethrin for ectoparasite control, in
feeding studies and in stable treatments.
Desirable
1. Additional residue data from supervised trials on citrus,
especially grapefruit, lemons, tangerines, etc., if uses on these
commodities are developed.
2. Data on residues in food in commerce or at consumption.
3. Information on national tolerances.
REFERENCES
Anonymous. Test to determine the toxicity in the chicken by oral
route. (1976a) Unpublished report RU-76.05.05/A, submitted by Roussel
Uclaf to WHO.
Anonymous. Toxicity of deltamethrin or DECIS in single ingestion in
game duck, Anas platyrhynchos L. (1976b) Unpublished report
INRA-76.21.12/A, Institut National de la Recherche Agronomique,
Jony-en-Josas, France, submitted by Roussel Uclaf to WHO.
Anonymous. Toxicity of deltamethrin or DECIS by single ingestion in
grey partridge, Perdix Perdix L. and red partridge, Alectous
rufa L. (1976c) Unpublished report INRA-76.28.09/A, Institut
National de la Recherche Agronomique, Jony-en-Josas, France, submitted
by Roussel Uclaf to WHO.
Anonymous. DECIS. Photostability to air and light. (1977b)
Unpublished report RU-22.08.77/A, submitted by Roussel Uclaf to WHO.
Anonymous. Deltamethrin. (1978a) Unpublished report RI-78.09.11/A,
submitted by Roussel Uclaf to WHO.
Anonymous. Deltamethrin/stability. (1978b) Unpublished report
RU-78.23.11/A, submitted by Roussel Uclaf to WHO.
Beavers, J.B. and Fink, R. Acute oral LD50 - Mallard duck Technical
DECIS. Final report. (1977a) Unpublished report WI-77.06.06/A,
Wildlife International, USA, submitted by Roussel Uclaf to WHO.
Beavers, J.B. and Fink, R. Eight-day dietary LD50 - Mallard duck.
Technical DECIS. Final report. (1977b) Unpublished report
VO-77.18.05/A. Wildlife International, USA, submitted by Roussel Uclaf
to WHO.
Beavers, J.B. and Fink, R. Eight-day dietary LD50 - Bobwhite quail.
Technical DECIS. Final report. (1977c) Unpublished report
WI-77.19.05/A, Wildlife International, USA, submitted by Roussel Uclaf
to WHO.
Chesterman, H., Heywood, R., Perkin, C.J., Beard, D., Street, E. and
Prentice, D.E. RU 22974. Oral toxicity study in Beagle dogs. (1977)
Unpublished report RSL 253/7751/A3, Huntingdon Research Centre,
Huntingdon, England, submitted by Roussel Uclaf to WHO.
Clair, M. RU 22974 - DECIS. Acute toxicity in the rabbit by
percutaneous administration. Unpublished report IFREB-R 770257.1/A,
Institut Français de Recherches et Essais Biologiques,
Joinville-le-Pont, France, submitted by Roussel Uclaf to WHO.
Coombs, D.W. and Clark, G.C. RU 22974. Acute inhalation toxicity in
rats. 6 Hour LC50. (1978) Unpublished report RSL 310/78453/A,
Huntingdon Research Centre, Huntingdon, England, submitted by Roussel
Uclaf to WHO.
Coquet B. RU 22974. Test to determine primary cutaneous irritation in
the rabbit. (1976a) Unpublished report no. IFREB-R 761157/A, Institut
Français de Recherches et Essais Biologiques, Joinville-le-Pont,
France, submitted by Roussel Uclaf to WHO.
Coquet B. RU 22974. Test to evaluate ocular irritation in the rabbit.
(1976b) Unpublished report no. IFREB 761158/A, Institut Français de
Recherches et Essais Biologiques, Joinville-le-Pont, France, submitted
by Roussel Uclaf to WHO.
Elliot, M. et al. Synthetic insecticide with a new order of activity.
Nature 248, 710-11.
Fouillet, X. RU 22974. Mutagenicity study of various preparations.
Salmonella-microsome test. (1976) Unpublished report no. IFREB-R
761153/A, Institut Français de Recherches et Essais Biologiques,
Joinville-le-Pont, France, submitted by Roussel Uclaf to WHO.
FP-78.27.04/A. Residues determination in plants. Stored wheat. (1978)
Research Report. Université de Montpellier.
FP-78.07.12/A. Residues determination in plants. Wheat (stored).
(1978) Research Report. Université de Montpellier.
FP-80.05.02/A. Residues determination in plants. Wheat (stored).
(1980) Research Report. Université de Montpellier.
FP-78.30.09/A. Residues determination in plants. Corn-stored grains.
(1978) Research Report. Université de Montpellier.
FP-79.16.07/A. Residues determination in plants. Maize (stored).
(1979) Research Report. Université de Montpellier.
FP-79.18.07/A. Residues determination in plants. Maize (stored).
(1979) Research Report. Université de Montpellier.
FP-78.30.05/A. Residues determination in plants. Corn (stored). (1978)
Research Report. Université de Montpellier.
FP-78.02.10/A. Residues determination in plants. Corn (stored). (1978)
Research Report. Université de Montpellier.
FP-79.07.06/A. Residues determination in plants. Peanut (stored).
(1979) Research Report. Université de Montpellier.
FP-78.29.09/A Residues determination in plants. Coffee. (1978)
Research Report. Université de Montpellier.
FP-79.18.10/A. Residues determination in plants. Coffee (stored).
(1979) Research Report. Université de Montpellier.
FP-79.19.10/A. Residues determination in plants. Coffee (stored).
(1979) Research Report. Université de Montpellier.
Glomot R. and Chevalier, B. RU 22974. Acute toxicity study mouse and
rat by oral route. (1976a) Unpublished report Tox 76810/A, submitted
by Roussel Uclaf to WHO.
Glomot, R. and Chevalier, B. RU 22974. Acute toxicity study mouse and
rat by intraperitoneal route. (1976b) Unpublished report Tox 76811/A,
submitted by Roussel Uclaf to WHO.
Glomot, R. and Chevalier, B. RU 22974. Acute toxicity study mouse and
rat by intravenous route. (1976c) Unpublished report Tox 76812/A,
submitted by Roussel Uclaf to WHO.
Glomot, R., Chevalier, B., Collas, E. and Audegond, L. RU 22974.
Acute toxicity study by oral route in male and female beagle dogs.
(1977) Unpublished report Toxico 77804/JL-5, submitted by Roussel
Uclaf to WHO.
Glomot, R. and Vannier, B. RU 22974. Teratological study in mouse,
rat and rabbit. (1977) Unpublished report no. Tox. 76534-76536/2/A,
submitted by Roussel Uclaf to WHO.
Glomot, R. and Vannier, B. RU 22974. Teratological study in rabbit.
Complementary information. (1978) Unpublished report no. Tox.
76534-76536/A3, submitted by Roussel Uclaf to WHO.
Goldenthal, E.I., Blair, M., Jefferson, N.D., Spicer, E.J.F., Arceo,
R.J. and Clark Kahn III, A. RU 22974. Two year toxicity and
carcinogenicity study in mice. (1980a) Unpublished report IRDC
406-001 A/4, International Research and Development Corporation,
Mattawan (Mich.) USA, submitted by Roussel Uclaf to WHO.
Goldenthal, E.I., Jefferson, N.D., Blair, M., Thorstenson, J.H.,
Spicer, E.J.F., Arceo, R.J. and Kahn III, A. CRU 22974. Two year oral
toxicity and carcinogenicity study in rats. (1980b) Unpublished report
IRDC 406-002/A4, International Research and Development Corporation,
Mattawan (Mich.), USA, submitted by Roussel Uclaf to WHO.
Guillot, J.P. and Guilaine, J. RU 22974-Decamethrine. Decis technical
Roussel Uclaf. Sensitization test in the guinea pig. (1977)
Unpublished report no. IFREB-R 709241/A, Institut Français de
Recherches et Essais Biologiques, Joinville-le-Pont, France, submitted
by Roussel Uclaf to WHO.
Herve, J.J., Smolikowski, S., Pastre, P., Piedallu, C. and Roa, L.
NRDC 161 (RU 22974): A New Pyrethroid Insecticide for Use in
Agricultural Crops. Proceedings 1977 British Crop Protection
Conference - Pests and Diseases.
Husson, J.M. Medical observations made on people working on the
manufacture and formulation of the pyrethroid insecticide decamethrin.
(1978) Unpublished report no. RU 78.25.08/A, submitted by Roussel
Uclaf to WHO.
Hunter, B., Jordan, J., Heywood, R., Hepworth, P., Street, A.E. and
Prentice, D.E. RU 22974. Assessment of toxicity to rats by oral
administration for 13 weeks, (followed by a 4-week withdrawal period).
(1977) Unpublished report by Roussel Uclaf to WHO.
Kynoch, S.R., Lloyd, G.K. and Andrews, C.D. Acute percutaneous
toxicity to rats of decamethrin. (1979) Unpublished report RSL-10098/D
147/79/A, Huntingdon Research Centre, Huntingdon, England, submitted
by Roussel Uclaf to WHO.
Mestres, R. Les Residus de Decamethrine dans lea Vegetaux
Consommables. Phytiatrie - Phytopharmboie 27, 81-84.
Mestres, R., Chevallier, C., Espinoza, C. and Cornet, R. Dosage des
Residus de Decamethrine dans les Produits Vegetaux. Travaux de la
Societe de Pharmacie de Montpellier 38, (2) 183-92.
Peyre, M., Chantot, J.F., Glomot, R. and Penasse, L. Detection of a
mutagenic potency of decamethrin (RU 22974). Bacterial tests. (1980)
Unpublished report no. RU/TOX/80.21.01/A, submitted by Roussel Uclaf
to WHO.
PRO 77.13.09/A. Preliminary Uptake-Trans-location Studies of RU 22974
on Cotton. (1977) Research Report. Procida, Groupe Roussel Uclaf.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E., Cooke, L.
and Gibson, W.A. RU 22974, (Decamethrine) LD50 determination and
assessment of neurotoxicity in the domestic hen. (1978) Unpublished
report no. RSL 293-NT/7830/A, Huntingdon Research Centre, Huntingdon,
England submitted by Roussel Uclaf to WHO.
Roussel Uclaf. Information on the general chemistry of the active
ingredient, the technical material, and some formulations. (1980a).
Roussel Uclaf. List of main world crops on which decamethrin is
applied at indicated rates. (1980b).
Roussel Uclaf. Information on residues of decamethrin resulting from
supervised trials. Assembled Research Reports. (1980c).
Roussel Uclaf. List of countries where DECIS is officially registered
for agricultural uses plus countries where DECIS is officially
admitted since no registration system exists. (1980d).
RU-78.II.07/A. Quantitative determination of Decamethrin residues in
plant tissues. (1978) Research Report. Procida, Groupe Roussel Uclaf.
RU-76.18.12/A. Gas Chromatographic Determination of DECIS. Development
of a Method for the Estimation of DECIS. Residues in Plants. (1976)
Research Report. Roussel Uclaf.
RU-80.01.26/A. Determination of Decamethrin Residues in Animal
Tissues. (1980) Research Report. Roussel Uclaf.
Ruzo L.O., Unai, T. and Casida, J.E. Decamethrin metabolism in rats.
(1977) Unpublished report UC-77.06.12/A, submitted by Roussel Uclaf to
WHO.
Ruzo, L.O., Unai, T. and Casida, J.E. Decamethrin metabolism in rats.
J. Agric. Food Chem. 26: 918.
Ruzo, L.O., Engel, J.L., and Casida, J.E. Oxidative, hydrolytic and
conjugative reactions in the metabolism of decamethrin in mice.
(1978b) Unpublished report UC.78.10.11/A, submitted by Roussel Uclaf
to WHO.
Ruzo, L.O. and Casida, J.E. Degradation of Decamethrin on Cotton
Plants. J. Agric. Food Chem. 27, (3) 572-75.
Ruzo, Luis O., Holmstead, R.L. and Casida, J.E. Pyrethroid
Photochemistry: Decamethrin. J. Agric. Food Chem. 25, (6) 1385-94.
Shono T., Ohsawa, K. and Casida, J.E. Metabolism of trans- and
cis-permethrin, trans- and cis-cypermethrin and decamethrin by
microsomal enzymes. Unpublished report UC 78.18.05/A, submitted by
Roussel Uclaf to WHO.
Sobels, F.H., Tates, A.D. and Vannier, B. Cytogenetic study with RU
22974. Detection of a mutagenic potency in mammalian cells. (1978)
Unpublished report no. ULN-782211/A, State University of Leiden, The
Netherlands, submitted by Roussel Uclaf to WHO.
Vannier, B. and Glomot, R. RU 22974. Mutagenic study. Dominant lethal
assay in the male mouse. (1977) Unpublished report no. Toxico
76533/DB9/A, submitted by Roussel Uclaf to WHO.
WELL-79.04HIBH/A. Residues in a cow's milk resulting from
intrarumenal, and later, dermal administration of carbon-14 labelled
decamethrin. Group Research and Development, The Wellcome Foundation,
Ltd. Research Report. (1979).
WELL/80.21.05/AL. The Fate of Decamethrin Residues on Wheat during
Storage and after Milling and Baking. An Interim Report after 3 Months
Storage. Group Research and Development, The Wellcome Foundation. Ltd.
(1980).
Wrenn, J.M., Rodwell, D.E., Goldenthal, E.I., Spider, E.J.C. and
Rajasekaran, D. Three generation reproduction study in rats. (1980)
Unpublished report no. IRDC-406.003/A4, International Research and
Development Corporation, Michigan, USA, submitted by Roussel Uclaf to
WHO.
