
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
DELTAMETHRIN
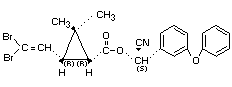 Explanation
In 1980 the JMPR reviewed deltamethrin (FAO/WHO 1981)1/, and
determined that an ADI could not be estimated without further
information. Additional data were received and reviewed by the JMPR in
1981 (2-year feeding study in dogs; 2-year mouse oncogenicity study;
mouse and rat teratology studies; additional mutagenicity evaluations
and information on humans). No ADI was estimated. Since then,
additional data has been submitted to FAO/WHO and are reviewed in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
Studies designed to investigate the potential carcinogenic
effects of deltamethrin have been initiated by IARC.
Groups of BDVI rats were administered orally 0-6 mg/kg bw
deltamethrin in arachis oil. Similarly, groups of C57 BL/6 mice
received orally 0-8 mg/kg bw of deltamethrin in arachis oil. Results
were not yet available to the Meeting.
1/ See Annex 2 for WHO and FAO documentation.
Special Studies on Eye Irritation
Male albino rats (9) weighing between 2 and 3 kg were
administered 0.1 ml of formulated deltamethrin (25 g/l mixofluid) into
the conjunctival sac. Six of the treated eyes remained unwashed, while
the remaining three were rinsed with lukewarm water 20-30 seconds
after instillation. There was only transient clouding of the cornea in
two animals one hour after dosing (1 washed, 1 unwashed), which
cleared by day 2. Also noted was a low grade conjunctival irritation
among all animals initially, which disappeared following day 2 of
observations (Glomot et al 1981d).
The 2.5% WP deltamethrin formulation diluted 1/10 in distilled
water (0.1 ml per rabbit) elicited a similar pattern of initial
transient corneal clouding in three of nine rabbits examined, which
cleared by day 4. The undiluted formulation, 100 mg, administered into
the conjunctival sac of rabbits produced increased involvement of
conjunctiva, iris and cornea in all animals, generally moderate in
severity, with low grade corneal opacity persisting in two rabbits
through day 7 (1 washed, 1 unwashed) (Glomot et al 1981e).
Special Study on Teratogenicity
Mouse
Groups of mated Swiss CD1, SPF mice (25/group) were orally
gavaged with deltamethrin dissolved in sesame oil at dose levels of 0,
0.1, 1 and 10 mg/kg bw/day administered during gestation days 6 to 17.
This was a complementary teratology study to the Glomot and Vannier
1977 evaluation and was experimentally designed to assess the effects
on foetal and post-natal development, including embryotoxicity. One
group of mated mice was sacrificed on day 18 of gestation and examined
for gravid uterine weight, number of implantation sites, viable
foetuses, dead foetuses and resorptions. Visceral and skeletal
examinations were performed on the foetuses. Some females were allowed
to produce litter, and full examinations of pups and dams were
conducted on day 1 post-partum. Similarly, a third group of females
was allowed to produce a litter and dams and pups were sacrificed on
day 21 post-partum. Pup weights were determined on days 1, 4, 10 and
21 post-partum.
Results demonstrate that the conception and pregnancy rates were
unaffected by treatment, except that maternal body weight gain was
decreased in the 10 mg/kg bw group. Skeletal variants, consisting of
delayed ossification of the sternebrae and phalanges in the 1 and
10 mg/kg bw/day group pups at gestation day 18, were not present at
day 1 or 21 post-partum. Extranumery ribs and reduced ossification of
skull bones were not observed in this complementary study. Litter
size, pup weight, viability and lactation index were all normal
through day 21 post-partum. Deltamethrin administered during the
period of major organogenesis in the mouse causes a moderate and
transient retardation of development of the foetus at 1 mg/kg bw/day
and higher doses, but these effects were not observed at day 1 or 21
post-partum. There were no teratogenic effects related to treatment
(Glomot and Vannier 1982).
Special Studies on Skin Sensitization
Male albino rabbits (6) weighing 2.5 to 2.9 kg were administered
0.5 ml of formulated deltamethrin (25 g/l mixofluid) to both shaved
intact and abraded skin. The Primary Irritation Index after 24 h
exposure to occluded sites was 1.2, slightly irritating (Glomot
et al 1981b).
An evaluation similar to the one described above was determined
for a 2.5% WP deltamethrin formulation. Rabbits had a Primary
Irritation Index of 2.41, moderately irritating. Moderate erythema
continued for 72 hours, while the oedema generally diminished, with
the exception of scarified skin sites (Glomot et al 1981c).
Acute Toxicity
Mouse
Mice presented far fewer symptoms than rats after oral dosing
at comparable levels, with diarrhoea being the only reportable
observation (Glomot et al 1980a).
Rat
The acute oral toxicity of low concentrations of deltamethrin to
rats was without noticeable mortality but included symptoms observed
at higher concentrations, including excessive grooming, diarrhoea,
drowsiness, piloerection, ptosis, difficulty in walking, general motor
incoordination and hypotonia (Glomot 1979; Glomot et al 1979,
1981a).
Rats were exposed (whole-body exposure) for 4 hours to an aerosol
concentration of deltamethrin equal to 2.8 g/m3, the highest
attainable airborne concentration of a 2-5% formulation. Approximately
80% of the total aerosol had a mean aerodynamic diameter less than
5.5 Ám. Dyspnoea and gasping were observed in exposed rats. Relative
lung weights and macroscopic pathology were normal. There were no
mortalities (Clark et al 1980).
Dog
Dogs orally dosed with a 2.5% formulation of deltamethrin
displayed no clinical symptoms related to treatment (Glomot
et al 1980b).
Table 1 Acute Toxicity of Deltamethrin in Animals
LD50
Species Route Sex (mg/kg bw) Reference
Rat Oral1/ M/F >5 000 Glomot et al
1981a
Rat Oral2/ M 22 700 Glomot et al 1979
(19 100-23 800)
Rat Oral2/ F 22 000 "
(18 800-22 700)
Rat Oral3/ M/F >15 000 Glomot 1979
Rat Inhalation3/ M/F >2.8 g/m3 Clark et al 1980
Mouse Oral3/ M/F >15 000 Glomot et al
1980a
Dog Oral3/ M/F >10 000 Glomot et al
1980b
1/ in an aqueous mixture of sodium carboxymethyl cellulose (0.25%)
and polysorbate 80 (0.20%);
2/ obtained for a 25 g/l flowable formulation;
3/ obtained for a 2.5% wettable powder formulation.
Observations in Humans
Persons exposed to deltamethrin for 7-8 years of its production,
syntheses and reformulation were subjected to medical observations,
including both clinical and haematological examinations. Evaluations
were conducted at several plants. There were no measurable effects
other than transient cutaneous and mucous membrane irritation which
was without sequelae. Adequate precautionary measures, such as gloves
and face masks, provide protection from exposure (Foulhouz 1981).
A medical survey of agricultural workers involved in the use and
application of EC and WP formulations of deltamethrin in Yugoslavia
revealed no untoward symptoms of exposure other than itching and
burning of the face, as well as nasal hypersecretion. Medical
examinations included chest x-ray, ECG, liver function tests,
neurological exam (eye tonometry, Goldman perimetry, dark adaptation
ability), kidney function tests, and whole blood and plasma
cholinesterase activity. No adverse effects were noted. Proper use of
masks and gloves, as well as good personal hygiene (e.g. washing),
were emphasized (Plestina 1981).
Four operators were evaluated during normal field applications
of deltamethrin lasting one day. Three of the operators wore no
protection on their heads or hands, while one wore hood, gloves and
respirator. Motor and sensory nerve conduction velocities were
determined as well as haematological and biochemical tests and
urinalysis. No changes were observed in blood parameters measured and
no residues were found in the urine samples. Furthermore, no decrease
in nerve conduction velocity occurred. Residues were primarily
confined to gloves and legs. None of the operators experienced
symptoms of facial sensation (Hewson and Burgess 1981).
COMMENTS
The additional information reviewed clarified previous concerns.
Embryotoxic effects observed are transient in nature, demonstrated
only in the foetuses, and without sequelae in newborn animals up
through 21 days post-partum.
Data accumulated on workers exposed to deltamethrin (e.g. plant
workers, field operators and supported by data in laboratory animals
demonstrate that deltamethrin is irritating to skin and mucous
membranes. This potential can be reduced by proper precautionary
measures such as gloves, face shield and other appropriate clothing.
Information would indicate that some of this irritation may be
partially caused or enhanced by the solvent (i.e. xylene) in the
EC formulation.
Evaluation of the histopathology in the 2-year rat feeding study
by independent pathologists confirmed that there were no dose-related
effects. The axonal degenerations at 20 ppm were not significantly
different from high dose or control animals. Historical data from the
laboratory performing the study re-affirmed that the reported
observation of testicular cell adenoma at 50 ppm were age related and
within the limits of 1 000 control animals of the same strain.
The Meeting noted with interest the neurological effects observed
in both acute and short-term studies, which were not observed in
longer-term studies. The high doses of the long-term studies in rat
and dog were equivalent to the lower doses utilized in the short-term
studies, which provides a possible reason for the absence of such
response.
The Meeting was made aware of ongoing long-term carcinogenicity
studies in the rat and mouse from which results are not yet available
and hoped that these would be made available to the JMPR when they are
completed. Additional data and information made available to the
Meeting provided sufficient data to recommend an ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 50 ppm in the diet, equivalent to 2.1 mg/kg bw.
Mouse: 100 ppm in the diet, equivalent to 12 mg/kg bw.
Dog: 40 ppm in the diet, equivalent to 1 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
1. Studies on the significance of neurological effects observed in
several animal species.
2. Results from the IARC carcinogenicity studies in mouse and rat.
3. Further information on exposure of humans to deltamethrin.
REFERENCES
Clark, G.C., Jackson, G.C. and Alexander, D.J. Acute inhalation
1980 toxicity in rats, 4-hour exposure (Decis PM 2.5 percent).
Report (ref.: RSL 437/80568) from Huntingdon Research
Centre, U.K., submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
Foulhouz, P. Medical observations of personnel working on synthesis
1981 or formulation of deltamethrin. Report (ref.: RU-81.18.06/A)
from Roussel Uclaf submitted to the World Health
Organization by Roussel Uclaf. (Unpublished)
Glomot, R. Acute toxicity study by oral route in the rat, Report
(ref.: RU-79 803-54/A2) 1979 from Roussel Uclaf, submitted
to the World Health Organization by Roussel Uclaf.
(Unpublished)
Glomot, R., Audegond, L. and Collas, E. Acute oral toxicity study
1979 in the rat (Decis 25 G/L Mixofluid). Report (ref.: RU
79824/A) from Roussel Uclaf submitted to the World Health
Organization by Roussel Uclaf. (Unpublished)
1980a Single administration by oral route in the mouse (Decis
Wettable Powder, 2.5%). Report (ref:RU-80.817/A) from
Roussel Uclaf submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1980b Single administration study by oral route in the dog (Decis
Wettable powder, 2.5%). Report (ref.: RU 80.194/A) from
Roussel Uclaf submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1981a Single administration study by oral route in the rat. Report
(ref.: RU-81239/A) from Roussel Uclaf, submitted to the
World Health Organization by Roussel Uclaf. (Unpublished)
1981b Primary dermal irritation study in the rabbit. Deltamethrin
25 G/L Mixofluid). Report (ref.: RU-79193/A) from Roussel
Uclaf, submitted to the World Health Organization by Roussel
Uclaf. (Unpublished)
1981c Primary dermal irritation study in the rabbit (Deltamethrin
wettable powder, 2.5%). Report (ref.: RU80200/A) from
Roussel Uclaf, submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1981d Primary eye irritation study in the rabbit (Deltamethrin in
25 G/L Mixofluid). Report (ref.: RU79192/A) from Roussel
Uclaf, submitted to the World Health Organization by Roussel
Uclaf. (Unpublished)
1981e Primary eye irritation study in the rabbit (Decis Wettable
Powder, 2.5%). Report (ref. RU79192/A) from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
Glomot, R. and Vannier, B. Complementary teratological study in the
1982 mouse. Report (ref.: RU-82.506-12/A), from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
Hewson, R.T. and Burgess, J.E. An operator study with deltamethrin
1981 including measurements of nerve conduction times. Report
(ref.: GB-NT-11.81/A) from Roussel Uclaf, submitted to the
World Health Organization by Roussel Uclaf. (Unpublished)
Plestina, R. An evaluation of the use of deltamethrin in public
1981 health. Report (ref.: ZAG. KOT. 81/A) from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
DELTAMETHRIN
Explanation
In 1980 the JMPR reviewed deltamethrin (FAO/WHO 1981)1/, and
determined that an ADI could not be estimated without further
information. Additional data were received and reviewed by the JMPR in
1981 (2-year feeding study in dogs; 2-year mouse oncogenicity study;
mouse and rat teratology studies; additional mutagenicity evaluations
and information on humans). No ADI was estimated. Since then,
additional data has been submitted to FAO/WHO and are reviewed in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
Studies designed to investigate the potential carcinogenic
effects of deltamethrin have been initiated by IARC.
Groups of BDVI rats were administered orally 0-6 mg/kg bw
deltamethrin in arachis oil. Similarly, groups of C57 BL/6 mice
received orally 0-8 mg/kg bw of deltamethrin in arachis oil. Results
were not yet available to the Meeting.
1/ See Annex 2 for WHO and FAO documentation.
Special Studies on Eye Irritation
Male albino rats (9) weighing between 2 and 3 kg were
administered 0.1 ml of formulated deltamethrin (25 g/l mixofluid) into
the conjunctival sac. Six of the treated eyes remained unwashed, while
the remaining three were rinsed with lukewarm water 20-30 seconds
after instillation. There was only transient clouding of the cornea in
two animals one hour after dosing (1 washed, 1 unwashed), which
cleared by day 2. Also noted was a low grade conjunctival irritation
among all animals initially, which disappeared following day 2 of
observations (Glomot et al 1981d).
The 2.5% WP deltamethrin formulation diluted 1/10 in distilled
water (0.1 ml per rabbit) elicited a similar pattern of initial
transient corneal clouding in three of nine rabbits examined, which
cleared by day 4. The undiluted formulation, 100 mg, administered into
the conjunctival sac of rabbits produced increased involvement of
conjunctiva, iris and cornea in all animals, generally moderate in
severity, with low grade corneal opacity persisting in two rabbits
through day 7 (1 washed, 1 unwashed) (Glomot et al 1981e).
Special Study on Teratogenicity
Mouse
Groups of mated Swiss CD1, SPF mice (25/group) were orally
gavaged with deltamethrin dissolved in sesame oil at dose levels of 0,
0.1, 1 and 10 mg/kg bw/day administered during gestation days 6 to 17.
This was a complementary teratology study to the Glomot and Vannier
1977 evaluation and was experimentally designed to assess the effects
on foetal and post-natal development, including embryotoxicity. One
group of mated mice was sacrificed on day 18 of gestation and examined
for gravid uterine weight, number of implantation sites, viable
foetuses, dead foetuses and resorptions. Visceral and skeletal
examinations were performed on the foetuses. Some females were allowed
to produce litter, and full examinations of pups and dams were
conducted on day 1 post-partum. Similarly, a third group of females
was allowed to produce a litter and dams and pups were sacrificed on
day 21 post-partum. Pup weights were determined on days 1, 4, 10 and
21 post-partum.
Results demonstrate that the conception and pregnancy rates were
unaffected by treatment, except that maternal body weight gain was
decreased in the 10 mg/kg bw group. Skeletal variants, consisting of
delayed ossification of the sternebrae and phalanges in the 1 and
10 mg/kg bw/day group pups at gestation day 18, were not present at
day 1 or 21 post-partum. Extranumery ribs and reduced ossification of
skull bones were not observed in this complementary study. Litter
size, pup weight, viability and lactation index were all normal
through day 21 post-partum. Deltamethrin administered during the
period of major organogenesis in the mouse causes a moderate and
transient retardation of development of the foetus at 1 mg/kg bw/day
and higher doses, but these effects were not observed at day 1 or 21
post-partum. There were no teratogenic effects related to treatment
(Glomot and Vannier 1982).
Special Studies on Skin Sensitization
Male albino rabbits (6) weighing 2.5 to 2.9 kg were administered
0.5 ml of formulated deltamethrin (25 g/l mixofluid) to both shaved
intact and abraded skin. The Primary Irritation Index after 24 h
exposure to occluded sites was 1.2, slightly irritating (Glomot
et al 1981b).
An evaluation similar to the one described above was determined
for a 2.5% WP deltamethrin formulation. Rabbits had a Primary
Irritation Index of 2.41, moderately irritating. Moderate erythema
continued for 72 hours, while the oedema generally diminished, with
the exception of scarified skin sites (Glomot et al 1981c).
Acute Toxicity
Mouse
Mice presented far fewer symptoms than rats after oral dosing
at comparable levels, with diarrhoea being the only reportable
observation (Glomot et al 1980a).
Rat
The acute oral toxicity of low concentrations of deltamethrin to
rats was without noticeable mortality but included symptoms observed
at higher concentrations, including excessive grooming, diarrhoea,
drowsiness, piloerection, ptosis, difficulty in walking, general motor
incoordination and hypotonia (Glomot 1979; Glomot et al 1979,
1981a).
Rats were exposed (whole-body exposure) for 4 hours to an aerosol
concentration of deltamethrin equal to 2.8 g/m3, the highest
attainable airborne concentration of a 2-5% formulation. Approximately
80% of the total aerosol had a mean aerodynamic diameter less than
5.5 Ám. Dyspnoea and gasping were observed in exposed rats. Relative
lung weights and macroscopic pathology were normal. There were no
mortalities (Clark et al 1980).
Dog
Dogs orally dosed with a 2.5% formulation of deltamethrin
displayed no clinical symptoms related to treatment (Glomot
et al 1980b).
Table 1 Acute Toxicity of Deltamethrin in Animals
LD50
Species Route Sex (mg/kg bw) Reference
Rat Oral1/ M/F >5 000 Glomot et al
1981a
Rat Oral2/ M 22 700 Glomot et al 1979
(19 100-23 800)
Rat Oral2/ F 22 000 "
(18 800-22 700)
Rat Oral3/ M/F >15 000 Glomot 1979
Rat Inhalation3/ M/F >2.8 g/m3 Clark et al 1980
Mouse Oral3/ M/F >15 000 Glomot et al
1980a
Dog Oral3/ M/F >10 000 Glomot et al
1980b
1/ in an aqueous mixture of sodium carboxymethyl cellulose (0.25%)
and polysorbate 80 (0.20%);
2/ obtained for a 25 g/l flowable formulation;
3/ obtained for a 2.5% wettable powder formulation.
Observations in Humans
Persons exposed to deltamethrin for 7-8 years of its production,
syntheses and reformulation were subjected to medical observations,
including both clinical and haematological examinations. Evaluations
were conducted at several plants. There were no measurable effects
other than transient cutaneous and mucous membrane irritation which
was without sequelae. Adequate precautionary measures, such as gloves
and face masks, provide protection from exposure (Foulhouz 1981).
A medical survey of agricultural workers involved in the use and
application of EC and WP formulations of deltamethrin in Yugoslavia
revealed no untoward symptoms of exposure other than itching and
burning of the face, as well as nasal hypersecretion. Medical
examinations included chest x-ray, ECG, liver function tests,
neurological exam (eye tonometry, Goldman perimetry, dark adaptation
ability), kidney function tests, and whole blood and plasma
cholinesterase activity. No adverse effects were noted. Proper use of
masks and gloves, as well as good personal hygiene (e.g. washing),
were emphasized (Plestina 1981).
Four operators were evaluated during normal field applications
of deltamethrin lasting one day. Three of the operators wore no
protection on their heads or hands, while one wore hood, gloves and
respirator. Motor and sensory nerve conduction velocities were
determined as well as haematological and biochemical tests and
urinalysis. No changes were observed in blood parameters measured and
no residues were found in the urine samples. Furthermore, no decrease
in nerve conduction velocity occurred. Residues were primarily
confined to gloves and legs. None of the operators experienced
symptoms of facial sensation (Hewson and Burgess 1981).
COMMENTS
The additional information reviewed clarified previous concerns.
Embryotoxic effects observed are transient in nature, demonstrated
only in the foetuses, and without sequelae in newborn animals up
through 21 days post-partum.
Data accumulated on workers exposed to deltamethrin (e.g. plant
workers, field operators and supported by data in laboratory animals
demonstrate that deltamethrin is irritating to skin and mucous
membranes. This potential can be reduced by proper precautionary
measures such as gloves, face shield and other appropriate clothing.
Information would indicate that some of this irritation may be
partially caused or enhanced by the solvent (i.e. xylene) in the
EC formulation.
Evaluation of the histopathology in the 2-year rat feeding study
by independent pathologists confirmed that there were no dose-related
effects. The axonal degenerations at 20 ppm were not significantly
different from high dose or control animals. Historical data from the
laboratory performing the study re-affirmed that the reported
observation of testicular cell adenoma at 50 ppm were age related and
within the limits of 1 000 control animals of the same strain.
The Meeting noted with interest the neurological effects observed
in both acute and short-term studies, which were not observed in
longer-term studies. The high doses of the long-term studies in rat
and dog were equivalent to the lower doses utilized in the short-term
studies, which provides a possible reason for the absence of such
response.
The Meeting was made aware of ongoing long-term carcinogenicity
studies in the rat and mouse from which results are not yet available
and hoped that these would be made available to the JMPR when they are
completed. Additional data and information made available to the
Meeting provided sufficient data to recommend an ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 50 ppm in the diet, equivalent to 2.1 mg/kg bw.
Mouse: 100 ppm in the diet, equivalent to 12 mg/kg bw.
Dog: 40 ppm in the diet, equivalent to 1 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
1. Studies on the significance of neurological effects observed in
several animal species.
2. Results from the IARC carcinogenicity studies in mouse and rat.
3. Further information on exposure of humans to deltamethrin.
REFERENCES
Clark, G.C., Jackson, G.C. and Alexander, D.J. Acute inhalation
1980 toxicity in rats, 4-hour exposure (Decis PM 2.5 percent).
Report (ref.: RSL 437/80568) from Huntingdon Research
Centre, U.K., submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
Foulhouz, P. Medical observations of personnel working on synthesis
1981 or formulation of deltamethrin. Report (ref.: RU-81.18.06/A)
from Roussel Uclaf submitted to the World Health
Organization by Roussel Uclaf. (Unpublished)
Glomot, R. Acute toxicity study by oral route in the rat, Report
(ref.: RU-79 803-54/A2) 1979 from Roussel Uclaf, submitted
to the World Health Organization by Roussel Uclaf.
(Unpublished)
Glomot, R., Audegond, L. and Collas, E. Acute oral toxicity study
1979 in the rat (Decis 25 G/L Mixofluid). Report (ref.: RU
79824/A) from Roussel Uclaf submitted to the World Health
Organization by Roussel Uclaf. (Unpublished)
1980a Single administration by oral route in the mouse (Decis
Wettable Powder, 2.5%). Report (ref:RU-80.817/A) from
Roussel Uclaf submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1980b Single administration study by oral route in the dog (Decis
Wettable powder, 2.5%). Report (ref.: RU 80.194/A) from
Roussel Uclaf submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1981a Single administration study by oral route in the rat. Report
(ref.: RU-81239/A) from Roussel Uclaf, submitted to the
World Health Organization by Roussel Uclaf. (Unpublished)
1981b Primary dermal irritation study in the rabbit. Deltamethrin
25 G/L Mixofluid). Report (ref.: RU-79193/A) from Roussel
Uclaf, submitted to the World Health Organization by Roussel
Uclaf. (Unpublished)
1981c Primary dermal irritation study in the rabbit (Deltamethrin
wettable powder, 2.5%). Report (ref.: RU80200/A) from
Roussel Uclaf, submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1981d Primary eye irritation study in the rabbit (Deltamethrin in
25 G/L Mixofluid). Report (ref.: RU79192/A) from Roussel
Uclaf, submitted to the World Health Organization by Roussel
Uclaf. (Unpublished)
1981e Primary eye irritation study in the rabbit (Decis Wettable
Powder, 2.5%). Report (ref. RU79192/A) from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
Glomot, R. and Vannier, B. Complementary teratological study in the
1982 mouse. Report (ref.: RU-82.506-12/A), from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
Hewson, R.T. and Burgess, J.E. An operator study with deltamethrin
1981 including measurements of nerve conduction times. Report
(ref.: GB-NT-11.81/A) from Roussel Uclaf, submitted to the
World Health Organization by Roussel Uclaf. (Unpublished)
Plestina, R. An evaluation of the use of deltamethrin in public
1981 health. Report (ref.: ZAG. KOT. 81/A) from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
DELTAMETHRIN
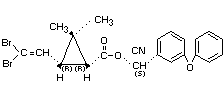 Explanation
Deltamethrin was reviewed by the Joint Meeting in 1980 and 1981
(FAO/ WHO 1981, 1982)1. In the absence of an ADI, guideline levels
were proposed on a wide variety of agricultural commodities. The
results of supervised trials on residues in meat, milk and eggs
arising from uses for ectoparasite control, in feeding studies and in
stable treatments were required by 1982. Also requested was more
information on the level and fate on various cereal grains treated
under different storage conditions, the effects of processing and
cooking, more residue data on kiwi fruit, currants and leafy
vegetables, the level and fate of residues in foods of animal origin
following feeding and more information on levels on cereal grains.
Information was received on residue levels on greenhouse
cucumbers and tomatoes and on stored wheat, wheat bran and wheat
flour. Data on residue levels in meat, milk and eggs arising from
ectoparasite control and stable treatments is under development but
not yet available.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Preharvest
New information was received from the Netherlands (Netherlands
1982) on preharvest treatments in use since 1981 on a wide variety of
crops, as shown in Table 1.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Deltamethrin Use Pattern in the Netherlands
Crop Application rate (a.i.) Preharvest
g/100 l g/ha Formulation Interval (days)
Apple, pear 2 0.5 75 EC 25 g/l 7
Strawberries 1 0.5 " -
Blackberries, 1 0.5 " 7
raspberries
Currants(black, 2 0.5 " 7
red,white),grapes,
gooseberries
Cherry, plums 1 0.5 " 7
Courgettes, 1 1.25 " 3
cucumbers,eggplant,
melons, sweet peppers
tomatoes
Lettuce, 1 1.25 25 EC 25 g/l 14
endive
Sweet fennel 1 7.5 " 7
Black radish, 1 7.5 " 7
radish, turnip,
rutabaga
Broccoli, 1 7.5 " 7
cauliflower,
Chinese cabbage,
cabbages, savoy,
curly kale,
kohlrabi
Table 1. (con't)
Crop Application rate (a.i.) Preharvest
g/100 l g/ha Formulation Interval (days)
Leek, 1 7.5 " 7
onions
Peas, 1 7.5 " 7
french beans
Potatoes 1 7.5 " 7
Sugar and fodder beet 1 7.5 " -
Poppy, 1 7.5 " 7
flax
Radish leaves, 1 7.5 " 7
cabbage greens,
turnip greens
Mushrooms 1 0.75-1.5 spray on beds,walk, 2
0.075 ceiling, swing or 2
pulsfog treatment
1 Small-scale use; 2 moderate-scale use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Some additional data were available from Finland (Finland 1982)
for residues on greenhouse cucumbers and tomatoes. Cucumbers were
sprayed at 2.1 mg a.i./plant; no residues (<0.05 mg/kg) were detected
in samples harvested at 1, 5 and 8 days post-treatment. Tomatoes were
sprayed at 1 mg a.i./plant; no residues (<0.05 mg/kg) were found in
samples collected at 1, 5 and 8 days post-treatment.
FATE OF RESIDUES
In Storage and Processing
Additional data were received on the behaviour of deltamethrin on
stored wheat arising from experiments conducted in Australia and
Greece (Table 2). Residues on wheat grain were roughly proportional to
half the treatment rate and showed no measurable losses during storage
for over a year. Residues in bran prepared from the stored wheat
treated at at 1 mg/kg ranged from 0.3 - 1.5 mg/kg, while corresponding
residues in flour (white) ranged from 0.01 - 0.07 mg/kg (UniversitÚ de
Montpellier 1981a,b).
NATIONAL MAXIMUM RESIDUE LIMITS
An updated list of national MRLs was provided to the Meeting by
the Netherlands (Netherlands 1982).
Commodity MRL (mg/kg)
Strawberries 0.05
Other fruits 0.1
Leafy vegetables 0.2
Other vegetables 0.05
Potatoes 0.05
Meat and milk 0.05
Other food commodities 0.05
APPRAISAL
The results of supervised trials in Finland on greenhouse
cucumbers and tomatoes sprayed with deltamethrin at 2.1 and
1.0 mg a.i./plant respectively showed no measurable residues;
<0.05 mg/kg were found even at 1 day post-treatment, and therefore
the guideline level of 0.05 mg/kg would not be exceeded.
Additional data on the behaviour of deltamethrin residues on
stored wheat treated at 0.25, 0.50 and 1.0 mg/kg confirmed that no
measurable degradation occurs on the grain over storage periods of
more than a year. Measured values ranged from 0.1 - 0.16, 0.25 - 0.27
Table 2. Residues of Deltamethrin on Stored Wheat 1
Location Rates Part Interval (days) following last application
(Year) Applied of
(mg/kg) Sample 7 90 180 270
Greece 0.25 Grain 0.13 0.10 0.13 0.16
1980 0.50 Grain 0.25 0.25 0.27 0.27
(Days) 40/48 77 81 118/137 186 223/242 281 318/337 383/420
>1 year
Australia 1 A Grain 0.3 0.5 0.25 0.4
1980-1982 Bran 1.2 1.3 0.5 0.3
Flour 0.06 0.06 0.06 0.01
(white)
1 B Grain 0.2 0.5 0.4 0.35 0.2
Bran 0.85 1.1 1.4 0.75 1.2
Flour 0.06 0.08 0.07 0.01 0.06
(white)
1 C Grain 0.25 0.35 0.3 0.35
Bran 1.1 0.8 1.4 1.5
Flour 0.05 0.07 0.07 0.01
(white)
1 D Grain 0.15 0.4 0.3 0.45
Bran 1.3 1.2 1 1
Flour 0.06 0.06 0.07 0.02
(white)
1 E Grain 0.2 0.3 0.4 0.4 0.25
Bran 1.4 0.9 1.2 1.2 1.3
Flour 0.07 0.07 0.07 0.06 0.06
(white)
1 All data based on one treatment.
and 0.15 - 0.5 mg/kg respectively. Bran obtained from the stored wheat
treated at the 1 mg/kg level contained most of the residue (range
0.3 - 1.5 mg/kg) while the corresponding white flour contained the
least (range 0.01 - 0.07 mg/kg). Taking existing or possible use
patterns into consideration, these data support the previously
proposed guideline levels for cereal grains, wheat bran and wheat
flour.
Studies are still under way for determining residues occurring in
meat, milk and eggs arising from ectoparasite and stable treatments
and will be made available by 1984.
RECOMMENDATIONS
The previous guideline levels are converted to temporary maximum
residue limits on the basis of the newly recommended temporary ADI for
deltamethrin.
FURTHER WORK OR INFORMATION
Desirable (as available)
1. Results of planned or ongoing studies on residue behaviour on
stored crop commodities for the purpose of recommending new or
revising previous MRLs on such commodities (estimated date -
1983),
2. Results of planned or ongoing supervised animal studies on
residues in meat, milk and eggs resulting from ectoparasite and
stable treatments for the purpose of recommending new MRLs on
those commodities (estimated date - 1984).
REFERENCES
Finland Table on deltamethrin residues (official testing),
1982 (Personal communication)
Netherlands Tables of preharvest treatments and national MRLs.
1982 (Personal communication)
UniversitÚ de Montpellier. Residues determination in plants. Wheat
1981a (stored). Research Report FP-81.27.04/A.
UniversitÚ de Montpellier, Residues determination in plants. Wheat
1981b (stored). Research Report FP-82.18.03/A.
Explanation
Deltamethrin was reviewed by the Joint Meeting in 1980 and 1981
(FAO/ WHO 1981, 1982)1. In the absence of an ADI, guideline levels
were proposed on a wide variety of agricultural commodities. The
results of supervised trials on residues in meat, milk and eggs
arising from uses for ectoparasite control, in feeding studies and in
stable treatments were required by 1982. Also requested was more
information on the level and fate on various cereal grains treated
under different storage conditions, the effects of processing and
cooking, more residue data on kiwi fruit, currants and leafy
vegetables, the level and fate of residues in foods of animal origin
following feeding and more information on levels on cereal grains.
Information was received on residue levels on greenhouse
cucumbers and tomatoes and on stored wheat, wheat bran and wheat
flour. Data on residue levels in meat, milk and eggs arising from
ectoparasite control and stable treatments is under development but
not yet available.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Preharvest
New information was received from the Netherlands (Netherlands
1982) on preharvest treatments in use since 1981 on a wide variety of
crops, as shown in Table 1.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Deltamethrin Use Pattern in the Netherlands
Crop Application rate (a.i.) Preharvest
g/100 l g/ha Formulation Interval (days)
Apple, pear 2 0.5 75 EC 25 g/l 7
Strawberries 1 0.5 " -
Blackberries, 1 0.5 " 7
raspberries
Currants(black, 2 0.5 " 7
red,white),grapes,
gooseberries
Cherry, plums 1 0.5 " 7
Courgettes, 1 1.25 " 3
cucumbers,eggplant,
melons, sweet peppers
tomatoes
Lettuce, 1 1.25 25 EC 25 g/l 14
endive
Sweet fennel 1 7.5 " 7
Black radish, 1 7.5 " 7
radish, turnip,
rutabaga
Broccoli, 1 7.5 " 7
cauliflower,
Chinese cabbage,
cabbages, savoy,
curly kale,
kohlrabi
Table 1. (con't)
Crop Application rate (a.i.) Preharvest
g/100 l g/ha Formulation Interval (days)
Leek, 1 7.5 " 7
onions
Peas, 1 7.5 " 7
french beans
Potatoes 1 7.5 " 7
Sugar and fodder beet 1 7.5 " -
Poppy, 1 7.5 " 7
flax
Radish leaves, 1 7.5 " 7
cabbage greens,
turnip greens
Mushrooms 1 0.75-1.5 spray on beds,walk, 2
0.075 ceiling, swing or 2
pulsfog treatment
1 Small-scale use; 2 moderate-scale use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Some additional data were available from Finland (Finland 1982)
for residues on greenhouse cucumbers and tomatoes. Cucumbers were
sprayed at 2.1 mg a.i./plant; no residues (<0.05 mg/kg) were detected
in samples harvested at 1, 5 and 8 days post-treatment. Tomatoes were
sprayed at 1 mg a.i./plant; no residues (<0.05 mg/kg) were found in
samples collected at 1, 5 and 8 days post-treatment.
FATE OF RESIDUES
In Storage and Processing
Additional data were received on the behaviour of deltamethrin on
stored wheat arising from experiments conducted in Australia and
Greece (Table 2). Residues on wheat grain were roughly proportional to
half the treatment rate and showed no measurable losses during storage
for over a year. Residues in bran prepared from the stored wheat
treated at at 1 mg/kg ranged from 0.3 - 1.5 mg/kg, while corresponding
residues in flour (white) ranged from 0.01 - 0.07 mg/kg (UniversitÚ de
Montpellier 1981a,b).
NATIONAL MAXIMUM RESIDUE LIMITS
An updated list of national MRLs was provided to the Meeting by
the Netherlands (Netherlands 1982).
Commodity MRL (mg/kg)
Strawberries 0.05
Other fruits 0.1
Leafy vegetables 0.2
Other vegetables 0.05
Potatoes 0.05
Meat and milk 0.05
Other food commodities 0.05
APPRAISAL
The results of supervised trials in Finland on greenhouse
cucumbers and tomatoes sprayed with deltamethrin at 2.1 and
1.0 mg a.i./plant respectively showed no measurable residues;
<0.05 mg/kg were found even at 1 day post-treatment, and therefore
the guideline level of 0.05 mg/kg would not be exceeded.
Additional data on the behaviour of deltamethrin residues on
stored wheat treated at 0.25, 0.50 and 1.0 mg/kg confirmed that no
measurable degradation occurs on the grain over storage periods of
more than a year. Measured values ranged from 0.1 - 0.16, 0.25 - 0.27
Table 2. Residues of Deltamethrin on Stored Wheat 1
Location Rates Part Interval (days) following last application
(Year) Applied of
(mg/kg) Sample 7 90 180 270
Greece 0.25 Grain 0.13 0.10 0.13 0.16
1980 0.50 Grain 0.25 0.25 0.27 0.27
(Days) 40/48 77 81 118/137 186 223/242 281 318/337 383/420
>1 year
Australia 1 A Grain 0.3 0.5 0.25 0.4
1980-1982 Bran 1.2 1.3 0.5 0.3
Flour 0.06 0.06 0.06 0.01
(white)
1 B Grain 0.2 0.5 0.4 0.35 0.2
Bran 0.85 1.1 1.4 0.75 1.2
Flour 0.06 0.08 0.07 0.01 0.06
(white)
1 C Grain 0.25 0.35 0.3 0.35
Bran 1.1 0.8 1.4 1.5
Flour 0.05 0.07 0.07 0.01
(white)
1 D Grain 0.15 0.4 0.3 0.45
Bran 1.3 1.2 1 1
Flour 0.06 0.06 0.07 0.02
(white)
1 E Grain 0.2 0.3 0.4 0.4 0.25
Bran 1.4 0.9 1.2 1.2 1.3
Flour 0.07 0.07 0.07 0.06 0.06
(white)
1 All data based on one treatment.
and 0.15 - 0.5 mg/kg respectively. Bran obtained from the stored wheat
treated at the 1 mg/kg level contained most of the residue (range
0.3 - 1.5 mg/kg) while the corresponding white flour contained the
least (range 0.01 - 0.07 mg/kg). Taking existing or possible use
patterns into consideration, these data support the previously
proposed guideline levels for cereal grains, wheat bran and wheat
flour.
Studies are still under way for determining residues occurring in
meat, milk and eggs arising from ectoparasite and stable treatments
and will be made available by 1984.
RECOMMENDATIONS
The previous guideline levels are converted to temporary maximum
residue limits on the basis of the newly recommended temporary ADI for
deltamethrin.
FURTHER WORK OR INFORMATION
Desirable (as available)
1. Results of planned or ongoing studies on residue behaviour on
stored crop commodities for the purpose of recommending new or
revising previous MRLs on such commodities (estimated date -
1983),
2. Results of planned or ongoing supervised animal studies on
residues in meat, milk and eggs resulting from ectoparasite and
stable treatments for the purpose of recommending new MRLs on
those commodities (estimated date - 1984).
REFERENCES
Finland Table on deltamethrin residues (official testing),
1982 (Personal communication)
Netherlands Tables of preharvest treatments and national MRLs.
1982 (Personal communication)
UniversitÚ de Montpellier. Residues determination in plants. Wheat
1981a (stored). Research Report FP-81.27.04/A.
UniversitÚ de Montpellier, Residues determination in plants. Wheat
1981b (stored). Research Report FP-82.18.03/A.
 Explanation
In 1980 the JMPR reviewed deltamethrin (FAO/WHO 1981)1/, and
determined that an ADI could not be estimated without further
information. Additional data were received and reviewed by the JMPR in
1981 (2-year feeding study in dogs; 2-year mouse oncogenicity study;
mouse and rat teratology studies; additional mutagenicity evaluations
and information on humans). No ADI was estimated. Since then,
additional data has been submitted to FAO/WHO and are reviewed in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
Studies designed to investigate the potential carcinogenic
effects of deltamethrin have been initiated by IARC.
Groups of BDVI rats were administered orally 0-6 mg/kg bw
deltamethrin in arachis oil. Similarly, groups of C57 BL/6 mice
received orally 0-8 mg/kg bw of deltamethrin in arachis oil. Results
were not yet available to the Meeting.
1/ See Annex 2 for WHO and FAO documentation.
Special Studies on Eye Irritation
Male albino rats (9) weighing between 2 and 3 kg were
administered 0.1 ml of formulated deltamethrin (25 g/l mixofluid) into
the conjunctival sac. Six of the treated eyes remained unwashed, while
the remaining three were rinsed with lukewarm water 20-30 seconds
after instillation. There was only transient clouding of the cornea in
two animals one hour after dosing (1 washed, 1 unwashed), which
cleared by day 2. Also noted was a low grade conjunctival irritation
among all animals initially, which disappeared following day 2 of
observations (Glomot et al 1981d).
The 2.5% WP deltamethrin formulation diluted 1/10 in distilled
water (0.1 ml per rabbit) elicited a similar pattern of initial
transient corneal clouding in three of nine rabbits examined, which
cleared by day 4. The undiluted formulation, 100 mg, administered into
the conjunctival sac of rabbits produced increased involvement of
conjunctiva, iris and cornea in all animals, generally moderate in
severity, with low grade corneal opacity persisting in two rabbits
through day 7 (1 washed, 1 unwashed) (Glomot et al 1981e).
Special Study on Teratogenicity
Mouse
Groups of mated Swiss CD1, SPF mice (25/group) were orally
gavaged with deltamethrin dissolved in sesame oil at dose levels of 0,
0.1, 1 and 10 mg/kg bw/day administered during gestation days 6 to 17.
This was a complementary teratology study to the Glomot and Vannier
1977 evaluation and was experimentally designed to assess the effects
on foetal and post-natal development, including embryotoxicity. One
group of mated mice was sacrificed on day 18 of gestation and examined
for gravid uterine weight, number of implantation sites, viable
foetuses, dead foetuses and resorptions. Visceral and skeletal
examinations were performed on the foetuses. Some females were allowed
to produce litter, and full examinations of pups and dams were
conducted on day 1 post-partum. Similarly, a third group of females
was allowed to produce a litter and dams and pups were sacrificed on
day 21 post-partum. Pup weights were determined on days 1, 4, 10 and
21 post-partum.
Results demonstrate that the conception and pregnancy rates were
unaffected by treatment, except that maternal body weight gain was
decreased in the 10 mg/kg bw group. Skeletal variants, consisting of
delayed ossification of the sternebrae and phalanges in the 1 and
10 mg/kg bw/day group pups at gestation day 18, were not present at
day 1 or 21 post-partum. Extranumery ribs and reduced ossification of
skull bones were not observed in this complementary study. Litter
size, pup weight, viability and lactation index were all normal
through day 21 post-partum. Deltamethrin administered during the
period of major organogenesis in the mouse causes a moderate and
transient retardation of development of the foetus at 1 mg/kg bw/day
and higher doses, but these effects were not observed at day 1 or 21
post-partum. There were no teratogenic effects related to treatment
(Glomot and Vannier 1982).
Special Studies on Skin Sensitization
Male albino rabbits (6) weighing 2.5 to 2.9 kg were administered
0.5 ml of formulated deltamethrin (25 g/l mixofluid) to both shaved
intact and abraded skin. The Primary Irritation Index after 24 h
exposure to occluded sites was 1.2, slightly irritating (Glomot
et al 1981b).
An evaluation similar to the one described above was determined
for a 2.5% WP deltamethrin formulation. Rabbits had a Primary
Irritation Index of 2.41, moderately irritating. Moderate erythema
continued for 72 hours, while the oedema generally diminished, with
the exception of scarified skin sites (Glomot et al 1981c).
Acute Toxicity
Mouse
Mice presented far fewer symptoms than rats after oral dosing
at comparable levels, with diarrhoea being the only reportable
observation (Glomot et al 1980a).
Rat
The acute oral toxicity of low concentrations of deltamethrin to
rats was without noticeable mortality but included symptoms observed
at higher concentrations, including excessive grooming, diarrhoea,
drowsiness, piloerection, ptosis, difficulty in walking, general motor
incoordination and hypotonia (Glomot 1979; Glomot et al 1979,
1981a).
Rats were exposed (whole-body exposure) for 4 hours to an aerosol
concentration of deltamethrin equal to 2.8 g/m3, the highest
attainable airborne concentration of a 2-5% formulation. Approximately
80% of the total aerosol had a mean aerodynamic diameter less than
5.5 Ám. Dyspnoea and gasping were observed in exposed rats. Relative
lung weights and macroscopic pathology were normal. There were no
mortalities (Clark et al 1980).
Dog
Dogs orally dosed with a 2.5% formulation of deltamethrin
displayed no clinical symptoms related to treatment (Glomot
et al 1980b).
Table 1 Acute Toxicity of Deltamethrin in Animals
LD50
Species Route Sex (mg/kg bw) Reference
Rat Oral1/ M/F >5 000 Glomot et al
1981a
Rat Oral2/ M 22 700 Glomot et al 1979
(19 100-23 800)
Rat Oral2/ F 22 000 "
(18 800-22 700)
Rat Oral3/ M/F >15 000 Glomot 1979
Rat Inhalation3/ M/F >2.8 g/m3 Clark et al 1980
Mouse Oral3/ M/F >15 000 Glomot et al
1980a
Dog Oral3/ M/F >10 000 Glomot et al
1980b
1/ in an aqueous mixture of sodium carboxymethyl cellulose (0.25%)
and polysorbate 80 (0.20%);
2/ obtained for a 25 g/l flowable formulation;
3/ obtained for a 2.5% wettable powder formulation.
Observations in Humans
Persons exposed to deltamethrin for 7-8 years of its production,
syntheses and reformulation were subjected to medical observations,
including both clinical and haematological examinations. Evaluations
were conducted at several plants. There were no measurable effects
other than transient cutaneous and mucous membrane irritation which
was without sequelae. Adequate precautionary measures, such as gloves
and face masks, provide protection from exposure (Foulhouz 1981).
A medical survey of agricultural workers involved in the use and
application of EC and WP formulations of deltamethrin in Yugoslavia
revealed no untoward symptoms of exposure other than itching and
burning of the face, as well as nasal hypersecretion. Medical
examinations included chest x-ray, ECG, liver function tests,
neurological exam (eye tonometry, Goldman perimetry, dark adaptation
ability), kidney function tests, and whole blood and plasma
cholinesterase activity. No adverse effects were noted. Proper use of
masks and gloves, as well as good personal hygiene (e.g. washing),
were emphasized (Plestina 1981).
Four operators were evaluated during normal field applications
of deltamethrin lasting one day. Three of the operators wore no
protection on their heads or hands, while one wore hood, gloves and
respirator. Motor and sensory nerve conduction velocities were
determined as well as haematological and biochemical tests and
urinalysis. No changes were observed in blood parameters measured and
no residues were found in the urine samples. Furthermore, no decrease
in nerve conduction velocity occurred. Residues were primarily
confined to gloves and legs. None of the operators experienced
symptoms of facial sensation (Hewson and Burgess 1981).
COMMENTS
The additional information reviewed clarified previous concerns.
Embryotoxic effects observed are transient in nature, demonstrated
only in the foetuses, and without sequelae in newborn animals up
through 21 days post-partum.
Data accumulated on workers exposed to deltamethrin (e.g. plant
workers, field operators and supported by data in laboratory animals
demonstrate that deltamethrin is irritating to skin and mucous
membranes. This potential can be reduced by proper precautionary
measures such as gloves, face shield and other appropriate clothing.
Information would indicate that some of this irritation may be
partially caused or enhanced by the solvent (i.e. xylene) in the
EC formulation.
Evaluation of the histopathology in the 2-year rat feeding study
by independent pathologists confirmed that there were no dose-related
effects. The axonal degenerations at 20 ppm were not significantly
different from high dose or control animals. Historical data from the
laboratory performing the study re-affirmed that the reported
observation of testicular cell adenoma at 50 ppm were age related and
within the limits of 1 000 control animals of the same strain.
The Meeting noted with interest the neurological effects observed
in both acute and short-term studies, which were not observed in
longer-term studies. The high doses of the long-term studies in rat
and dog were equivalent to the lower doses utilized in the short-term
studies, which provides a possible reason for the absence of such
response.
The Meeting was made aware of ongoing long-term carcinogenicity
studies in the rat and mouse from which results are not yet available
and hoped that these would be made available to the JMPR when they are
completed. Additional data and information made available to the
Meeting provided sufficient data to recommend an ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 50 ppm in the diet, equivalent to 2.1 mg/kg bw.
Mouse: 100 ppm in the diet, equivalent to 12 mg/kg bw.
Dog: 40 ppm in the diet, equivalent to 1 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
1. Studies on the significance of neurological effects observed in
several animal species.
2. Results from the IARC carcinogenicity studies in mouse and rat.
3. Further information on exposure of humans to deltamethrin.
REFERENCES
Clark, G.C., Jackson, G.C. and Alexander, D.J. Acute inhalation
1980 toxicity in rats, 4-hour exposure (Decis PM 2.5 percent).
Report (ref.: RSL 437/80568) from Huntingdon Research
Centre, U.K., submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
Foulhouz, P. Medical observations of personnel working on synthesis
1981 or formulation of deltamethrin. Report (ref.: RU-81.18.06/A)
from Roussel Uclaf submitted to the World Health
Organization by Roussel Uclaf. (Unpublished)
Glomot, R. Acute toxicity study by oral route in the rat, Report
(ref.: RU-79 803-54/A2) 1979 from Roussel Uclaf, submitted
to the World Health Organization by Roussel Uclaf.
(Unpublished)
Glomot, R., Audegond, L. and Collas, E. Acute oral toxicity study
1979 in the rat (Decis 25 G/L Mixofluid). Report (ref.: RU
79824/A) from Roussel Uclaf submitted to the World Health
Organization by Roussel Uclaf. (Unpublished)
1980a Single administration by oral route in the mouse (Decis
Wettable Powder, 2.5%). Report (ref:RU-80.817/A) from
Roussel Uclaf submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1980b Single administration study by oral route in the dog (Decis
Wettable powder, 2.5%). Report (ref.: RU 80.194/A) from
Roussel Uclaf submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1981a Single administration study by oral route in the rat. Report
(ref.: RU-81239/A) from Roussel Uclaf, submitted to the
World Health Organization by Roussel Uclaf. (Unpublished)
1981b Primary dermal irritation study in the rabbit. Deltamethrin
25 G/L Mixofluid). Report (ref.: RU-79193/A) from Roussel
Uclaf, submitted to the World Health Organization by Roussel
Uclaf. (Unpublished)
1981c Primary dermal irritation study in the rabbit (Deltamethrin
wettable powder, 2.5%). Report (ref.: RU80200/A) from
Roussel Uclaf, submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1981d Primary eye irritation study in the rabbit (Deltamethrin in
25 G/L Mixofluid). Report (ref.: RU79192/A) from Roussel
Uclaf, submitted to the World Health Organization by Roussel
Uclaf. (Unpublished)
1981e Primary eye irritation study in the rabbit (Decis Wettable
Powder, 2.5%). Report (ref. RU79192/A) from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
Glomot, R. and Vannier, B. Complementary teratological study in the
1982 mouse. Report (ref.: RU-82.506-12/A), from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
Hewson, R.T. and Burgess, J.E. An operator study with deltamethrin
1981 including measurements of nerve conduction times. Report
(ref.: GB-NT-11.81/A) from Roussel Uclaf, submitted to the
World Health Organization by Roussel Uclaf. (Unpublished)
Plestina, R. An evaluation of the use of deltamethrin in public
1981 health. Report (ref.: ZAG. KOT. 81/A) from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
DELTAMETHRIN
Explanation
In 1980 the JMPR reviewed deltamethrin (FAO/WHO 1981)1/, and
determined that an ADI could not be estimated without further
information. Additional data were received and reviewed by the JMPR in
1981 (2-year feeding study in dogs; 2-year mouse oncogenicity study;
mouse and rat teratology studies; additional mutagenicity evaluations
and information on humans). No ADI was estimated. Since then,
additional data has been submitted to FAO/WHO and are reviewed in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
Studies designed to investigate the potential carcinogenic
effects of deltamethrin have been initiated by IARC.
Groups of BDVI rats were administered orally 0-6 mg/kg bw
deltamethrin in arachis oil. Similarly, groups of C57 BL/6 mice
received orally 0-8 mg/kg bw of deltamethrin in arachis oil. Results
were not yet available to the Meeting.
1/ See Annex 2 for WHO and FAO documentation.
Special Studies on Eye Irritation
Male albino rats (9) weighing between 2 and 3 kg were
administered 0.1 ml of formulated deltamethrin (25 g/l mixofluid) into
the conjunctival sac. Six of the treated eyes remained unwashed, while
the remaining three were rinsed with lukewarm water 20-30 seconds
after instillation. There was only transient clouding of the cornea in
two animals one hour after dosing (1 washed, 1 unwashed), which
cleared by day 2. Also noted was a low grade conjunctival irritation
among all animals initially, which disappeared following day 2 of
observations (Glomot et al 1981d).
The 2.5% WP deltamethrin formulation diluted 1/10 in distilled
water (0.1 ml per rabbit) elicited a similar pattern of initial
transient corneal clouding in three of nine rabbits examined, which
cleared by day 4. The undiluted formulation, 100 mg, administered into
the conjunctival sac of rabbits produced increased involvement of
conjunctiva, iris and cornea in all animals, generally moderate in
severity, with low grade corneal opacity persisting in two rabbits
through day 7 (1 washed, 1 unwashed) (Glomot et al 1981e).
Special Study on Teratogenicity
Mouse
Groups of mated Swiss CD1, SPF mice (25/group) were orally
gavaged with deltamethrin dissolved in sesame oil at dose levels of 0,
0.1, 1 and 10 mg/kg bw/day administered during gestation days 6 to 17.
This was a complementary teratology study to the Glomot and Vannier
1977 evaluation and was experimentally designed to assess the effects
on foetal and post-natal development, including embryotoxicity. One
group of mated mice was sacrificed on day 18 of gestation and examined
for gravid uterine weight, number of implantation sites, viable
foetuses, dead foetuses and resorptions. Visceral and skeletal
examinations were performed on the foetuses. Some females were allowed
to produce litter, and full examinations of pups and dams were
conducted on day 1 post-partum. Similarly, a third group of females
was allowed to produce a litter and dams and pups were sacrificed on
day 21 post-partum. Pup weights were determined on days 1, 4, 10 and
21 post-partum.
Results demonstrate that the conception and pregnancy rates were
unaffected by treatment, except that maternal body weight gain was
decreased in the 10 mg/kg bw group. Skeletal variants, consisting of
delayed ossification of the sternebrae and phalanges in the 1 and
10 mg/kg bw/day group pups at gestation day 18, were not present at
day 1 or 21 post-partum. Extranumery ribs and reduced ossification of
skull bones were not observed in this complementary study. Litter
size, pup weight, viability and lactation index were all normal
through day 21 post-partum. Deltamethrin administered during the
period of major organogenesis in the mouse causes a moderate and
transient retardation of development of the foetus at 1 mg/kg bw/day
and higher doses, but these effects were not observed at day 1 or 21
post-partum. There were no teratogenic effects related to treatment
(Glomot and Vannier 1982).
Special Studies on Skin Sensitization
Male albino rabbits (6) weighing 2.5 to 2.9 kg were administered
0.5 ml of formulated deltamethrin (25 g/l mixofluid) to both shaved
intact and abraded skin. The Primary Irritation Index after 24 h
exposure to occluded sites was 1.2, slightly irritating (Glomot
et al 1981b).
An evaluation similar to the one described above was determined
for a 2.5% WP deltamethrin formulation. Rabbits had a Primary
Irritation Index of 2.41, moderately irritating. Moderate erythema
continued for 72 hours, while the oedema generally diminished, with
the exception of scarified skin sites (Glomot et al 1981c).
Acute Toxicity
Mouse
Mice presented far fewer symptoms than rats after oral dosing
at comparable levels, with diarrhoea being the only reportable
observation (Glomot et al 1980a).
Rat
The acute oral toxicity of low concentrations of deltamethrin to
rats was without noticeable mortality but included symptoms observed
at higher concentrations, including excessive grooming, diarrhoea,
drowsiness, piloerection, ptosis, difficulty in walking, general motor
incoordination and hypotonia (Glomot 1979; Glomot et al 1979,
1981a).
Rats were exposed (whole-body exposure) for 4 hours to an aerosol
concentration of deltamethrin equal to 2.8 g/m3, the highest
attainable airborne concentration of a 2-5% formulation. Approximately
80% of the total aerosol had a mean aerodynamic diameter less than
5.5 Ám. Dyspnoea and gasping were observed in exposed rats. Relative
lung weights and macroscopic pathology were normal. There were no
mortalities (Clark et al 1980).
Dog
Dogs orally dosed with a 2.5% formulation of deltamethrin
displayed no clinical symptoms related to treatment (Glomot
et al 1980b).
Table 1 Acute Toxicity of Deltamethrin in Animals
LD50
Species Route Sex (mg/kg bw) Reference
Rat Oral1/ M/F >5 000 Glomot et al
1981a
Rat Oral2/ M 22 700 Glomot et al 1979
(19 100-23 800)
Rat Oral2/ F 22 000 "
(18 800-22 700)
Rat Oral3/ M/F >15 000 Glomot 1979
Rat Inhalation3/ M/F >2.8 g/m3 Clark et al 1980
Mouse Oral3/ M/F >15 000 Glomot et al
1980a
Dog Oral3/ M/F >10 000 Glomot et al
1980b
1/ in an aqueous mixture of sodium carboxymethyl cellulose (0.25%)
and polysorbate 80 (0.20%);
2/ obtained for a 25 g/l flowable formulation;
3/ obtained for a 2.5% wettable powder formulation.
Observations in Humans
Persons exposed to deltamethrin for 7-8 years of its production,
syntheses and reformulation were subjected to medical observations,
including both clinical and haematological examinations. Evaluations
were conducted at several plants. There were no measurable effects
other than transient cutaneous and mucous membrane irritation which
was without sequelae. Adequate precautionary measures, such as gloves
and face masks, provide protection from exposure (Foulhouz 1981).
A medical survey of agricultural workers involved in the use and
application of EC and WP formulations of deltamethrin in Yugoslavia
revealed no untoward symptoms of exposure other than itching and
burning of the face, as well as nasal hypersecretion. Medical
examinations included chest x-ray, ECG, liver function tests,
neurological exam (eye tonometry, Goldman perimetry, dark adaptation
ability), kidney function tests, and whole blood and plasma
cholinesterase activity. No adverse effects were noted. Proper use of
masks and gloves, as well as good personal hygiene (e.g. washing),
were emphasized (Plestina 1981).
Four operators were evaluated during normal field applications
of deltamethrin lasting one day. Three of the operators wore no
protection on their heads or hands, while one wore hood, gloves and
respirator. Motor and sensory nerve conduction velocities were
determined as well as haematological and biochemical tests and
urinalysis. No changes were observed in blood parameters measured and
no residues were found in the urine samples. Furthermore, no decrease
in nerve conduction velocity occurred. Residues were primarily
confined to gloves and legs. None of the operators experienced
symptoms of facial sensation (Hewson and Burgess 1981).
COMMENTS
The additional information reviewed clarified previous concerns.
Embryotoxic effects observed are transient in nature, demonstrated
only in the foetuses, and without sequelae in newborn animals up
through 21 days post-partum.
Data accumulated on workers exposed to deltamethrin (e.g. plant
workers, field operators and supported by data in laboratory animals
demonstrate that deltamethrin is irritating to skin and mucous
membranes. This potential can be reduced by proper precautionary
measures such as gloves, face shield and other appropriate clothing.
Information would indicate that some of this irritation may be
partially caused or enhanced by the solvent (i.e. xylene) in the
EC formulation.
Evaluation of the histopathology in the 2-year rat feeding study
by independent pathologists confirmed that there were no dose-related
effects. The axonal degenerations at 20 ppm were not significantly
different from high dose or control animals. Historical data from the
laboratory performing the study re-affirmed that the reported
observation of testicular cell adenoma at 50 ppm were age related and
within the limits of 1 000 control animals of the same strain.
The Meeting noted with interest the neurological effects observed
in both acute and short-term studies, which were not observed in
longer-term studies. The high doses of the long-term studies in rat
and dog were equivalent to the lower doses utilized in the short-term
studies, which provides a possible reason for the absence of such
response.
The Meeting was made aware of ongoing long-term carcinogenicity
studies in the rat and mouse from which results are not yet available
and hoped that these would be made available to the JMPR when they are
completed. Additional data and information made available to the
Meeting provided sufficient data to recommend an ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 50 ppm in the diet, equivalent to 2.1 mg/kg bw.
Mouse: 100 ppm in the diet, equivalent to 12 mg/kg bw.
Dog: 40 ppm in the diet, equivalent to 1 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
1. Studies on the significance of neurological effects observed in
several animal species.
2. Results from the IARC carcinogenicity studies in mouse and rat.
3. Further information on exposure of humans to deltamethrin.
REFERENCES
Clark, G.C., Jackson, G.C. and Alexander, D.J. Acute inhalation
1980 toxicity in rats, 4-hour exposure (Decis PM 2.5 percent).
Report (ref.: RSL 437/80568) from Huntingdon Research
Centre, U.K., submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
Foulhouz, P. Medical observations of personnel working on synthesis
1981 or formulation of deltamethrin. Report (ref.: RU-81.18.06/A)
from Roussel Uclaf submitted to the World Health
Organization by Roussel Uclaf. (Unpublished)
Glomot, R. Acute toxicity study by oral route in the rat, Report
(ref.: RU-79 803-54/A2) 1979 from Roussel Uclaf, submitted
to the World Health Organization by Roussel Uclaf.
(Unpublished)
Glomot, R., Audegond, L. and Collas, E. Acute oral toxicity study
1979 in the rat (Decis 25 G/L Mixofluid). Report (ref.: RU
79824/A) from Roussel Uclaf submitted to the World Health
Organization by Roussel Uclaf. (Unpublished)
1980a Single administration by oral route in the mouse (Decis
Wettable Powder, 2.5%). Report (ref:RU-80.817/A) from
Roussel Uclaf submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1980b Single administration study by oral route in the dog (Decis
Wettable powder, 2.5%). Report (ref.: RU 80.194/A) from
Roussel Uclaf submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1981a Single administration study by oral route in the rat. Report
(ref.: RU-81239/A) from Roussel Uclaf, submitted to the
World Health Organization by Roussel Uclaf. (Unpublished)
1981b Primary dermal irritation study in the rabbit. Deltamethrin
25 G/L Mixofluid). Report (ref.: RU-79193/A) from Roussel
Uclaf, submitted to the World Health Organization by Roussel
Uclaf. (Unpublished)
1981c Primary dermal irritation study in the rabbit (Deltamethrin
wettable powder, 2.5%). Report (ref.: RU80200/A) from
Roussel Uclaf, submitted to the World Health Organization by
Roussel Uclaf. (Unpublished)
1981d Primary eye irritation study in the rabbit (Deltamethrin in
25 G/L Mixofluid). Report (ref.: RU79192/A) from Roussel
Uclaf, submitted to the World Health Organization by Roussel
Uclaf. (Unpublished)
1981e Primary eye irritation study in the rabbit (Decis Wettable
Powder, 2.5%). Report (ref. RU79192/A) from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
Glomot, R. and Vannier, B. Complementary teratological study in the
1982 mouse. Report (ref.: RU-82.506-12/A), from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
Hewson, R.T. and Burgess, J.E. An operator study with deltamethrin
1981 including measurements of nerve conduction times. Report
(ref.: GB-NT-11.81/A) from Roussel Uclaf, submitted to the
World Health Organization by Roussel Uclaf. (Unpublished)
Plestina, R. An evaluation of the use of deltamethrin in public
1981 health. Report (ref.: ZAG. KOT. 81/A) from Roussel Uclaf,
submitted to the World Health Organization by Roussel Uclaf.
(Unpublished)
DELTAMETHRIN
 Explanation
Deltamethrin was reviewed by the Joint Meeting in 1980 and 1981
(FAO/ WHO 1981, 1982)1. In the absence of an ADI, guideline levels
were proposed on a wide variety of agricultural commodities. The
results of supervised trials on residues in meat, milk and eggs
arising from uses for ectoparasite control, in feeding studies and in
stable treatments were required by 1982. Also requested was more
information on the level and fate on various cereal grains treated
under different storage conditions, the effects of processing and
cooking, more residue data on kiwi fruit, currants and leafy
vegetables, the level and fate of residues in foods of animal origin
following feeding and more information on levels on cereal grains.
Information was received on residue levels on greenhouse
cucumbers and tomatoes and on stored wheat, wheat bran and wheat
flour. Data on residue levels in meat, milk and eggs arising from
ectoparasite control and stable treatments is under development but
not yet available.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Preharvest
New information was received from the Netherlands (Netherlands
1982) on preharvest treatments in use since 1981 on a wide variety of
crops, as shown in Table 1.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Deltamethrin Use Pattern in the Netherlands
Crop Application rate (a.i.) Preharvest
g/100 l g/ha Formulation Interval (days)
Apple, pear 2 0.5 75 EC 25 g/l 7
Strawberries 1 0.5 " -
Blackberries, 1 0.5 " 7
raspberries
Currants(black, 2 0.5 " 7
red,white),grapes,
gooseberries
Cherry, plums 1 0.5 " 7
Courgettes, 1 1.25 " 3
cucumbers,eggplant,
melons, sweet peppers
tomatoes
Lettuce, 1 1.25 25 EC 25 g/l 14
endive
Sweet fennel 1 7.5 " 7
Black radish, 1 7.5 " 7
radish, turnip,
rutabaga
Broccoli, 1 7.5 " 7
cauliflower,
Chinese cabbage,
cabbages, savoy,
curly kale,
kohlrabi
Table 1. (con't)
Crop Application rate (a.i.) Preharvest
g/100 l g/ha Formulation Interval (days)
Leek, 1 7.5 " 7
onions
Peas, 1 7.5 " 7
french beans
Potatoes 1 7.5 " 7
Sugar and fodder beet 1 7.5 " -
Poppy, 1 7.5 " 7
flax
Radish leaves, 1 7.5 " 7
cabbage greens,
turnip greens
Mushrooms 1 0.75-1.5 spray on beds,walk, 2
0.075 ceiling, swing or 2
pulsfog treatment
1 Small-scale use; 2 moderate-scale use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Some additional data were available from Finland (Finland 1982)
for residues on greenhouse cucumbers and tomatoes. Cucumbers were
sprayed at 2.1 mg a.i./plant; no residues (<0.05 mg/kg) were detected
in samples harvested at 1, 5 and 8 days post-treatment. Tomatoes were
sprayed at 1 mg a.i./plant; no residues (<0.05 mg/kg) were found in
samples collected at 1, 5 and 8 days post-treatment.
FATE OF RESIDUES
In Storage and Processing
Additional data were received on the behaviour of deltamethrin on
stored wheat arising from experiments conducted in Australia and
Greece (Table 2). Residues on wheat grain were roughly proportional to
half the treatment rate and showed no measurable losses during storage
for over a year. Residues in bran prepared from the stored wheat
treated at at 1 mg/kg ranged from 0.3 - 1.5 mg/kg, while corresponding
residues in flour (white) ranged from 0.01 - 0.07 mg/kg (UniversitÚ de
Montpellier 1981a,b).
NATIONAL MAXIMUM RESIDUE LIMITS
An updated list of national MRLs was provided to the Meeting by
the Netherlands (Netherlands 1982).
Commodity MRL (mg/kg)
Strawberries 0.05
Other fruits 0.1
Leafy vegetables 0.2
Other vegetables 0.05
Potatoes 0.05
Meat and milk 0.05
Other food commodities 0.05
APPRAISAL
The results of supervised trials in Finland on greenhouse
cucumbers and tomatoes sprayed with deltamethrin at 2.1 and
1.0 mg a.i./plant respectively showed no measurable residues;
<0.05 mg/kg were found even at 1 day post-treatment, and therefore
the guideline level of 0.05 mg/kg would not be exceeded.
Additional data on the behaviour of deltamethrin residues on
stored wheat treated at 0.25, 0.50 and 1.0 mg/kg confirmed that no
measurable degradation occurs on the grain over storage periods of
more than a year. Measured values ranged from 0.1 - 0.16, 0.25 - 0.27
Table 2. Residues of Deltamethrin on Stored Wheat 1
Location Rates Part Interval (days) following last application
(Year) Applied of
(mg/kg) Sample 7 90 180 270
Greece 0.25 Grain 0.13 0.10 0.13 0.16
1980 0.50 Grain 0.25 0.25 0.27 0.27
(Days) 40/48 77 81 118/137 186 223/242 281 318/337 383/420
>1 year
Australia 1 A Grain 0.3 0.5 0.25 0.4
1980-1982 Bran 1.2 1.3 0.5 0.3
Flour 0.06 0.06 0.06 0.01
(white)
1 B Grain 0.2 0.5 0.4 0.35 0.2
Bran 0.85 1.1 1.4 0.75 1.2
Flour 0.06 0.08 0.07 0.01 0.06
(white)
1 C Grain 0.25 0.35 0.3 0.35
Bran 1.1 0.8 1.4 1.5
Flour 0.05 0.07 0.07 0.01
(white)
1 D Grain 0.15 0.4 0.3 0.45
Bran 1.3 1.2 1 1
Flour 0.06 0.06 0.07 0.02
(white)
1 E Grain 0.2 0.3 0.4 0.4 0.25
Bran 1.4 0.9 1.2 1.2 1.3
Flour 0.07 0.07 0.07 0.06 0.06
(white)
1 All data based on one treatment.
and 0.15 - 0.5 mg/kg respectively. Bran obtained from the stored wheat
treated at the 1 mg/kg level contained most of the residue (range
0.3 - 1.5 mg/kg) while the corresponding white flour contained the
least (range 0.01 - 0.07 mg/kg). Taking existing or possible use
patterns into consideration, these data support the previously
proposed guideline levels for cereal grains, wheat bran and wheat
flour.
Studies are still under way for determining residues occurring in
meat, milk and eggs arising from ectoparasite and stable treatments
and will be made available by 1984.
RECOMMENDATIONS
The previous guideline levels are converted to temporary maximum
residue limits on the basis of the newly recommended temporary ADI for
deltamethrin.
FURTHER WORK OR INFORMATION
Desirable (as available)
1. Results of planned or ongoing studies on residue behaviour on
stored crop commodities for the purpose of recommending new or
revising previous MRLs on such commodities (estimated date -
1983),
2. Results of planned or ongoing supervised animal studies on
residues in meat, milk and eggs resulting from ectoparasite and
stable treatments for the purpose of recommending new MRLs on
those commodities (estimated date - 1984).
REFERENCES
Finland Table on deltamethrin residues (official testing),
1982 (Personal communication)
Netherlands Tables of preharvest treatments and national MRLs.
1982 (Personal communication)
UniversitÚ de Montpellier. Residues determination in plants. Wheat
1981a (stored). Research Report FP-81.27.04/A.
UniversitÚ de Montpellier, Residues determination in plants. Wheat
1981b (stored). Research Report FP-82.18.03/A.
Explanation
Deltamethrin was reviewed by the Joint Meeting in 1980 and 1981
(FAO/ WHO 1981, 1982)1. In the absence of an ADI, guideline levels
were proposed on a wide variety of agricultural commodities. The
results of supervised trials on residues in meat, milk and eggs
arising from uses for ectoparasite control, in feeding studies and in
stable treatments were required by 1982. Also requested was more
information on the level and fate on various cereal grains treated
under different storage conditions, the effects of processing and
cooking, more residue data on kiwi fruit, currants and leafy
vegetables, the level and fate of residues in foods of animal origin
following feeding and more information on levels on cereal grains.
Information was received on residue levels on greenhouse
cucumbers and tomatoes and on stored wheat, wheat bran and wheat
flour. Data on residue levels in meat, milk and eggs arising from
ectoparasite control and stable treatments is under development but
not yet available.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Preharvest
New information was received from the Netherlands (Netherlands
1982) on preharvest treatments in use since 1981 on a wide variety of
crops, as shown in Table 1.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Deltamethrin Use Pattern in the Netherlands
Crop Application rate (a.i.) Preharvest
g/100 l g/ha Formulation Interval (days)
Apple, pear 2 0.5 75 EC 25 g/l 7
Strawberries 1 0.5 " -
Blackberries, 1 0.5 " 7
raspberries
Currants(black, 2 0.5 " 7
red,white),grapes,
gooseberries
Cherry, plums 1 0.5 " 7
Courgettes, 1 1.25 " 3
cucumbers,eggplant,
melons, sweet peppers
tomatoes
Lettuce, 1 1.25 25 EC 25 g/l 14
endive
Sweet fennel 1 7.5 " 7
Black radish, 1 7.5 " 7
radish, turnip,
rutabaga
Broccoli, 1 7.5 " 7
cauliflower,
Chinese cabbage,
cabbages, savoy,
curly kale,
kohlrabi
Table 1. (con't)
Crop Application rate (a.i.) Preharvest
g/100 l g/ha Formulation Interval (days)
Leek, 1 7.5 " 7
onions
Peas, 1 7.5 " 7
french beans
Potatoes 1 7.5 " 7
Sugar and fodder beet 1 7.5 " -
Poppy, 1 7.5 " 7
flax
Radish leaves, 1 7.5 " 7
cabbage greens,
turnip greens
Mushrooms 1 0.75-1.5 spray on beds,walk, 2
0.075 ceiling, swing or 2
pulsfog treatment
1 Small-scale use; 2 moderate-scale use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Some additional data were available from Finland (Finland 1982)
for residues on greenhouse cucumbers and tomatoes. Cucumbers were
sprayed at 2.1 mg a.i./plant; no residues (<0.05 mg/kg) were detected
in samples harvested at 1, 5 and 8 days post-treatment. Tomatoes were
sprayed at 1 mg a.i./plant; no residues (<0.05 mg/kg) were found in
samples collected at 1, 5 and 8 days post-treatment.
FATE OF RESIDUES
In Storage and Processing
Additional data were received on the behaviour of deltamethrin on
stored wheat arising from experiments conducted in Australia and
Greece (Table 2). Residues on wheat grain were roughly proportional to
half the treatment rate and showed no measurable losses during storage
for over a year. Residues in bran prepared from the stored wheat
treated at at 1 mg/kg ranged from 0.3 - 1.5 mg/kg, while corresponding
residues in flour (white) ranged from 0.01 - 0.07 mg/kg (UniversitÚ de
Montpellier 1981a,b).
NATIONAL MAXIMUM RESIDUE LIMITS
An updated list of national MRLs was provided to the Meeting by
the Netherlands (Netherlands 1982).
Commodity MRL (mg/kg)
Strawberries 0.05
Other fruits 0.1
Leafy vegetables 0.2
Other vegetables 0.05
Potatoes 0.05
Meat and milk 0.05
Other food commodities 0.05
APPRAISAL
The results of supervised trials in Finland on greenhouse
cucumbers and tomatoes sprayed with deltamethrin at 2.1 and
1.0 mg a.i./plant respectively showed no measurable residues;
<0.05 mg/kg were found even at 1 day post-treatment, and therefore
the guideline level of 0.05 mg/kg would not be exceeded.
Additional data on the behaviour of deltamethrin residues on
stored wheat treated at 0.25, 0.50 and 1.0 mg/kg confirmed that no
measurable degradation occurs on the grain over storage periods of
more than a year. Measured values ranged from 0.1 - 0.16, 0.25 - 0.27
Table 2. Residues of Deltamethrin on Stored Wheat 1
Location Rates Part Interval (days) following last application
(Year) Applied of
(mg/kg) Sample 7 90 180 270
Greece 0.25 Grain 0.13 0.10 0.13 0.16
1980 0.50 Grain 0.25 0.25 0.27 0.27
(Days) 40/48 77 81 118/137 186 223/242 281 318/337 383/420
>1 year
Australia 1 A Grain 0.3 0.5 0.25 0.4
1980-1982 Bran 1.2 1.3 0.5 0.3
Flour 0.06 0.06 0.06 0.01
(white)
1 B Grain 0.2 0.5 0.4 0.35 0.2
Bran 0.85 1.1 1.4 0.75 1.2
Flour 0.06 0.08 0.07 0.01 0.06
(white)
1 C Grain 0.25 0.35 0.3 0.35
Bran 1.1 0.8 1.4 1.5
Flour 0.05 0.07 0.07 0.01
(white)
1 D Grain 0.15 0.4 0.3 0.45
Bran 1.3 1.2 1 1
Flour 0.06 0.06 0.07 0.02
(white)
1 E Grain 0.2 0.3 0.4 0.4 0.25
Bran 1.4 0.9 1.2 1.2 1.3
Flour 0.07 0.07 0.07 0.06 0.06
(white)
1 All data based on one treatment.
and 0.15 - 0.5 mg/kg respectively. Bran obtained from the stored wheat
treated at the 1 mg/kg level contained most of the residue (range
0.3 - 1.5 mg/kg) while the corresponding white flour contained the
least (range 0.01 - 0.07 mg/kg). Taking existing or possible use
patterns into consideration, these data support the previously
proposed guideline levels for cereal grains, wheat bran and wheat
flour.
Studies are still under way for determining residues occurring in
meat, milk and eggs arising from ectoparasite and stable treatments
and will be made available by 1984.
RECOMMENDATIONS
The previous guideline levels are converted to temporary maximum
residue limits on the basis of the newly recommended temporary ADI for
deltamethrin.
FURTHER WORK OR INFORMATION
Desirable (as available)
1. Results of planned or ongoing studies on residue behaviour on
stored crop commodities for the purpose of recommending new or
revising previous MRLs on such commodities (estimated date -
1983),
2. Results of planned or ongoing supervised animal studies on
residues in meat, milk and eggs resulting from ectoparasite and
stable treatments for the purpose of recommending new MRLs on
those commodities (estimated date - 1984).
REFERENCES
Finland Table on deltamethrin residues (official testing),
1982 (Personal communication)
Netherlands Tables of preharvest treatments and national MRLs.
1982 (Personal communication)
UniversitÚ de Montpellier. Residues determination in plants. Wheat
1981a (stored). Research Report FP-81.27.04/A.
UniversitÚ de Montpellier, Residues determination in plants. Wheat
1981b (stored). Research Report FP-82.18.03/A.
