
PESTICIDE RESIDUES IN FOOD - 1981
Sponsored jointly by FAO and WHO
EVALUATIONS 1981
Food and Agriculture Organization of the United Nations
Rome
FAO PLANT PRODUCTION AND PROTECTION PAPER 42
pesticide residues in food:
1981 evaluations
the monographs
data and recommendations
of the joint meeting
of the
FAO panel of experts on pesticide residues
in food and the environment
and the
WHO expert group on pesticide residues
Geneva, 23 November-2 December 1981
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
Rome 1982
DELTAMETHRIN
Explanation
Deltamethrin was first evaluated in 1980 when, in the absence of
an ADI, recommendations were made for guidelines for residue levels in
a number of commodities.
Additional information received since 1980 has been evaluated by
the Meeting.
DATA FOR THE ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Rat
Studies on rats to investigate uptake, distribution and excretion
of deltamethrin were conducted with deltamethrin labelled with 14C in
three positions (Ruzo et al 1978a). The labelled deltamethrin was
administered to male rats orally at levels of 0.64 to 1.6 mg/kg and
resulted in the elimination of deltamethrin and various metabolites
derived from its acid and alcohol fragments within 2 to 4 days.
Metabolites of the cyano substituent were eliminated more slowly,
especially from the skin and stomach, in the latter case owing to
temporary retention of thiocyanate, which was formed from released
cyanide. The excreted metabolites included: esters monohydroxylated at
the 2',4' and 5 positions of the alcohol moiety; 2,2-dimethyl-3-
(2,2,-dibromovinyl) cyclopropane carboxylic acid and its glucuronide
and glycine conjugates and a hydroxylated derivative of this acid,
with the hydroxymethyl group trans to the carboxyl and its
glucuronide; 3-phenoxybenzoic acid and its glucuronide and glycine
conjugates; 3-(4'-hydroxyphenoxy)benzoic acid and its glucuronide and
sulphate conjugate and 3-(2'-hydroxyphenoxy)-benzoic acid sulphate,
thiocyanate and 2-imino-thiazolidine-4-carboxylic acid. The trans
isomer of deltamethrin was also rapidly metabolized in rats.
Figure 1 illustrates metabolic pathways for deltamethrin in rats.
The principal mechanisms of metabolism are ester cleavage and
oxidation at the 4'-position of the alcohol moiety. Minor oxidation
sites are the 5 and 2' positions of the alcohol moiety and the methyl
group trans to the carboxyl. The ester metabolites are not conjugated,
but the corresponding acids undergo extensive conjugation at both the
* See Annex II for FAO and WHO documentation.
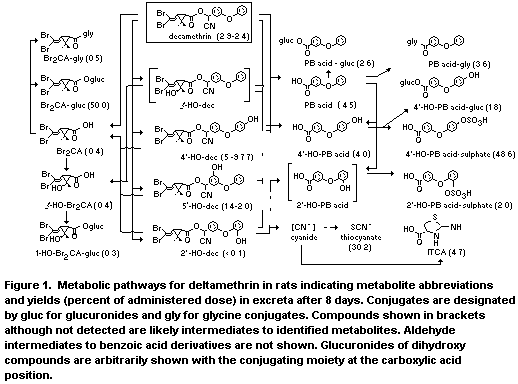 phenolic hydroxyl and carboxylic acid groups. The acid moiety is
rapidly excreted as the glucuronide, with smaller amounts as free and
as the glycine conjugate. The trans-hydroxymethyl derivative is also
excreted both free and as the glucuronide.
All major metabolites of the aromatic portion of the alcohol
moiety are rapidly excreted and probably arise from ester cleavage of
deltamethrin or its ester metabolites, conversion of the released
cyanohydrins to the aldehydes which rapidly yield the corresponding
acids and conjugates of these acids. Table 1 illustrates the
radiocarbon in the urine, faeces, carbon dioxide and tissues of rats
up to 8 days after oral administration of labelled deltamethrin.
TABLE 1. Radiocarbon in the urine, faeces, carbon dioxide, and
tissues of rats up to 8 days after oral administration
of 14Cv, 14Cai.e, alpha &: 14CN deltamethrin.
Labeling position
Sample analyzed 14Cva,b 14Calpha 14CN
Administered Dose, mg/kg
0.90 1.60 0.64
% of Administered Dose
Urine
0-1 day 45.1 67.6 10.4
1-2 days 5.6 3.0 8.1
2-4 days 2.7 2.1 11.4
4-8 days 1.0 1.0 13.0
Feces
Methanol extract
0-1 day 35.6 22.7 12.1
1-2 days 2.0 1.8 2.3
2-4 days 1.7 0.3 3.3
4-8 days 0.4 0.0 3.3
Unextractable, 0-8 days 4.4 0.4 14.7
14CO2, 0-2 days 0.0 0.0 0.0
Carcass and tissues, 8 days 1.5 1.1 21.4
TABLE 1. (con't)
Labeling position
Sample analyzed 14Cva,b 14Calpha 14CN
Tissue Residue at 8 Days, ppb of Decamethrin Equiv
Blood 12 89 103
Bone 12 11 57
Brain 4 20 5
Fat 59 182 94
Heart 8 9 41
Intestine
Large 9 5 77
Small 10 5 129
Kidney 8 10 66
Liver 12 38 66
Lung 4 5 73
Muscle 5 5 57
Skin 16 16 603
Spleen 2 5 49
Stomach 8 3 654
Testes 5 3 54
a With (1RS)-trans-[14Cv]decamethrin administered orally at
0.94 mg/kg, the 14C balance sheet at 8 days as percent of dose is
72.6% in urine (48.6% at 1 day, 62.3% at 2 days, and 71.2% at
4 days), 23.2% in the methanol extract of feces (12.3% at 1 day,
18.6% at 2 days, and 21.7% at 4 days), 3.0% in the unextractable
portion of feces (0-8 days), and 1.2% in carcass and tissues (all
individual tissues as above < 10 ppb decamethrin equivalents),
b With [14Cv]Br2CA administered orally at 3.74 mg/kg, the 14C
balance sheet at 8 days as percent of dose is 94.0% in urine (70% at
1 day, 82% at 2 days, and 91% at 3 days), 5.8% in feees (5.4% in methanol
extract), 0.0% 14CO2, and 0.2% in carcass and tissues. The tissue
residues are < 3 ppb decamethrin equiv except for liver which is
27 ppb.
The pathways involved in rat metabolism of deltamethrin isomers
are similar to those utilized for other pyrethroids in many segments
of the ecosystem (Elliott 1977; Gaughan et al 1977a, b).
The tissue distribution of toxic doses of 14C-acid,
14C-alcohol-, and 14C-cyano labelled deltamethrin after i.v.
administration to rats has been studied (Gray and Richard 1981). All
three radiolabelled preparations were found in every tissue examined
1 min. after injection. Peak CNS levels were achieved within 1 to
5 min. but did not correspond to the onset of choreoathetosis. In the
majority of tissues examined, the levels of deltamethrin label were
similar, proportionately to those found after administration of
alcohol labelled cismethrin. A major exception was the CNS, which
contained approximately 20% of the anticipated level of radiolabel,
suggesting that the CNS threshold for deltamethrin was approximately
0.5 to 1.0 nmol/g. Progressive accumulation of radioactivity in the
erythrocytes fraction of the blood was observed following
administration of cyano-labelled deltamethrin; it was suggested that
this may contribute to the selective retention of 14C with
radiolabelled preparations.
Mouse
Studies on mice to determine oxidative, hydrolytic and
conjugative reactions were conducted with (14Calpha) and (14Cv)
labelled deltamethrin (Ruzo et al 1979). The labelled deltamethrins
were orally administered to two mice each at 3.1 and 3.6 mg/kg
respectively and the animals sacrificed 6 h later. The 14C label was
rapidly and almost completely excreted, with little tissue retention
after 8 days (Table 2).
Deltamethrin metabolism in mice involved four sites of oxidative
attack (trans-methyl group of the acid moiety and 2', 4' and 5
positions of the alcohol moiety, hydrolysis and a variety of
conjugation process) (Figure 2). Mice excrete less unmetabolized
deltamethrin than rats (Ruzo et al 1978) suggesting more efficient
absorption and/or metabolism. Mice produce considerable amounts of the
trans-, 2'- and 5'-hydroxy derivatives, whereas rats hydroxylate
deltamethrin predominantly at the 4'-position.
The acid moiety is rapidly excreted as the glucuronide with
smaller amounts free and as the glycine conjugate. The trans hydroxy
methyl derivative is also excreted free, as the glucuronide and as the
sulphate conjugate; the latter was not detected in rats.
Metabolites in mouse, but not rat, excreta include: 3-2(2,2-
dibromovinyl)-2-trans-hydroxymethyl-2-methylcyclopropanecarboxylic
acid sulphate; 3-phenoxybenzaldehyde; 3-phenoxybenzyl alcohol and its
glucuronide; glucuronides of 4'-hydroxy-3-phenoxybenzyl alcohol and
5-hydroxy-3-phenoxybenzoic acid and 3-phenoxybenzoyltaurine.
Intraperitoneal (i.p.) administration of deltamethrin to the mouse
yielded the same metabolites, but in different ratios. Deltamethrin is
detoxified in mice by both oxidative and hydrolytic processes.
TABLE 2. Radiocarbon in the urine, faeces, and tissues of mice up
to 8 days after oral administration of 14Cv, 14Ca
i.e, alpha & 14C deltamethrin.
labeling position
sample analyzed 14Cv 14Calpha 14CN
Administered Dose, mg/kg
4.4 1.7 2.2
% of Administered Doseb
urine
0-1 day 28.9 40.5 13.1
1-2 days 13.8 5.0 8.5
2-5 days 12.0 15.7 10.3
5-8 days 2.6 3.9 3.6
feces
methanol extract
0-1 day 19.0 18.6 20.8
1-2 days 9.5 4.0 16.4
2-5 days 72 5.1 4.5
5-8 days 0.9 0.4 2.2
unextractable, 0-8 days 5.1 5.8 13.8
carcass and tissues, 8 days 1.0 1.0 6.8
Tissue Residue at 8 Days, ppb of Decamethrin Equivc
blood 4 5 54
brain 17 0 4
fat 273 115 79
kidney 39 27 20
liver 47 20 19
skin 77 3 778
stomach 28 9 175
a Values at 3 days are given in Table 1 of the microfiche
supplement to this report.
b 14CO2 values are <0.1% for 0-2-day samples with each labeled
preparation.
c Comparable values for bone, heart, intestine, lung, muscle,
spleen, and testes are <60 ppb.
phenolic hydroxyl and carboxylic acid groups. The acid moiety is
rapidly excreted as the glucuronide, with smaller amounts as free and
as the glycine conjugate. The trans-hydroxymethyl derivative is also
excreted both free and as the glucuronide.
All major metabolites of the aromatic portion of the alcohol
moiety are rapidly excreted and probably arise from ester cleavage of
deltamethrin or its ester metabolites, conversion of the released
cyanohydrins to the aldehydes which rapidly yield the corresponding
acids and conjugates of these acids. Table 1 illustrates the
radiocarbon in the urine, faeces, carbon dioxide and tissues of rats
up to 8 days after oral administration of labelled deltamethrin.
TABLE 1. Radiocarbon in the urine, faeces, carbon dioxide, and
tissues of rats up to 8 days after oral administration
of 14Cv, 14Cai.e, alpha &: 14CN deltamethrin.
Labeling position
Sample analyzed 14Cva,b 14Calpha 14CN
Administered Dose, mg/kg
0.90 1.60 0.64
% of Administered Dose
Urine
0-1 day 45.1 67.6 10.4
1-2 days 5.6 3.0 8.1
2-4 days 2.7 2.1 11.4
4-8 days 1.0 1.0 13.0
Feces
Methanol extract
0-1 day 35.6 22.7 12.1
1-2 days 2.0 1.8 2.3
2-4 days 1.7 0.3 3.3
4-8 days 0.4 0.0 3.3
Unextractable, 0-8 days 4.4 0.4 14.7
14CO2, 0-2 days 0.0 0.0 0.0
Carcass and tissues, 8 days 1.5 1.1 21.4
TABLE 1. (con't)
Labeling position
Sample analyzed 14Cva,b 14Calpha 14CN
Tissue Residue at 8 Days, ppb of Decamethrin Equiv
Blood 12 89 103
Bone 12 11 57
Brain 4 20 5
Fat 59 182 94
Heart 8 9 41
Intestine
Large 9 5 77
Small 10 5 129
Kidney 8 10 66
Liver 12 38 66
Lung 4 5 73
Muscle 5 5 57
Skin 16 16 603
Spleen 2 5 49
Stomach 8 3 654
Testes 5 3 54
a With (1RS)-trans-[14Cv]decamethrin administered orally at
0.94 mg/kg, the 14C balance sheet at 8 days as percent of dose is
72.6% in urine (48.6% at 1 day, 62.3% at 2 days, and 71.2% at
4 days), 23.2% in the methanol extract of feces (12.3% at 1 day,
18.6% at 2 days, and 21.7% at 4 days), 3.0% in the unextractable
portion of feces (0-8 days), and 1.2% in carcass and tissues (all
individual tissues as above < 10 ppb decamethrin equivalents),
b With [14Cv]Br2CA administered orally at 3.74 mg/kg, the 14C
balance sheet at 8 days as percent of dose is 94.0% in urine (70% at
1 day, 82% at 2 days, and 91% at 3 days), 5.8% in feees (5.4% in methanol
extract), 0.0% 14CO2, and 0.2% in carcass and tissues. The tissue
residues are < 3 ppb decamethrin equiv except for liver which is
27 ppb.
The pathways involved in rat metabolism of deltamethrin isomers
are similar to those utilized for other pyrethroids in many segments
of the ecosystem (Elliott 1977; Gaughan et al 1977a, b).
The tissue distribution of toxic doses of 14C-acid,
14C-alcohol-, and 14C-cyano labelled deltamethrin after i.v.
administration to rats has been studied (Gray and Richard 1981). All
three radiolabelled preparations were found in every tissue examined
1 min. after injection. Peak CNS levels were achieved within 1 to
5 min. but did not correspond to the onset of choreoathetosis. In the
majority of tissues examined, the levels of deltamethrin label were
similar, proportionately to those found after administration of
alcohol labelled cismethrin. A major exception was the CNS, which
contained approximately 20% of the anticipated level of radiolabel,
suggesting that the CNS threshold for deltamethrin was approximately
0.5 to 1.0 nmol/g. Progressive accumulation of radioactivity in the
erythrocytes fraction of the blood was observed following
administration of cyano-labelled deltamethrin; it was suggested that
this may contribute to the selective retention of 14C with
radiolabelled preparations.
Mouse
Studies on mice to determine oxidative, hydrolytic and
conjugative reactions were conducted with (14Calpha) and (14Cv)
labelled deltamethrin (Ruzo et al 1979). The labelled deltamethrins
were orally administered to two mice each at 3.1 and 3.6 mg/kg
respectively and the animals sacrificed 6 h later. The 14C label was
rapidly and almost completely excreted, with little tissue retention
after 8 days (Table 2).
Deltamethrin metabolism in mice involved four sites of oxidative
attack (trans-methyl group of the acid moiety and 2', 4' and 5
positions of the alcohol moiety, hydrolysis and a variety of
conjugation process) (Figure 2). Mice excrete less unmetabolized
deltamethrin than rats (Ruzo et al 1978) suggesting more efficient
absorption and/or metabolism. Mice produce considerable amounts of the
trans-, 2'- and 5'-hydroxy derivatives, whereas rats hydroxylate
deltamethrin predominantly at the 4'-position.
The acid moiety is rapidly excreted as the glucuronide with
smaller amounts free and as the glycine conjugate. The trans hydroxy
methyl derivative is also excreted free, as the glucuronide and as the
sulphate conjugate; the latter was not detected in rats.
Metabolites in mouse, but not rat, excreta include: 3-2(2,2-
dibromovinyl)-2-trans-hydroxymethyl-2-methylcyclopropanecarboxylic
acid sulphate; 3-phenoxybenzaldehyde; 3-phenoxybenzyl alcohol and its
glucuronide; glucuronides of 4'-hydroxy-3-phenoxybenzyl alcohol and
5-hydroxy-3-phenoxybenzoic acid and 3-phenoxybenzoyltaurine.
Intraperitoneal (i.p.) administration of deltamethrin to the mouse
yielded the same metabolites, but in different ratios. Deltamethrin is
detoxified in mice by both oxidative and hydrolytic processes.
TABLE 2. Radiocarbon in the urine, faeces, and tissues of mice up
to 8 days after oral administration of 14Cv, 14Ca
i.e, alpha & 14C deltamethrin.
labeling position
sample analyzed 14Cv 14Calpha 14CN
Administered Dose, mg/kg
4.4 1.7 2.2
% of Administered Doseb
urine
0-1 day 28.9 40.5 13.1
1-2 days 13.8 5.0 8.5
2-5 days 12.0 15.7 10.3
5-8 days 2.6 3.9 3.6
feces
methanol extract
0-1 day 19.0 18.6 20.8
1-2 days 9.5 4.0 16.4
2-5 days 72 5.1 4.5
5-8 days 0.9 0.4 2.2
unextractable, 0-8 days 5.1 5.8 13.8
carcass and tissues, 8 days 1.0 1.0 6.8
Tissue Residue at 8 Days, ppb of Decamethrin Equivc
blood 4 5 54
brain 17 0 4
fat 273 115 79
kidney 39 27 20
liver 47 20 19
skin 77 3 778
stomach 28 9 175
a Values at 3 days are given in Table 1 of the microfiche
supplement to this report.
b 14CO2 values are <0.1% for 0-2-day samples with each labeled
preparation.
c Comparable values for bone, heart, intestine, lung, muscle,
spleen, and testes are <60 ppb.
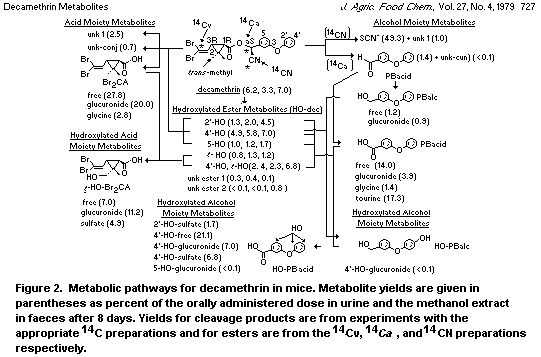 TABLE 3. Acute toxicity of deltamethrin
LD50
Species Sex Route (mg/kg bw) References
Mouse M+F iv 41 Glomot and Chevalier 1976c
M ip 181 ibid 1976b
M ip 1712 ibid 1976b
M oral 211 ibid 1976a
M oral 332 ibid 1976a
F io 121 ibid 1976b
F ip 1662 ibid 1976b
F oral 191 ibid 1976a
F oral 342 ibid 1976a
Rat M+F iv 311 ibid 1976c
M ip 241 ibid 1976b
M ip 2092 ibid 1976b
M oral 671 ibid 1976a
M oral 1282 ibid 1976a
M+F inhal. 0.63 Coombs and Clark 1978
M+F dermal <29404 Kynoch et al 1979
F ip 251 Glomot and Chevalier 1976b
F ip 1862 ibid 1976b
F oral 861 ibid 1976a
F oral 1392 ibid 1976a
Rabbit M+F dermal >20005 Clair 1977
Dog (beagle) M+F oral >3001 Glomot et al 1977
M+F oral >3006 ibid
Chicken oral ca 10002 Roussel Uclaf 1976a
Hen (adult) F oral >25002 Ross et al 1978
F oral >50007 ibid
Mallard duck oral >46407 Beavers and Fink 1977a
Game duck oral >40006 Roussel Uclaf 1976b
Grey Partridge M+F oral >18007 ibid
Red Partridge M+F oral >30007 ibid
1 Suspended in polyethylene glycol 200; 2 dissolved in sesame oil;
3 expressed for LC50 in mg dust/m3 air; 4 60% w/v suspension in aqueous
methyl-cellulose in occlusion; 5 as paste in PEG 400 on occlusion;
6 in capsules or cachets; 7 dissolved in maize oil.
Pretreatment with either the oxidase inhibitor piperonyl butoxide or
the esterase inhibitor S,S,S-tributyl phosphorotrithioate (DEF)
further increased deltamethrin in mice, reduced its rate of in vivo
metabolism by hydroxylation and hydrolysis, reduced the rate of
product excretion and elevated deltamethrin levels in fat and brain
(Ruzo et al. 1979; Soderlund and Casida 1977).
Deltamethrin-hydrolysing esterases are generally sensitive to
organo-phosphate inhibition (e.g. TEPP inhibition of hydrolysis by
all tissue preparations except stomach). The two major factors
contributing to the rapid detoxification of decamethrin are: a) the
relevant esterases present in many tissues and the oxidases in, at
least, liver microsomes, and b) many molecular sites that are
susceptible to metabolic attack (Ruzo et al 1979).
There are large differences in the toxicity of deltamethrin to
mice, depending on the route of administration, the carrier vehicle
and previous exposure to piperonyl butoxide or DEF. Irrespective of
these factors, there appears to be a critical concentration of
deltamethrin in brain that correlated with the onset of tremors or the
time of death. Additionally, direct intracerebral administration of
deltamethrin at approximately this brain level gave a similar
poisoning effect. The brain appears to be a primary target in
deltamethrin poisoning of mice (Ruzo et al 1979).
Rat
The signs of toxicity observed in rats after deltamethrin
administration included salivation and choreoathetosis (a writhing
type of toxicity) (Barnes and Verschoyle 1974; Ray 1980; Ray and
Cremer 1979; Verschoyle and Aldridge 1980) and have been designated as
the CS syndrome of toxicity (Verschoyle and Aldridge 1980).
Rats injected i.v. with deltamethrin showed muscular
contractions, piloerection, respiratory defects, convulsions and
paresis of the hind quarters immediately following treatment. Death
occurred within 10 min. After 24 h only piloerection was visible;
after 48 h surviving animals showed normal behaviour. After i.p.
injection immediate tremors, convulsions, prostration and cyanosis
were observed. After 48 h surviving animals showed normal behaviour.
Gavage with deltamethrin shortly after dosing induced motor
incoordination, convulsions and respiratory defects. After 24 and
48 h, hypomotility and convulsions were still observed. After 3 days
surviving animals showed normal behaviour (Glomot and Chevalier
1976a,b,c).
In an earlier study, the acute i.v. lethal dose in young adult
female rats was 2 to 2.5 mg/kg when administered as a solution in
glycerol formal (Barnes and Verschoyle 1974). Signs of poisoning
included excessive salivation without lacrimation, rapidly followed by
continuous jerking movements of the limbs, occasionally progressing to
convulsions. The toxic effects were rapid in onset and brief in
duration. Death occurred in some animals within 12 min of dosing while
survivors were generally symptom-free within 6 h. In a more recent
study (Kavlock et al 1979, see Table 4), the acute LD50 for adult
female rats was 31 mg/kg by the oral route and 4 mg/kg by the i.v.
route. The LD50 was observed to be sex and age dependent, with higher
values found for weanlings and males. Initial signs of deltamethrin
poisoning included profuse salivation and convulsive movements;
weakness, dyspnoea, anorexia and staining of the fur were observed
beyond the first day following compound administration.
Rats (7 males and 7 females/group) were exposed (whole body)
during 6 h to aerosols of a.i., aerosol concentration being 0.049,
0.43, 0.54 and 0.72 g/m3. The aerosol contained 66 to 86% of
particles < 5.5 µ. During exposure, hyperactivity and dose-dependent
increase in grooming and irritation were observed. The animals were
hypersensitive to touch and noise and showed uncoordinated movements.
During the observation period of 14 days following exposure, all
animals except those from the lowest dose group developed poor motor
coordination and hypersensitivity. At the end of the period all
animals were recovered to normal. In these groups the body weight gain
and food intake was depressed during 3 days following exposure. In
rats (4 of control and of highest dose group) killed immediately after
exposure, the stomach and small intestine were gas filled. In treated
rats, as result of exposure, massive haemorrhage and oedema in lungs
were observed. Stomachs were filled with gas, blood and mucus. White
deposits were visible in the trachea. In animals killed after the
observation period, a dose-dependent increase of lung degeneration
(coloured spots to congestion) was observed (Coombs and Clark 1978).
Mouse
Mice injected i.v. with deltamethrin showed intense tremors,
convulsions and ataxia immediately after administration. Also,
tachycardia and respiratory defects were observed at higher doses.
Surviving animals showed normal appearance after 4 to 5 h. Immediately
after i.p. injection, jumping movements, slight convulsions and
prostration, ptosis, tail hypertonicity and cyanosis were observed.
Surviving animals appeared normal after 72 h. Animals gavaged with
deltamethrin showed muscular stiffening and convulsions 1 h after
dosing. After 24 h hypermotility, stereotype movements of the head,
tachycardia, hypertonicity of the tail and a few convulsions were
noted. Normal behaviour and appearance were seen after 48 h (Glomot
and Chevalier 1976a,b,c).
TABLE 4. Acute toxicity of deltamethrin to rats1
95 % Confidence Lowest dose Minimum Toxic
Route Strain Sex Age LD50 limit of tested Dose
mg/kg LD50 (mg/kg) (mg/kg) (mg/kg)
Oral2 Sherman M Adult 52 46 - 58 30.00 306
Oral2 Sherman F Adult 31 29 - 34 5.00 107
Oral2 Sherman F Weanling 50 42- 60 7.50 157
Intravenous3 Sherman F Adult 4 2.9-5.3 1.57 1.576
Intravenous3 Sherman F Weanling 1.8 1.5-2.1 0.78 0.76
Dermal4 Sherman F Adult >800 --- 800:0 --- 8
Inhalation5 Sprague-Dawley M Adult 940 mg/m3 --- ---
Inhalation5 Sprague-Dawley F Adult 785 mg/m3 --- ---
1 Reference-Kavlock et al 1979;
2 Dissolved in peanut oil and administered via gavage at a rate of 5 ml/kg bw;
3 Dissolved in acetone and administered via a single injection into tail vein of rat at 0.313 ml/kg bw;
4 Dissolved in xylene and administered at a rate of 3.2 ml/kg bw;
5 Aerosols generated from 10% DMSO solution. Based on exposure time of 120 to 150 min.;
6 Moderate to severe salivation and convulsions;
7 Mild salivation;
8 No signs of toxicity at 800 mg/kg.
Rabbit
Rabbits (10 males and 10 females) were treated with 2 g
deltamethrin in 2 ml PEG 400/kg bw on 80 cm3 shaven skin for 24 h on
occlusion. The animals were observed for 14 days. One animal showed
obvious erythema and another congested skin. No weight changes or
abnormal behaviour were observed. On histological observation of
liver, kidneys and skin, small changes were observed that were common
for this strain of rabbits and not related to treatment (Clair 1977).
Dog
Dogs showed, at non-lethal doses, transient hyperexcitability,
akynesia, vomiting and stiffness of the hind legs (Glomot et al
1977).
Birds
Oral administration of a.i. to hen, game duck or partridge
produced no distinct symptoms except a small initial weight loss
occasionally. In chickens diarrhoea, convulsions and jerky movements
of the head were observed. Mallard ducks, at lethal doses, exhibited
signs of neurotoxic effects, which included ataxia, loss of
equilibrium and loss of coordination. The effect was dose-related: at
lower dose levels only some hyperexcitability and imbalance were
observed (Beavers and Fink 1977a).
Short-term studies
Rat
Male and female weanling Sprague-Dawley rats (20/sex/group)
were daily dosed by oral gavage with 0, 0.1, 1.0, 2.5 or 10.0 mg
deltamethrin in PEG 200/kg bw/day for 13 weeks (Hunter et al 1977).
No treatment-related effects on food and water consumption, mortality,
urinalysis and haematology were observed. Neurological examinations
and ophthalmoscopy revealed no abnormalities. In the 10 mg/kg bw
group, some hypersensitivity was observed in week 6 in males. Body
weight gain among males receiving deltamethrin was significantly lower
at 2.5 and 10 mg/kg/day. The body weight of the females was not
affected by the treatment. The male animals of the 1 mg/kg group
showed a tendency to a reduced body weight gain. In the females, blood
glucose and urea were significantly increased in week 6, but no
significant changes occurred in week 12 or with other blood chemistry
parameters. No clear effects were noted on the weights of the organs.
Gross and microscopic examination of a variety of tissues and organs
showed no treatment-related alterations.
Following the 13-week dosage period, 5 males and 5 females per
group were allowed to recover for 4 weeks. Autopsy was performed at
the end of this recovery period. The body weights of all previously
treated animals appeared not to be different from controls. Thyroid
weights were not dose-related but were increased in males. This
increase was significantly higher in the 1.0 mg/kg and 10.0 mg/kg
group. Marginal no-effect level was 1 mg/kg bw (Hunter et al 1977).
Dog
Male and female beagle dogs, 3-5/sex/group, 25 weeks of age, were
daily dosed orally with 0, 0.1, 1.0, 2.5 and 10.0 mg deltamethrin in
PEG 200/kg bw/day in gelatin capsules. Dosage was continued for 13
weeks, followed by a recovery period of 20 weeks for 2 dogs/sex from
the groups receiving 1.0, 2.5 and 10.0 mg/kg bw/day (Chesterman
et al 1977). Observations were made on behaviour, mortality, body
weight and food and water consumption. Haematology, blood chemistry,
urinalysis and six channel EEG-analysis were performed at week 0, 6
and 1, and ophthalmoscopy at week 0, 5 and 12. Special attention was
paid to the muscular and nervous systems.
Liquid faeces were associated with all groups of treated dogs
throughout the dosing period. All groups of animals receiving
deltamethrin gained less weight than the controls. The effects were
not strictly dose related. The dogs from the control group left
smaller quantities of the offered food than those of the treated
groups, whereas the water consumption was not dose-relatedly decreased
in all treated groups.
Dilation of the pupils was seen to occur in the dogs receiving
2.5 and 10.0 mg/kg/day. The sign was first seen 4 to 7 h after dosing
and persisted throughout the day. They reacted normally prior to
dosing on the following day. The incidence of vomiting was dose-
related increased in all treated groups, except the 0.1 mg dose level.
The incidence decreased in all the animals affected as the dosing
period progressed. In the highest dose group, unsteadiness, body
tremors and jerking movements were seen, particularly in males in
weeks 2, 3 and 4. These effects were reduced during week 5 to 9 and
were seen only in one dog in week 13. Excessive salivation was seen
initially but diminished during dosing period. After 5 and 12 weeks,
depression of the gag reflex was noted in a proportion of animals in
all treated groups. Depression of the patellar reflex was observed in
all treated groups except the dogs administered 0.1 mg/kg. In the
animals given 1 or 2.5 mg/kg/day, exaggeration of the patellar reflex
was noted only after 5 weeks. Some animals of all treated groups
showed variations in the flexor reflex. A high proportion of the
animals had depression of the hind limb tactile placing reaction.
At dosage levels of 2.5 and 10 mg/kg/day, deltamethrin caused
modification of the EEG pattern in some animals following 12 weeks
administration. Histopathological evaluations of tissues and organs,
including nervous system and muscle, did not reveal abnormalities that
could be related to dosage with the test compound. During recovery the
gag reflex continued to be depressed, whereas exaggeration of the
patellar reflex was still seen in some dogs that had previously
received 1.0 mg/kg/day. One animal continued to show an abnormal EEG
pattern (Chesterman et al 1977).
Quail and duck
Deltamethrin was given in the diet to 14-day old mallard ducks
for 5 days, at doses of 0, 464, 1 000, 2 150, 4 640 and 10 000 mg/kg
feed. The number of animals per group was 10. There was some mortality
in the two highest dose groups. Birds of the highest dose group showed
ataxia and loss of coordination. There were dose-related decreased
weight gain and food consumption (Beavers and Fink 1977a).
An identical experiment was performed with 14-day old bobwhite
quails (10 animals/group). The effects were the same as in the mallard
ducks, except there was no mortality (Beavers and Fink 1977b).
Long-term studies
Rat
Male and female Charles River CD rats (90/sex/group) were dietary
fed with 0 (control), 2, 20 and 50 mg deltamethrin/kg in the diet for
two years. Sixty males and 60 females were used in a second control
group. After 6, 12 and 18 months of compound administration, 10
animals/sex/group were sacrificed, except for the second control group
(Goldenthal et al 1980b).
No changes in general behaviour and appearance in relation to
compound treatment were recorded. Survival was similar in control and
treated rats (50 to 67%). Rats at 50 mg/kg feed gained slightly less
weight than control rats, whereas the food consumption was essentially
the same. Ophthalmoscopic findings generally were similar for control
and treated rats. No haematological and biochemical parameters were
changed in a biologically significant way in relation to treatment at
any time, except for a decreased SGPT activity at 6 months in the mid-
and high-dose groups. No treatment-related effects were observed on
organ weights. The macroscopy and microscopy findings were common for
the animals of the species and strain, except for a slightly increased
incidence of axonal degenerations in sciatic, tibial and/or plantar
nerves at 18 months in the 20 and 50 mg/kg groups. Evaluation of
incidence and/or severity of these degenerations at termination of the
study was obscured by the age of the animals.
Seven interstitial cell adenomas were observed in the testes of
the 50 mg/kg feed group compared to 0 and 4 in the two control groups.
Only from some animals of the 2 and 20 mg/kg groups were some organs
and tissues, including the testes, studied histopathologically.
Evaluation of a possible dose-response effect on the testes was
therefore not possible. The no-effect level was suggested to be
2 mg/kg (Goldenthal et al 1980b).
Mouse
Male and female Charles River CD-1 mice (80/sex/group) were fed
dosage levels of 0(control), 1, 5, 25 and 100 mg deltamethrin/kg in
the diet for 24 months. In a second control group 60 mice/sex were
used. After 12 and 18 months 10 mice/sex/group, except for two only in
the control group, were sacrificed.
There were no clear effects related to the administration of
deltamethrin on general behaviour, mortality, body weight and food
consumption. Blood chemistry, haematology and urine analysis
parameters were normal after 12, 18 and 24 months. Increases or
decreases in absolute and/or relative organ weights occurred in a few
organs at each dosage level at any time of sacrifice. Microscopic
examination of tissues did not reveal any lesions indicative of a
compound-related effect. The tumour incidence was unaffected by
deltamethrin administration. The no-effect level was suggested to be
100 mg/kg (Goldenthal et al 1980a).
Studies designed to investigate the potential carcinogenic
effects of deltamethrin have been initiated (Cabral 1981). Groups of
C57B1 mice have been administered orally 0-8 mg/kg bw deltamethrin in
arachis oil.
Dog
Deltamethrin (RU 22974) dissolved in maize oil was administered
in the diet to 64 beagle dogs at dosage levels of 1, 10 and 40 ppm for
24 months. This corresponds to 0.025, 0.25 and 1 mg/kg bw respectively
(IRDC 1980). Eight males and eight females were used at each dosage
level and in a control group. Individual body weights and food
consumption values were determined weekly. Ophthalmic, haematologic,
biochemical and urinalysis examinations were conducted during the pre-
test period at 6, 12, 18 and 24 months of the study. Neurological
exams were conducted at approximately 1 year and before termination.
These later parameters included: for cranial nerves and segmental
nerves, tests for postural reactions, placing reactions and hopping
reactions.
No signs of overt toxicity were observed in any of the dogs. Body
weights and food consumption values were similar for control and
treated dogs. No compound-related effects were observed during the
ophthalmoscopic and physical examinations. Although there were some
randomly statistically significant differences between the control and
other dose groups in the haematologic and biochemical tests, there
were not any physiologically significant changes observed at any
interval in the study. Two treated and two control animals died during
the study.
No compound-related gross or microscopic changes were observed in
the surviving dogs that were sacrificed and necropsied. Inflammatory,
degenerative and proliferative changes described were spontaneous in
nature or related to theoestrous phase of the menstrual cycle and
unrelated to compound administration.
On the basis of the results from this study, it was concluded
that the no-effect level is 40 ppm in the diet (equivalent to
1 mg/kg bw/day) administered RU 22974 over a 2-year period (IRDC
1980).
Special studies on primary irritation
Cutaneous irritation
Male albino rabbits (12/group) weighing 2.5 to 3.5 kg were
administered 0.5 g deltamethrin to either shaven intact or abraded
skin. The occlusive patch was fixed on the skin for 23h. Scoring for
erythematous and oedematous lesions occurred 1 h and 49h after removal
of the patches. Technical deltamethrin, 98% a.i., showed no irritant
effect (Coquet 1976a).
Ocular irritation
Deltamethrin (0.1 g/animal) was administered into the
conjunctival sac of the eye of 6 male albino rabbits, weighing 2.5 kg,
with or without rinsing 60 sec. after instillation. Observations for
conjunctival lesions, chemosis, discharge, conjunctival enanthema,
opacity and affected cornea were made 1 h, 24h, 2,3,4 and 7 days
following instillation. Deltamethrin showed, both with and without
rinsing, transient irritating effects (Coquet 1976b).
Special studies on sensitization
Deltamethrin (0.5 g/animal) was applied topically to the skin of
albino guinea pigs with a 2-day interval for 3 weeks, and once at the
start of the 4th week. The preparation was covered with an occlusive
patch for 48h. On days 1 and 10 the guinea pigs received an
intradermal injection of 0.1 ml of Freund's adjuvant. The animals were
challenged 12 days after the last application with 0.5 g deltamethrin.
The macroscopic and histological examination did not reveal evidence
of sensitization (Guillot and Guilaine 1977).
Special studies on reproduction
Rat
Groups of 10 female and 10 male Charles River rats were fed
deltamethrin in the diet at 0, 2, 20 and 50 mg/kg and mated to begin a
three-generation, 2 litter (first generation, 3 litter) standard
reproduction study. Parental body weights and food consumption were
recorded during the study. After weaning of the second litter, the
survival parental rats were sacrificed and necropsied. Five male and
5 female pups of the F3b were necropsied. No changes in general
behaviour or survival of parental rats or pups relevant to the test
material were observed. The body weight of F0 males of the 50 mg/kg
group was decreased from week eleven onwards. There was some slight
decreases in mean food consumption of the parental F1 male rats in
the 50 mg/kg feed group.
The basic reproduction indices (fertility, gestation, lactation,
viability and litter size) were not affected by the treatment.
However, the mean pup weight was affected in some litters; especially
that of the 50 mg/kg group was slightly decreased in comparison to the
controls. Gross external examination revealed no abnormalities. No
gross or microscopic lesions of treatment-related significance or
significant effects on the organ weights of the F3b generation were
observed (Wrenn et al 1980).
Special studies on teratogenicity
Mouse
Mated female Swiss CDI.SPF mice (24/group) were given orally
deltamethrin dissolved in sesame oil at dose levels of 0, 0.1, 1 and
10 mg/kg bw/day during days 6 to 17 of pregnancy. The animals were
necropsied on day 18 of gestation. No teratogenic effects could be
detected. Total implantation sites, foetal losses, living foetuses and
examinations of skeletal tissue were normal. Minor embryotoxic effects
were observed, e.g. dose-dependent decrease of average foetal weight
and delayed ossifications at all dose levels tested (Glomot and
Vannier 1977).
Technical deltamethrin (Roussel UCLAF), dissolved in corn oil was
administered to CD-1 mice by gastric intubation at doses of 12.0, 6.0,
3.0 or 0 mg/kg during days 7 to 16 of gestation (Kavlock et al
1979). Maternal weight on day 6 was used for calculation of doses and
intubation was 0.2 ml; control animals received vehicle alone. Animals
were sacrificed on day 16 of gestation. Administration of deltamethrin
to pregnant mice resulted in a dose-related (p < 0.001) reduction in
maternal weight gain during pregnancy. Pregnant dams in the high
dosage group (12.0 mg/kg/day) gained 58% less weight than did those in
the control group. No dose-related occurrences of maternal mortality
were observed, but most animals in the high dosage group and some in
the middle dosage group became convulsive soon after dosing. No
effects were observed on the number of implantation sites, foetal
mortality, or foetal weights, or in the number of sternal and caudal
ossification centers. A significant (p < 0.01) dose-related increase
in the occurrence of supernumerary ribs was observed. No other dose-
related skeletal or visceral anomalies were observed in deltamethrin-
treated mouse foetuses. No evidence of teratogenic activity was found
in mice at dose levels that produced maternal toxicity (Kavlock
et al 1979).
Rat
Mated female Sprague-Dawley rats (24/group) were administered
orally 0, 0.1, 1 and 10 mg deltamethrin/kg bw/day during days 6 to 18
of pregnancy. Examination occurred on day 21 of gestation. Twelve
females in the control and 10 mg/kg bw groups were allowed to deliver.
There were no effects on the reproduction or teratogenicity parameters
examined, with the exception of a slight delayed ossification in the
highest dose level (Glomot and Vannier 1977).
Technical deltamethrin (Roussel UCLAF) dissolved in maize oil was
administered to Sprague-Dawley rats by gastric intubation at doses of
5.0, 2.5, 1.25 or 0 mg/kg during days 7 to 20 of gestation (Kavlock
et al 1979), Maternal weights on day 6 were used for the calculation
of doses and intubation volume was 0.2 ml; control animals received
the vehicle alone. Rats were sacrificed on day 21 of gestation.
Administration of deltamethrin to pregnant rats resulted in a dose-
related reduction (p > 0.01) in maternal weight gain during
pregnancy, with animals in the high dosage group (5.0 mg/kg) gaining
only 80% of the control value. This dose produced a mild salivation
for up to 4 h after dosing in approximately 50% of the animals. No
effects were observed on the number of implantation sites, foetal
mortality, foetal weight or number of sternal and caudal ossification
centres. For post-natal studies, an additional group of pregnant rats
was housed individually and intubated from day 7 of gestation to day
15 of lactation with dosages of either 5.0, 2.5 or 0 mg/kg
deltamethrin dissolved in maize oil. No persistent toxicity was
observed in neonatal rats that received perinatal exposure to
deltamethrin (Kavlock et al 1979).
Rabbit
Groups of fifteen mated females received deltamethrin dissolved
in sesame oil at levels of 0, 1, 4 or 16 mg/kg bw/day from days 6 to
19 of pregnancy. Examination was carried out on day 28 of gestation.
The average foetal losses were not dose-related and increased at all
doses tested. This effect was mainly caused by a higher rate of
expelling traces. The average foetal weight in the highest dose group
was decreased. Some malformations (one animal with hydrocephalia, and
one with exencephalia and thoracogastroschisis) were observed in
2 animals of the highest dose level. A complementary study with
15 mg/kg bw/day was performed. In this study one foetus with spina
bifida and shortened tail was detected among 69 parent normal
foetuses. It was concluded that the malformations were within the
normal limits of the strain used and were not related to the
treatment, despite the occurrence at the highest dose level only
(Glomot and Vannier 1977, 1978).
Special studies on neurotoxicity
Adult hens (10/group) were gavaged with a single dose of 0, 500,
1 250 or 5 000 mg/kg bw deltamethrin suspended in maize oil or 0 and
1 000 mg/kg bw dissolved in sesame oil. Tri-o-cresylphosphate (TOCP)
(500 mg/kg bw) was used as positive control for delayed neurotoxicity.
During 21 days, observations were made on mortality, health,
neurotoxic signs and body weight.
In the TOCP group, 8 out of 10 animals died whereas only
2 mortalities were observed in the group dosed at 1 000 mg
deltamethrin/kg with sesame oil as the vehicle. Deltamethrin
induced no clinical, macroscopic or histological signs of delayed
neurotoxicity. TOCP-treated hens showed severe ataxia and degenerative
changes in the spinal cord and, occasionally, in the sciatic nerve
(Ross et al 1978).
Special studies on potentiation
Mice
Deltamethrin is hydrolysed in vitro by esterases in blood and in
brain, kidney, liver and stomach preparations of mice. Pre-treatment
of mice with oxidase inhibitor, piperonyl butoxide (PB), or esterase
inhibitor, S, S, S-tributylphosphorotrithioate (DEF), delayed
metabolism of i.p. administered deltamethrin. Using oxidase or
esterase inhibitor, different vehicles and different administration
routes, it was possible to induce similar toxic effects with a wide
range of deltamethrin doses (6 to 191 mg/kg bw). The different
treatments showed that PB or DEF made mice more sensitive to
deltamethrin (Ruzo et al 1978b).
Special studies in mutagenicity
Bacteria and yeast
In a growth inhibition test with Escherichia coli, deltamethrin
at levels of 1 250, 2 500 and 5 000 µg/ml DMSO (0.1 ml per plate) had
the same marginal inhibitor effect on the mother strains (W 3110 and
WP2) as on their mutants (p 3478 and CM 611). Chloramphenicol and
N-methyl N'-nitro-N-nitroguanidine (MNNG) were used as positive
controls and induced clear inhibition (Peyre et al 1980).
Deltamethrin was compared with MNNG, 9-aminoacridine,
2-nitrofluorene and 2-amino-anthracene for mutagenic activity in the
Ames test with Salmonella typhimurium, strains TA 1535, 100, 1537,
1528 and 98. The concentrations of deltamethrin used were 2, 10, 50,
200, 500, 1 000 and 5 000 µg/plate. Deltamethrin began to precipitate
at 200 µg/plate. The mean number of revertants was not influenced by
any concentration of deltamethrin in any strain with or without S9-mix
(metabolic activation), whereas the positive control mutagens produced
an increase of the number of spontaneous revertants (Peyre et al
1980).
In a similar experiment, deltamethrin (0.2, 2, 20, 200 and
400 µg/plate dissolved in DMSO) in the presence of activated
microsomal enzymes did not influence the number of revertants of
S. typhimurium strains TA 1535, 1537, 1538, 98 and 100.
2-Aminoanthracene, 3-methylcholanthrene, benzo(a)pyrene and acridine
orange showed mutagenic activity, whereas thio-TEPA was negative
(Fouillet 1976).
Deltamethrin at levels of 10, 50, 100, 500 and 1 000 µg/plate was
not mutagenic in assays with S. typhimurium strains TA 1535, 1537,
1538, 98 or 100 either in the presence or absence of a rat-liver
homogenate metabolic activation system (the positive controls were
2-anthramine and 9-aminoacridine) (Kavlock et al 1979).
Deltamethrin was not mutagenic in assays with E. coli WP2 when
tested at levels of 10, 50, 100, 500 and 1 000 µg/plate with or
without metabolic activation (the positive control was 2-anthramine)
(Kavlock et al 1979).
Deltamethrin was not mutagenic at levels of 1 to 5% when tested
with and without metabolic activation with Saccharomyces cerevisiae
(the positive control was 1, 2,3,4-diepoxybutane) (Kavlock et al
1979).
Mammalian cells
Deltamethrin dissolved in Cremophor oil (0, 0.4, 0.2, 1 and 5 mM,
10, 0.08, 0.4, 2 and 10% v/v respectively) in the presence of a
metabolic activation system increased the incidence of chromosome and
chromatid aberrations and SCEs, after incubation with Chinese hamster
ovary cells at 1 mMol. In the absence of S9-mix (metabolic
activation), no higher rate of aberration was observed.
It was shown that this increased incidence was due to a subtoxic
effect of some reaction product of Cremaphor oil and S9-mix.
Deltamethrin in Cremaphor oil without S9-mix and dissolved in DMSO
(1%) at levels of 0, 0.001, 0.01, 0.1 and 0.2 mM with or without
metabolic activation had no effect on the number of aberrations and
SCEs. Due to insolubility, no higher concentrations were tested
(Sobels et al 1978).
Animals
Mice, 3 males and 3 females per dose, were gavaged for two
consecutive days with 5 or 10 mg deltamethrin dissolved in sesame
oil/kg bw. Control mice were gavaged with 0.3 ml sesame oil. The
incidence of chromatid aberrations in bone marrow cells or of
micronuclei in polychromic erythrocytes did not show any significant
statistical difference in treated and control groups (Sobels et al
1978).
A single oral administration of 15 mg/kg deltamethrin was given
to Swiss mice. Groups of 2 animals were sacrificed every 3 h during a
24-h period. Several animals died after treatment. The incidence of
chromatid aberrations in the femoral bone marrow was low. There was no
consistent time-related trend in the distribution of these aberrations
(Sobels et al 1978).
Deltamethrin dissolved in sesame oil in groups of 9 to 13 male
mice dosed orally with 0 or 3 mg/kg bw for 7 days and 6 or 15 mg/kg bw
in a single dose showed no effect on the rate of pre- or post-
implantation losses after mating with 6 to 18 non-treated females.
The highest dose tested was toxic to the males, e.g. 7 out of 20
animals died shortly after treatment. Histological examination of
the testes of all animals revealed no abnormalities. Triethylene
thiophosphoramide (10 mg/kg bw), used as a positive control, reduced
considerably the rate of pregnancies in the second and third week and
increased the number of embryonal losses (Vannier and Glomot 1977).
Special studies on humans
Cutaneo-mucuous manifestations have been observed among plant
workers dermally exposed to technical deltamethrin or its
formulations. Initial lesions were tenacious and painful pruritus
(pricking sensation), especially observed after exposure to hot water
or on perspiration, followed by a blotchy local burning sensation with
blotchy erythema for about 2 days. Thereafter, slight and regular
desquamation, restricted to the contaminated area, occurred. The
cutaneous signs are sometimes accompanied by itching of the face
(mainly around the mouth) and/or rhinorrhoea or lacrimation. No other
symptoms related to exposure were observed (Husson 1978).
The effects described, which were observed before 1978 in a few
plant workers, have not been seen since a new plant was specifically
built for the entire automatization of deltamethrin production, which
does not involve workers being in direct contact with intermediates
and the final product. The only workers exposed are those involved in
the packaging of the technical product (>98% active ingredient) and
they are fully protected by special hermetic clothing (Glomot 1981).
At the level of formulation plants, steps have been taken to
avoid direct contact by workers involved in introducing technical
powder in the basic solvent. No toxic effects have been reported in
the past several years in these formulation plants, although a very
limited number of workers have complained about some irritating
effects. However, these were always transient and without any further
consequences (Glomot 1981).
The formulation which has been most frequently used for
deltamethrin application is the EC. The use of both EC and ULV
formulations corresponds in 1981 to a treated area covering several
million hectares in the world without any case of intoxication. The
majority of users are plant growers who apply the formulations in the
fields and are not in contact with the technical product per se
(Glomot 1981). A few cases of irritation problems have been reported
in some workers, due primarily to the use of the EC formulation. This
has been observed when treatments were made in orchards with high
trees needing high volumes of mixture (e.g. in South Africa) or under
greenhouses in confined spaces. In all cases, the reported problems
were not severe and could be treated using antihistaminic syrups or
with pomades based on cocaine derivatives. The irritation is most
probably enhanced by the presence of aromatic solvent, e.g. xylene, in
the EC formulation. Flowable experimental formulations without any
solvent did not cause irritating effects in human volunteers (Glomot
1981). No cases of neurological effects have been reported with
formulation workers nor have fatal intoxications been reported (Glomot
1981).
RESIDUES IN FOOD
USE PATTERN
Pre-harvest treatments
Information was received from The Netherlands on pre-harvest
treatment of several crops with deltamethrin, which is summarized in
Table 5.
RESIDUES ARISING FROM SUPERVISED TRIALS
Results of supervised trials with deltamethrin on various crops
are summarized in Table 6.
Assorted fruit, inedible peel
Kiwi fruit
Data from two new field trials conducted in France show that
residue levels for the whole fruit are below 0.05 mg/kg, the residue
being entirely in the peel.
TABLE 5. Deltamethrin use in The Netherlands
Application Rate
Crop g/100 l g/ha P.H.I.
Apple 0.5 (from before blossom to
Pear 0.5 (to 14 days P.H.
Grape 0.5 7
Cherry 0.5 7
Plum 0.5 7
Strawberry 0.5 7
Currant 0.5 7
Gooseberry 0.5 7
Raspberry 0.5 7
Cucumber 1.25 3
Melon 25 14
Bell pepper 25 14
Eggplant 25 14
Lettuce 1.25 14
Endive 25 14
Radish 7.5 7
Turnip 7.5 7
Rutabaga 7.5 7
Brussels sprouts 7.5 7
Cauliflower 7.5 7
Broccoli 7.5 7
Kohlrabi 7.5 7
Leek 7.5 7
Onion 7.5 7
Peas 7.5 7
Dwarf french bean 7.5 7
Sugarbeet 7.5 7
Mushroom 0.75-1.5 75/100 2
Hops
Several trials conducted in the UK and the Federal Republic of
Germany show a high level of variation between samples, but relatively
little decline with time over the period of 7 to 14 days following
treatment. No information was provided on the fate of such residues
when the hops were used in brewing.
TABLE 6. Supervised trials with deltamethrin in various crops
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) Formulation of sample 0 1 2/3 4 7 14
Kiwi fruit France 1980 2 12.5 peel 0.05 0.06 0.06 0.08 0.07
whole fruit 0.01 0.01 0.01 0.01 0.01
pulp ND ND ND ND ND
12.5 peel 0.4 0.22 0.23 0.25 0.1
whole fruit 0.06 0.04 0.04 0.04 0.01
pulp ND ND ND ND ND
Hops England 1978 7 12.5 0.02
W. Germany 1978 1 25 0.01
3 62.5 0.1 0.3 1.4 1.1 1.9
3 62.5 16 5.5 0.1 1.8 1.9
3 62.5 3.3 1.3 0.4 2.0 2.5
1980 5 6.25-37.5 1.3 0.2 0.4 0.3 0.7
5 " 0.2 0.7 0.3 0.7 1.6
5 " 1.0 0.7 0.2 0.6 2.7
5 " 0.5 0.3 0.2 0.7 1.1
Mushrooms1 Netherlands 1978 2 6.25 0.0025% whole 0.003 0.002 0.001
1978 2 25 0.01% " 0.009 0.004 0.003
(DAYS)
0 1 3 5 7 14
Black currant Finland 1979 1 6.3mg/bush. 0.1 0.1
W. Germany 1979 2 18.5g/ha 0.3 0.2 0.2 0.1
" 0.3 0.3 0.3 0.3
" 0.3 0.2 0.2 0.2
2 11.25g/ha 0.2 0.2 0.1 0.1
0.3 0.4 0.3 0.2
TABLE 6. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) of sample 0 1 2 4 8 14 21
Spinach France 1978 1 17.5g/ha Fresh leaf 0.48 0.33 0.22
Cooked leaf 0.27 0.21
Cooking water 0.0001 0.00009
17.5g/ha Fresh leaf 0.52 0.30 0.155
Cooked leaf 0.28 0.12
Cooking water 0.00015 0.000,05
1979 1 12.5g/ha Fresh leaf 0.4 0.2 0.14 0.12
Cooked leaf 0.12 0.10
Cooking water 0.0001 ND
12.5g/ha Fresh leaf 0.4 0.2 0.17 0.15
Cooked leaf 0.15 0.12
Cooking water 0.00015 0.0001
1980 1 17.5g/ha Fresh leaf 0.18 0.22 0.35
Cooked leaf 0.16 0.20 0.28
Cooking water ND ND ND
W.Germany 1980 3 12.5g/ha Fresh leaf 0.3 0.7 0.2 0.09
0.7 0.3 0.3 0.1
14 21 28/39 49 61 74 80
Wheat straw France 1978 1 7.5 EC 0.015
1 15 EC 0.05
1 7.5 EC 0.025
1 15 EC 0.025
1 7.5 EC 0.025
1 15 EC 0.025
1 7.5 EC ND
1 7.5 EC ND
1 12.5 EC 0.15
1 12.5 EC 0.06
1 12.5 EC 0.2
1 12.5 EC 0.1
TABLE 6. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) of sample 14 21 28/39 49 61 74 80
Oat Straw W.Germany 1978 1 12.5 EC 0.06
1 12.5 EC 0.06
1 12.5 EC ND
1 12.5 EC 0.08
1979 1 12.5 EC 0.6
1 12.5 0.2
1 12.5 0.2
1 12.5 0.5
Rice straw Surinam 1977 1 6.25 ND
1 12.5 0.010
1 18.75 0.01
1 25 0.08
Phillipines 1978 1 17.5 0.2
25 0.1
1 After oven drying at 7 days.
Small fruits and berries
Black currant
From trials conducted in the Federal Republic of Germany, it
would appear that residues on these berries can range up to 0.3 to
0.4 mg/kg, but as yet, there is no registered use of deltamethrin on
black currants.
Leafy vegetables
Spinach
Additional data from trials carried out in France and the Federal
Republic of Germany indicate that residues range around 0.3 to
0.4 mg/kg, 7 or 8 days after the last treatment. These levels do not
decline significantly during cooking.
Fodder and cereal straws
Additional data were available to indicate the level of
deltamethrin on wheat straw, oat straw and rice straw. Though the bulk
of the values were below 0.2 mg/kg, oat straw sampled 28 to 39 days
after treatment showed residues ranging up to 0.6 mg/kg. The amount of
data is, however, limited and in view of the potential for the wide
application of deltamethrin to cereal crops further data are required.
Cereal grains
A report was received on trials to determine the fate of
deltamethrin on wheat during storage and after milling and baking
(Halls and Periam 1980).
Batches of an English hard wheat were sprayed on a conveyor with
diluted deltamethrin/piperonyl butoxide liquid grain protectant to
give 250 kg at each of two deltamethrin treatment levels. These 250 kg
batches were then placed in 1 ton capacity metal silos. Target
treatment levels for the two batches of grain were 1 mg/kg
deltamethrin + 10 mg/kg piperonyl butoxide and 2 mg/kg deltamethrin +
10 mg/kg piperonyl butoxide respectively, but chemical analysis
revealed that the actual treatment levels achieved were only about 40
to 75% of these.
Samples of the treated wheat were taken after spraying and
thereafter at monthly intervals for nine months and analysed for
deltamethrin content. Replicate samples were taken from each silo,
analysed separately and the result expressed as the mean. Wheat from
the initial sampling and from samplings after 3, 6 and 9 months was
submitted to the Flour Milling and Baking Research Association,
Chorleywood, Buckinghamshire to be milled and baked.
At Chorleywood the wheat was first cleaned of extraneous
material, then divided into two samples and each subjected to a
different milling procedure. The first produced whole-meal flour, the
second produced white flour, bran and fine offal.
Two different samples of white flour were taken from this
procedure, the first reduction flour (the cleanest flour mainly from
the centre of the grain and used in the baking of cakes) and total
white flour (straight-run flour plus flour from the bran and fine
offal fractions). These samples, together with the wholemeal flour,
bran and fine offal were analysed for total deltamethrin content for
both batches of treated wheat. In addition, bread was baked from both
wholemeal and total white flours to produce wholemeal and white bread,
respectively, and these loaves were also analysed for deltamethrin
content.
Results for residues of deltamethrin on wheat, milling data and
residues of NRDC 161 on flour fractions and bread are given in Tables
7, 8 and 9, respectively.
In both the deltamethrin treatments, levels were initially found
by analysis to be only about 40% of the expected application. This
could be due predominantly to inter alia incorrect spraying rates,
inadequate extraction, or loss of compound at some stage between the
nozzle and the grain.
The results given in Table 7 show that for both application rates
there was no detectable breakdown of deltamethrin on wheat over a
nine-month storage period. The fluctuation in levels between different
samplings was within the inherent error of the analytical method
demonstrating that deltamethrin is very stable on wheat. In this
respect deltamethrin differs from many organophosphate grain
protectants such as fenitrothion (Desmarchelier 1978).
The moisture content of the wheat remained fairly consistent over
the storage period of nine months. Deltamethrin analysis data for the
milling fractions and bread derived from treated wheat are given in
Tables 8 and 9.
For all sampling times at both treatment levels there was no
significant change in deltamethrin residues when wheat was milled to
produce wholemeal flour. When this flour was baked, the wholemeal
bread produced contained lower, or identical, deltamethrin residues
but this can be explained by the greater moisture content (up to 45%)
present in bread.
TABLE 7. Deltamethrin residues on wheat after indicated periods of storage in the UK1
Residue analysis (mg/kg deltamethrin)
Target
Application Initial2 1 month 2 months 3 months 4 months 5 months 6 months 7 months 8 months 9 months
rate (mg/kg) (15.11.79) (13.12.79) (17.1.80) (11.2.80) (12.3.80) (21.4.80) (12.5.80) (10.6.80) (10.7.80) (6.8.80)
1.0 0.44 0.53 0.48 0.50 0.50 0.41 0.48 0.49 0.42 0.41
2.0 0.80 1.25 1.33 1.46 1.21 1.01 0.96 1.12 0.94 1.08
1 Analytical results are subject to overall confidence limits of 30%
2 Dates refer to day of sampling.
TABLE 8. Milling data for wheat freshly treated with deltamethrin and from wheat after 3, 6 and 9 months storage1
Target application Sampling Clean wheat Bran Fine Offal Total white flour
rate period
(mg/kg) (months) Weight Prop. of Weight Prop. of Weight Prop. of Weight Prop. of
(kg) total(%) (kg) total (%) (kg) total (%) (kg) total (%)
0 3.835 100 0.596 15.54 0.306 7.98 2.849 74.29
3 4.921 100 0.779 15.83 0.519 10.55 3.502 71.16
1.0
6 3.700 100 0.621 16.78 0.340 9.19 2.670 72.16
9 3.700 100 0.626 16.92 0.288 7.78 2.746 74.22
0 3.786 100 0.590 15.58 0.292 7.71 2.809 74.19
3 4.884 100 0.769 15.75 0.514 10.52 3.489 71.44
2.0
6 3.700 100 0.630 17.03 0.306 8.27 2.688 72.65
9 3.700 100 0.650 17.57 0.275 7.43 2.692 72.76
0 (Control) 0 2.010 100 0.312 15.52 0.175 8.71 1.525 75.87
1 There is a loss of up to 3% in the laboratory milling process
TABLE 9. Deltamethrin residues on milling fractions and bread made from freshly treated wheat and from wheat after 3, 6 and
9 months storage1
Target application Sampling First Total
rate period Wholemeal Wholemeal Bran Fine reduction white White
(mg/kg) (months) Wheat flour bread offal flour flour bread
0 0.44 0.42 0.26 3.00 nd2 nd 0.09 0.10
3 0.50 0.43 0.27 1.80 approx. 0.5 nd approx. 0.05 nd
1.0
6 0.48 0.42 0.26 1.70 0.35 0.05 0.09 0.10
9 0.41 0.36 0.36 1.90 0.46 0.03 0.03 0.05
0 0.80 0.73 0.63 5.40 nd nd 0.20 0.29
3 1.46 0.80 0.57 4.70 0.75 nd 0.20 0.15
2.0
6 0.96 0.83 0.66 3.60 0.75 0.10 0.16 0.20
9 1.08 0.95 0.70 4.40 1.36 0.05 0.17 0.11
1 Analytical results are subject to confidence limits of ± 30%
2 nd = not detectable (<0.03 mg/kg).
When the treated wheats were milled to produce white flour,
relatively high deltamethrin residues were found on the bran fraction,
which is derived from the superficial layers of the grain. Initially,
deltamethrin residues on the bran were 6 to 7 times higher than those
on the wheat at both treatment rates. The levels declined to be about
4 to 5 times higher on the bran than on the wheat after 9 months'
storage.
This observation, together with the appearance of deltamethrin on
the fine offal fractions after 3 months' storage, and on the first-
reduction flours after 6 months' storage, suggests that although there
is no change in the total deltamethrin level on the wheat over 9
months, there is a subtle change in its distribution. Thus, initially,
as might be expected, the insecticide residues were found
predominantly in the outer layers of the grain, mainly in the bran
fraction, but also to a lesser extent in the total white flour
fraction. The first-reduction flour, which tends not to come from the
superficial layers of the grain, and the fine offal, consisting
largely of wheatgerm, is uncontaminated initially but residues move
into these areas as the period of storage is increased.
The deltamethrin levels on total white flour milled from treated
wheat after different storage periods remained quite constant at about
one sixth of the applied rates. Some fluctuation in this proportion
was detectable at the lower application rate, but this is probably
because in these cases the deltamethrin levels on the flour were close
to the limits of detection. When baked in white bread, there is no
significant change in residue levels, showing that no breakdown of
deltamethrin occurred during baking.
Consideration of the levels found on each fraction, and the
weights of each fraction produced by unit weight of wheat, leads to
the conclusion that there is no breakdown of deltamethrin during the
break, reduction and finishing systems of the milling process that
produces white flour.
The following conclusions may be drawn from the results of this
trial:
Deltamethrin is not degraded on wheat over a nine-month storage
period.
There is no degradation of deltamethrin when treated wheat is milled
to produce either wholemeal or white flour.
When wholemeal flour made from deltamethrin treated wheat is baked,
there is an apparent reduction in deltamethrin residues, but this is
due to the greater moisture content of the bread.
In the production of white flour and bread from deltamethrin treated
wheat, there is a reduction in the deltamethrin level to about 10 to
20% of the level applied to the wheat. This is because the
deltamethrin is predominantly found on the bran fraction with a
concentration increase of 4 to 7 fold. There is no evidence for
deltamethrin degradation when white flour is baked to produce white
bread.
Over a 9-month storage period, there appears to be a migration of
deltamethrin to the fine offal fraction (including the wheat germ
which is used in health foods and "Hovis" bread) and the first-
reduction flour (used in the production of cakes) presumably from the
bran fraction (predominantly used for animal feed).
NATIONAL MAXIMUM RESIDUE LIMITS
The Meeting was advised of the following limits established by The
Netherlands:
Strawberry 0.2 mg/kg
Other fruit 0.1 mg/kg
Leafy vegetables 0.2 mg/kg
Other vegetables 0.05 mg/kg
EVALUATION
COMMENTS AND APPRAISAL
Deltamethrin was first evaluated in 1980 but, in the absence of
some essential toxicological information, it was not possible to
establish an ADI. The Meeting proposed a series of guideline levels
reflecting residues likely to occur on various food commodities
following the use of deltamethrin with present good agricultural
practice. A small amount of additional information was available for
consideration at the Meeting.
Residue data from trials on kiwi fruit were considered suitable
to propose guideline levels, but further data are needed to confirm
whether these levels are typical of those occurring in other regions
where these crops are grown. In the case of kiwi fruit, the residues
are entirely confined to the inedible peel. Data were received from
residue trials on black currants, but as there was evidence that
deltamethrin was not yet approved for use on currants, no guideline
levels were proposed.
Results of several trials on spinach indicate that cooking has
little or no effect on the level of deltamethrin residues on such
leafy vegetables. These studies do not, however, provide an adequate
basis for determining whether the guidelines proposed in 1980 for
spinach should be amended.
There is a suggestion that residues of deltamethrin on some
cereal straws used for animal feed could be higher than the 0.5 mg/kg
limit proposed for legume animal feeds, but the amount of information
is inadequate to provide a basis for amending the recommendation.
A report on one further study of the level and fat of
deltamethrin on wheat, milled products and bread confirm that
deltamethrin is not degraded significantly during storage, nor is it
destroyed in cooking. A substantial proportion is, however, removed on
bran during the milling of white flour. In view of the vast experience
with other grain protectant insecticides and the considerations
discussed in 1980, the Meeting deemed it necessary to revise the
recommended guideline levels for deltamethrin on raw cereals and
milled cereal products to accommodate, realistically, all the
situations likely to arise from normal applications under good storage
practice, although in most cases, levels would be considerably lower.
Such provisions are also intended to accommodate the variations due to
sampling difficulties and analytical error.
The Meeting, however, expressed the view that considerably more
information was required about the level and fate of deltamethrin on
different grains under different storage conditions and especially the
effect of processing and cooking of all cereal grains.
RECOMMENDATIONS OF GUIDELINE LEVELS
As no ADI has been allocated, the Meeting proposed the following
guideline levels determined and expressed as deltamethrin.
Commodity Guideline level (mg/kg)
Kiwi fruit 0.05
Hops (dry) 5
Cereal straw (animal feed) 0.5
Cereal grains 2
Wheat bran (unprocessed) 5
Wheat flour (wholemeal) 2
FURTHER WORK OR INFORMATION
Required (by 1982 and before an acceptable daily intake can be
allocated) (as listed in the 1980 JMPR Report).
1. Results of the two-year feeding study in dogs currently in
progress.
2. Clarification of possible embryotoxic effects in animals.
3. Further observations on reported effects in man.
4. Studies on the significance of neurological effects observed in
several animal species.
5. Results of supervised trials on residues in meat, milk, and eggs
arising from the use of deltamethrin for ectoparasite control, in
feeding studies and in stable treatments. Also required before MRLs
can be recommended:
6. Considerably more information on the level and fate of
deltamethrin on various cereal grains treated under different storage
conditions, and especially the effect of processing and cooking of all
cereal grains.
7. Additional residue data from supervised trials on kiwi fruit,
currants and leafy vegetables.
8. Information on the level and fate of deltamethrin residues in
foods of animal origin following the feeding of cattle, pigs and
poultry with rations containing deltamethrin at levels of the order
likely to be encountered in practice.
9. Further information on the level of deltamethrin on cereal grains
following treatment of crops in conformity with approved or proposed
use patterns.
REFERENCES
Barnes, J.M. and Verschoyle, R.D. Toxicity of a new pyrethroid
1974 insecticide. Nature, 248:711 1974
Beavers, J.B. and Fink, R. Eight-day dietary LC50-mallard duck,
1977a technical DECIS. Final report W1-77.18.05/A, Wildlife
International, USA, submitted by Roussel Uclaf to WHO.
(Unpublished)
Beavers, J.B. and Fink, R. Eight-day dietary LC50-bobwhite quail,
1977b technical DECIS. Final report. W1-77-19.05/A, Wildlife
International, USA, submitted by Roussel Uclaf to WHO.
(Unpublished)
Cabral, J.R. In: IARC Annual Report - 1981. International Agency for
1981 Research on Cancer, Lyon, in press.
Chesterman, H,, Heywood, R., Perkin, C.J., Beard, D., Street, E. and
1977 Prentice, D.E. RU 22974. Oral toxicity study in beagle dogs.
Report RSL 253/7751/A 3, Huntingdon Research Centre,
Huntingdon, England, submitted by Roussel Uclaf to WHO.
(Unpublished)
Clair, M. RU 22974 - DECIS. Acute toxicity in the rabbit by
1977 percutaneous administration. Report IFREB-R 770256.1/A,
Institut Francais de Recherches et Essais Biologiques,
Joinville-le-Pont, France, submitted by Roussel Uclaf to
WHO. (Unpublished)
Coombs, D.W. and Clark, G.C. RU 22974. Acute inhalation toxicity in
1978 rats, 6 hour LC50. Report RLS 310/78453/A, Huntingdon
Research Centre, Huntingdon, England, submitted by Roussel
Uclaf to WHO. (Unpublished)
Coquet, B. RU 22974. Test to determine primary cutaneous irritation in
1976a the rabbit. Report no. IFREB-R 761157/A, Institut Francais
de Recherches et Essais Biologiques, Joinville-le-Pont,
France, submitted by Roussel Uclaf to WHO. (Unpublished)
1976b RU 22974. Test to evaluate ocular irritation in the rabbit.
Report no. IFREB-R 761158/A, Institut Francais de Recherches
et Essais Biologiques, Joinville-le-Pont France, submitted
by Roussel Uclaf to WHO. (Unpublished)
Desmarchelier, J., Bengston, M., Connell, M., Minett, W., Moore, B.,
1977 Phillips, M., Snelson, J., Sticka, R. and Tucker, K. A
collaborative study of residues on wheat of
chlorpyrifosmethyl, fenitrothion, malathion, methacrifos and
pirimiphos-methyl. 1. Method Development. Pesticide Science,
8: 473-483.
Desmarchelier, J. Loss of fenitrothion on grains in storage. Pesticide
1978 Science, 9:33-38.
Elliott, M. Synthetic pyrethroids, American Chemical Society
1977 Symposium, 42:1-29.
Fouillet, X. RU 22974. Mutagenicity study of various preparations.
1976 Salmonella microsome test. Report no. IFREB-R 761153/A,
Institut Francais de Recherches et Essais Biologiques,
Joinville-de-Pont, France, submitted by Roussel Uclaf to
WHO. (Unpublished)
Gaughan, L.C., Unai, T. and Casida, J.E. Permethrin metabolism in
1977a rats. Journal of Agricultural and Food Chemistry, 25:9-17
1977b Permethrin metabolism in rats and cows and in bean and
cotton plants. American Chemical Society Symposium,
42:186-193.
Glomot, R. and Chevalier, B. RU 22974. Acute toxicity study, mouse and
1976a rat by oral route. Report Tox 76810/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
1976b RU 22974. Acute toxicity study mouse and rat by
intraperitoneal route. Report Tox 76811/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
1976c RU 22974. Acute toxicity study, mouse and rat by intravenous
route. Report Tox 76812/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
Glomot, R., Chevalier, B., Collas, E. and Audegond, L. RU 22974. Acute
1977 toxicity study by oral route in male and female beagle dogs.
Report Toxico 77804/JL-5, submitted by Roussel Uclaf to WHO.
(Unpublished)
Glomot, R. and Vannier, B. RU 22974. Teratological study in mouse, rat
1977 and rabbit. Report no. Tox 76534-76536/2/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
Glomot, R. and Vannier, B. RU 22974. Teratological study in mouse.
1978 Complementary Information. Report no. Tox. 76534.76536/A3,
submitted by Roussel Uclaf to WHO. (Unpublished)
Glomot, R. Personal communication to WHO. Roussel, Uclaf, Paris,
1981 France.
Goldenthal, E.I., Blair, M., Jefferson, N.D., Spicer, E.J.F., Arceo,
1980a R.J. and Clark Kahn III, A., RU 22974. Two-year toxicity and
carcinogenicity study in mice. Report IRDC 406-001/A/4,
International Research and Development Corp., Mattawan,
Michigan, submitted by Roussel Uclaf to WHO. (Unpublished)
Goldenthal, E.I., Jefferson, N.D., Blair, M., Thorstenson, J.H.,
1980b Spicer, E., J.F. Arceo, and Kahn, III, A. RU 22974. Two year
oral toxicity and carcinogenicity study in rats. Report IRDC
406-002/A 4, International research and Development Corp.,
Mattawan, Michigan, submitted by Roussel Uclaf to WHO.
(Unpublished)
Gray, A.J. and Rickard, J. Distribution of radiolabel in rats after
1981 intravenous injection of a toxic dose of 14C-acid-,
14C-alcohol- or 14C-cyano-labelled deltamethrin. Pesticide
Biochemistry and Physiology, 16:79-85
Guillot, J.P. and Guilaine, J. RU 22974 - Decamethrine. Decis
1977 technical Roussel Uclaf. Sensitization test in the guinea
pig. Report no. IFREB-R 709241/A, Institut Francais de
Recherches et Essais Biologiques, Joinville-le-Pont, France,
submitted by Roussel Uclaf to WHO. (Unpublished)
Halls, G.R.H., and Periam, A.W.. The fate of residues of NRDC 161 on
1980 wheat during storage and after milling and baking - Report
after 9 months storage. Wellcome Research Laboratories
Report HEFH 80-4, November 1980. (Unpublished)
Husson, J.M. Medical observations made on people working on the
1978 manufacture and formulation of the pyrethroid insecticide
decamethrin. Report no. RU 78.25.08/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
Hunter, B., Jordan, J., Heywood, R., Hepworth, P., Street, A.E. and
1977 Prentice, D. RU 22974. Assessment of toxicity to rats by
oral administration for 13 weeks (followed by a 4 week
withdrawal period). Report RSL 254/76938/A3, Huntingdon
Research Centre, Huntingdon, England, submitted by Roussel
Uclaf to WHO. (Unpublished)
IRDC Laboratories. Two year chronic dog feeding study. Report IRDC
1980 406-004/A1, International Research and Development
Corporation, Mattawan, Michigan, submitted by Roussel Uclaf
to WHO. (Unpublished)
Kavlock, R., Chernoff, N., Baron, R., Linder, R., Rogers, E. and
1979 Carver, B. Toxicity studies with decamethrin, a synthetic
pyrethroid insecticide. Journal of Environmental Pathology
and Toxicology, 2:751-765.
Kynoch, S.R., Lloyd, G.K. and Andrews, C.D. Acute percutaneous
1979 toxicity to rats of decamethrin. Report RSL-10098/D
147/79/A, Huntingdon Research Centre, Huntingdon England,
submitted by Roussel Uclaf to WHO. (Unpublished)
Peyre, M., Chantot, J.F., Glomot, R. and Penasse, L. Detection of a
1982 mutagenic potency of decamethrin (RU 22974). Bacterial
tests. Report no. RU/TOX/80.21.01/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
Ray, D.E. and Cremer, J.E. The action of decamethrin (a synthetic
1979 pyrethroid) on the rat. Pesticide Biochemistry and
Physiology, 10:33.
Ray, D.E. An EEG investigation of decamethrin induced choreoathetosis
1980 in the rat. Experimental Brain Research, 38:221.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E., Cooke, L.
1978 and Gibson, W.A. RU 22974 (Decamethrin) LD50 determination
and assessment of neurotoxicity in the domestic hen. Report
no. RSL 293-NI/830/A. Huntingdon Research Centre,
Huntingdon, England, submitted by Roussel Uclaf to WHO.
(Unpublished)
Roussel Uclaf. Test to determine the toxicity in the chicken by oral
1976a route. Report RU-76.05.05/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
1976b Toxicity of decamethrin or DECIS in single ingestion in
game ducks, Anas platyrhynchos L. Report INRA-76.21.12/A.
Institut National de la Recherche Agronomique. Jony-En-
Josas, France, submitted by Roussel Uclaf to WHO.
(Unpublished)
Roussel Uclaf. Toxicity of decamethrin or DECIS by single ingestion
1976c in grey partridge, Perdix perdix l., and Red Partridge
Alectous rufa l. Report INRA-76.28.09/A. Institut National
de la Recherche Agronomique. Joney-en-Josas, France,
submitted by Roussel Uclaf to WHO. (Unpublished)
Ruzo, L.O., Unai, T. and Casida, J.E. decamethrin metabolism in rats.
1977 Report UC-77.06.12/A, submitted by Roussel Uclaf to WHO.
(Unpublished)
1978a Decamethrin metabolism in rats. Journal of Agriculture and
Food Chemistry, 26:918-925.
Ruzo, L.O., Engel, J.L. and Casida, J.E. Oxidative, hydrolytic and
1978b conjugative reactions in the metabolism of decamethrin in
mice. Report UC 78.10.11/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
1979 Decamethrin metabolism from oxidative, hydrolytic and
conjugative reactions in mice. Journal of Agricultural Food
and Chemistry, 27:725-731.
Shono, T., Oshawa, K. and Casida, J.E. Metabolism of trans- and cis
1978 permethrin, trans- and cis-cypermethrin and decamethrin by
microsomal enzymes. Report UC 78.18.05/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
Sobels, F.H., Tates, A.D. and Vannier, B. Cytogenetic study with RU
1978 22974. Detection of a mutagenic potency in mammalian cells.
Report no. ULN-782211/A, State university of Leiden, The
Netherlands, submitted by Roussel Uclaf to WHO.
(Unpublished)
Soderland, D.M. and Casida, J.E. Substrate specificity of mouse liver
1977 microsomal enzymes in pyrethroid metabolism. In: Synthetic
Pyrethroids, American Chemical Society Symposium,
42:152-162.
Vannier, B. and Glomot, R. RU 22974. Mutagenic study. Dominant lethal
1977 assay in the male mouse. Report no. Toxico 76533/DB9/A,
submitted by Roussel Uclaf to WHO. (Unpublished)
Wrenn, J.M., Rodwell, D.E., Goldenthal, E.I., Spider, E.J.C. and
1980 Rajasekaran, B. Three-generation reproduction study in rats.
Report no. IRDC-406-003/A4, International Research and
Development Corporation, Michigan, USA, submitted by Roussel
Uclaf to WHO. (Unpublished)
TABLE 3. Acute toxicity of deltamethrin
LD50
Species Sex Route (mg/kg bw) References
Mouse M+F iv 41 Glomot and Chevalier 1976c
M ip 181 ibid 1976b
M ip 1712 ibid 1976b
M oral 211 ibid 1976a
M oral 332 ibid 1976a
F io 121 ibid 1976b
F ip 1662 ibid 1976b
F oral 191 ibid 1976a
F oral 342 ibid 1976a
Rat M+F iv 311 ibid 1976c
M ip 241 ibid 1976b
M ip 2092 ibid 1976b
M oral 671 ibid 1976a
M oral 1282 ibid 1976a
M+F inhal. 0.63 Coombs and Clark 1978
M+F dermal <29404 Kynoch et al 1979
F ip 251 Glomot and Chevalier 1976b
F ip 1862 ibid 1976b
F oral 861 ibid 1976a
F oral 1392 ibid 1976a
Rabbit M+F dermal >20005 Clair 1977
Dog (beagle) M+F oral >3001 Glomot et al 1977
M+F oral >3006 ibid
Chicken oral ca 10002 Roussel Uclaf 1976a
Hen (adult) F oral >25002 Ross et al 1978
F oral >50007 ibid
Mallard duck oral >46407 Beavers and Fink 1977a
Game duck oral >40006 Roussel Uclaf 1976b
Grey Partridge M+F oral >18007 ibid
Red Partridge M+F oral >30007 ibid
1 Suspended in polyethylene glycol 200; 2 dissolved in sesame oil;
3 expressed for LC50 in mg dust/m3 air; 4 60% w/v suspension in aqueous
methyl-cellulose in occlusion; 5 as paste in PEG 400 on occlusion;
6 in capsules or cachets; 7 dissolved in maize oil.
Pretreatment with either the oxidase inhibitor piperonyl butoxide or
the esterase inhibitor S,S,S-tributyl phosphorotrithioate (DEF)
further increased deltamethrin in mice, reduced its rate of in vivo
metabolism by hydroxylation and hydrolysis, reduced the rate of
product excretion and elevated deltamethrin levels in fat and brain
(Ruzo et al. 1979; Soderlund and Casida 1977).
Deltamethrin-hydrolysing esterases are generally sensitive to
organo-phosphate inhibition (e.g. TEPP inhibition of hydrolysis by
all tissue preparations except stomach). The two major factors
contributing to the rapid detoxification of decamethrin are: a) the
relevant esterases present in many tissues and the oxidases in, at
least, liver microsomes, and b) many molecular sites that are
susceptible to metabolic attack (Ruzo et al 1979).
There are large differences in the toxicity of deltamethrin to
mice, depending on the route of administration, the carrier vehicle
and previous exposure to piperonyl butoxide or DEF. Irrespective of
these factors, there appears to be a critical concentration of
deltamethrin in brain that correlated with the onset of tremors or the
time of death. Additionally, direct intracerebral administration of
deltamethrin at approximately this brain level gave a similar
poisoning effect. The brain appears to be a primary target in
deltamethrin poisoning of mice (Ruzo et al 1979).
Rat
The signs of toxicity observed in rats after deltamethrin
administration included salivation and choreoathetosis (a writhing
type of toxicity) (Barnes and Verschoyle 1974; Ray 1980; Ray and
Cremer 1979; Verschoyle and Aldridge 1980) and have been designated as
the CS syndrome of toxicity (Verschoyle and Aldridge 1980).
Rats injected i.v. with deltamethrin showed muscular
contractions, piloerection, respiratory defects, convulsions and
paresis of the hind quarters immediately following treatment. Death
occurred within 10 min. After 24 h only piloerection was visible;
after 48 h surviving animals showed normal behaviour. After i.p.
injection immediate tremors, convulsions, prostration and cyanosis
were observed. After 48 h surviving animals showed normal behaviour.
Gavage with deltamethrin shortly after dosing induced motor
incoordination, convulsions and respiratory defects. After 24 and
48 h, hypomotility and convulsions were still observed. After 3 days
surviving animals showed normal behaviour (Glomot and Chevalier
1976a,b,c).
In an earlier study, the acute i.v. lethal dose in young adult
female rats was 2 to 2.5 mg/kg when administered as a solution in
glycerol formal (Barnes and Verschoyle 1974). Signs of poisoning
included excessive salivation without lacrimation, rapidly followed by
continuous jerking movements of the limbs, occasionally progressing to
convulsions. The toxic effects were rapid in onset and brief in
duration. Death occurred in some animals within 12 min of dosing while
survivors were generally symptom-free within 6 h. In a more recent
study (Kavlock et al 1979, see Table 4), the acute LD50 for adult
female rats was 31 mg/kg by the oral route and 4 mg/kg by the i.v.
route. The LD50 was observed to be sex and age dependent, with higher
values found for weanlings and males. Initial signs of deltamethrin
poisoning included profuse salivation and convulsive movements;
weakness, dyspnoea, anorexia and staining of the fur were observed
beyond the first day following compound administration.
Rats (7 males and 7 females/group) were exposed (whole body)
during 6 h to aerosols of a.i., aerosol concentration being 0.049,
0.43, 0.54 and 0.72 g/m3. The aerosol contained 66 to 86% of
particles < 5.5 µ. During exposure, hyperactivity and dose-dependent
increase in grooming and irritation were observed. The animals were
hypersensitive to touch and noise and showed uncoordinated movements.
During the observation period of 14 days following exposure, all
animals except those from the lowest dose group developed poor motor
coordination and hypersensitivity. At the end of the period all
animals were recovered to normal. In these groups the body weight gain
and food intake was depressed during 3 days following exposure. In
rats (4 of control and of highest dose group) killed immediately after
exposure, the stomach and small intestine were gas filled. In treated
rats, as result of exposure, massive haemorrhage and oedema in lungs
were observed. Stomachs were filled with gas, blood and mucus. White
deposits were visible in the trachea. In animals killed after the
observation period, a dose-dependent increase of lung degeneration
(coloured spots to congestion) was observed (Coombs and Clark 1978).
Mouse
Mice injected i.v. with deltamethrin showed intense tremors,
convulsions and ataxia immediately after administration. Also,
tachycardia and respiratory defects were observed at higher doses.
Surviving animals showed normal appearance after 4 to 5 h. Immediately
after i.p. injection, jumping movements, slight convulsions and
prostration, ptosis, tail hypertonicity and cyanosis were observed.
Surviving animals appeared normal after 72 h. Animals gavaged with
deltamethrin showed muscular stiffening and convulsions 1 h after
dosing. After 24 h hypermotility, stereotype movements of the head,
tachycardia, hypertonicity of the tail and a few convulsions were
noted. Normal behaviour and appearance were seen after 48 h (Glomot
and Chevalier 1976a,b,c).
TABLE 4. Acute toxicity of deltamethrin to rats1
95 % Confidence Lowest dose Minimum Toxic
Route Strain Sex Age LD50 limit of tested Dose
mg/kg LD50 (mg/kg) (mg/kg) (mg/kg)
Oral2 Sherman M Adult 52 46 - 58 30.00 306
Oral2 Sherman F Adult 31 29 - 34 5.00 107
Oral2 Sherman F Weanling 50 42- 60 7.50 157
Intravenous3 Sherman F Adult 4 2.9-5.3 1.57 1.576
Intravenous3 Sherman F Weanling 1.8 1.5-2.1 0.78 0.76
Dermal4 Sherman F Adult >800 --- 800:0 --- 8
Inhalation5 Sprague-Dawley M Adult 940 mg/m3 --- ---
Inhalation5 Sprague-Dawley F Adult 785 mg/m3 --- ---
1 Reference-Kavlock et al 1979;
2 Dissolved in peanut oil and administered via gavage at a rate of 5 ml/kg bw;
3 Dissolved in acetone and administered via a single injection into tail vein of rat at 0.313 ml/kg bw;
4 Dissolved in xylene and administered at a rate of 3.2 ml/kg bw;
5 Aerosols generated from 10% DMSO solution. Based on exposure time of 120 to 150 min.;
6 Moderate to severe salivation and convulsions;
7 Mild salivation;
8 No signs of toxicity at 800 mg/kg.
Rabbit
Rabbits (10 males and 10 females) were treated with 2 g
deltamethrin in 2 ml PEG 400/kg bw on 80 cm3 shaven skin for 24 h on
occlusion. The animals were observed for 14 days. One animal showed
obvious erythema and another congested skin. No weight changes or
abnormal behaviour were observed. On histological observation of
liver, kidneys and skin, small changes were observed that were common
for this strain of rabbits and not related to treatment (Clair 1977).
Dog
Dogs showed, at non-lethal doses, transient hyperexcitability,
akynesia, vomiting and stiffness of the hind legs (Glomot et al
1977).
Birds
Oral administration of a.i. to hen, game duck or partridge
produced no distinct symptoms except a small initial weight loss
occasionally. In chickens diarrhoea, convulsions and jerky movements
of the head were observed. Mallard ducks, at lethal doses, exhibited
signs of neurotoxic effects, which included ataxia, loss of
equilibrium and loss of coordination. The effect was dose-related: at
lower dose levels only some hyperexcitability and imbalance were
observed (Beavers and Fink 1977a).
Short-term studies
Rat
Male and female weanling Sprague-Dawley rats (20/sex/group)
were daily dosed by oral gavage with 0, 0.1, 1.0, 2.5 or 10.0 mg
deltamethrin in PEG 200/kg bw/day for 13 weeks (Hunter et al 1977).
No treatment-related effects on food and water consumption, mortality,
urinalysis and haematology were observed. Neurological examinations
and ophthalmoscopy revealed no abnormalities. In the 10 mg/kg bw
group, some hypersensitivity was observed in week 6 in males. Body
weight gain among males receiving deltamethrin was significantly lower
at 2.5 and 10 mg/kg/day. The body weight of the females was not
affected by the treatment. The male animals of the 1 mg/kg group
showed a tendency to a reduced body weight gain. In the females, blood
glucose and urea were significantly increased in week 6, but no
significant changes occurred in week 12 or with other blood chemistry
parameters. No clear effects were noted on the weights of the organs.
Gross and microscopic examination of a variety of tissues and organs
showed no treatment-related alterations.
Following the 13-week dosage period, 5 males and 5 females per
group were allowed to recover for 4 weeks. Autopsy was performed at
the end of this recovery period. The body weights of all previously
treated animals appeared not to be different from controls. Thyroid
weights were not dose-related but were increased in males. This
increase was significantly higher in the 1.0 mg/kg and 10.0 mg/kg
group. Marginal no-effect level was 1 mg/kg bw (Hunter et al 1977).
Dog
Male and female beagle dogs, 3-5/sex/group, 25 weeks of age, were
daily dosed orally with 0, 0.1, 1.0, 2.5 and 10.0 mg deltamethrin in
PEG 200/kg bw/day in gelatin capsules. Dosage was continued for 13
weeks, followed by a recovery period of 20 weeks for 2 dogs/sex from
the groups receiving 1.0, 2.5 and 10.0 mg/kg bw/day (Chesterman
et al 1977). Observations were made on behaviour, mortality, body
weight and food and water consumption. Haematology, blood chemistry,
urinalysis and six channel EEG-analysis were performed at week 0, 6
and 1, and ophthalmoscopy at week 0, 5 and 12. Special attention was
paid to the muscular and nervous systems.
Liquid faeces were associated with all groups of treated dogs
throughout the dosing period. All groups of animals receiving
deltamethrin gained less weight than the controls. The effects were
not strictly dose related. The dogs from the control group left
smaller quantities of the offered food than those of the treated
groups, whereas the water consumption was not dose-relatedly decreased
in all treated groups.
Dilation of the pupils was seen to occur in the dogs receiving
2.5 and 10.0 mg/kg/day. The sign was first seen 4 to 7 h after dosing
and persisted throughout the day. They reacted normally prior to
dosing on the following day. The incidence of vomiting was dose-
related increased in all treated groups, except the 0.1 mg dose level.
The incidence decreased in all the animals affected as the dosing
period progressed. In the highest dose group, unsteadiness, body
tremors and jerking movements were seen, particularly in males in
weeks 2, 3 and 4. These effects were reduced during week 5 to 9 and
were seen only in one dog in week 13. Excessive salivation was seen
initially but diminished during dosing period. After 5 and 12 weeks,
depression of the gag reflex was noted in a proportion of animals in
all treated groups. Depression of the patellar reflex was observed in
all treated groups except the dogs administered 0.1 mg/kg. In the
animals given 1 or 2.5 mg/kg/day, exaggeration of the patellar reflex
was noted only after 5 weeks. Some animals of all treated groups
showed variations in the flexor reflex. A high proportion of the
animals had depression of the hind limb tactile placing reaction.
At dosage levels of 2.5 and 10 mg/kg/day, deltamethrin caused
modification of the EEG pattern in some animals following 12 weeks
administration. Histopathological evaluations of tissues and organs,
including nervous system and muscle, did not reveal abnormalities that
could be related to dosage with the test compound. During recovery the
gag reflex continued to be depressed, whereas exaggeration of the
patellar reflex was still seen in some dogs that had previously
received 1.0 mg/kg/day. One animal continued to show an abnormal EEG
pattern (Chesterman et al 1977).
Quail and duck
Deltamethrin was given in the diet to 14-day old mallard ducks
for 5 days, at doses of 0, 464, 1 000, 2 150, 4 640 and 10 000 mg/kg
feed. The number of animals per group was 10. There was some mortality
in the two highest dose groups. Birds of the highest dose group showed
ataxia and loss of coordination. There were dose-related decreased
weight gain and food consumption (Beavers and Fink 1977a).
An identical experiment was performed with 14-day old bobwhite
quails (10 animals/group). The effects were the same as in the mallard
ducks, except there was no mortality (Beavers and Fink 1977b).
Long-term studies
Rat
Male and female Charles River CD rats (90/sex/group) were dietary
fed with 0 (control), 2, 20 and 50 mg deltamethrin/kg in the diet for
two years. Sixty males and 60 females were used in a second control
group. After 6, 12 and 18 months of compound administration, 10
animals/sex/group were sacrificed, except for the second control group
(Goldenthal et al 1980b).
No changes in general behaviour and appearance in relation to
compound treatment were recorded. Survival was similar in control and
treated rats (50 to 67%). Rats at 50 mg/kg feed gained slightly less
weight than control rats, whereas the food consumption was essentially
the same. Ophthalmoscopic findings generally were similar for control
and treated rats. No haematological and biochemical parameters were
changed in a biologically significant way in relation to treatment at
any time, except for a decreased SGPT activity at 6 months in the mid-
and high-dose groups. No treatment-related effects were observed on
organ weights. The macroscopy and microscopy findings were common for
the animals of the species and strain, except for a slightly increased
incidence of axonal degenerations in sciatic, tibial and/or plantar
nerves at 18 months in the 20 and 50 mg/kg groups. Evaluation of
incidence and/or severity of these degenerations at termination of the
study was obscured by the age of the animals.
Seven interstitial cell adenomas were observed in the testes of
the 50 mg/kg feed group compared to 0 and 4 in the two control groups.
Only from some animals of the 2 and 20 mg/kg groups were some organs
and tissues, including the testes, studied histopathologically.
Evaluation of a possible dose-response effect on the testes was
therefore not possible. The no-effect level was suggested to be
2 mg/kg (Goldenthal et al 1980b).
Mouse
Male and female Charles River CD-1 mice (80/sex/group) were fed
dosage levels of 0(control), 1, 5, 25 and 100 mg deltamethrin/kg in
the diet for 24 months. In a second control group 60 mice/sex were
used. After 12 and 18 months 10 mice/sex/group, except for two only in
the control group, were sacrificed.
There were no clear effects related to the administration of
deltamethrin on general behaviour, mortality, body weight and food
consumption. Blood chemistry, haematology and urine analysis
parameters were normal after 12, 18 and 24 months. Increases or
decreases in absolute and/or relative organ weights occurred in a few
organs at each dosage level at any time of sacrifice. Microscopic
examination of tissues did not reveal any lesions indicative of a
compound-related effect. The tumour incidence was unaffected by
deltamethrin administration. The no-effect level was suggested to be
100 mg/kg (Goldenthal et al 1980a).
Studies designed to investigate the potential carcinogenic
effects of deltamethrin have been initiated (Cabral 1981). Groups of
C57B1 mice have been administered orally 0-8 mg/kg bw deltamethrin in
arachis oil.
Dog
Deltamethrin (RU 22974) dissolved in maize oil was administered
in the diet to 64 beagle dogs at dosage levels of 1, 10 and 40 ppm for
24 months. This corresponds to 0.025, 0.25 and 1 mg/kg bw respectively
(IRDC 1980). Eight males and eight females were used at each dosage
level and in a control group. Individual body weights and food
consumption values were determined weekly. Ophthalmic, haematologic,
biochemical and urinalysis examinations were conducted during the pre-
test period at 6, 12, 18 and 24 months of the study. Neurological
exams were conducted at approximately 1 year and before termination.
These later parameters included: for cranial nerves and segmental
nerves, tests for postural reactions, placing reactions and hopping
reactions.
No signs of overt toxicity were observed in any of the dogs. Body
weights and food consumption values were similar for control and
treated dogs. No compound-related effects were observed during the
ophthalmoscopic and physical examinations. Although there were some
randomly statistically significant differences between the control and
other dose groups in the haematologic and biochemical tests, there
were not any physiologically significant changes observed at any
interval in the study. Two treated and two control animals died during
the study.
No compound-related gross or microscopic changes were observed in
the surviving dogs that were sacrificed and necropsied. Inflammatory,
degenerative and proliferative changes described were spontaneous in
nature or related to theoestrous phase of the menstrual cycle and
unrelated to compound administration.
On the basis of the results from this study, it was concluded
that the no-effect level is 40 ppm in the diet (equivalent to
1 mg/kg bw/day) administered RU 22974 over a 2-year period (IRDC
1980).
Special studies on primary irritation
Cutaneous irritation
Male albino rabbits (12/group) weighing 2.5 to 3.5 kg were
administered 0.5 g deltamethrin to either shaven intact or abraded
skin. The occlusive patch was fixed on the skin for 23h. Scoring for
erythematous and oedematous lesions occurred 1 h and 49h after removal
of the patches. Technical deltamethrin, 98% a.i., showed no irritant
effect (Coquet 1976a).
Ocular irritation
Deltamethrin (0.1 g/animal) was administered into the
conjunctival sac of the eye of 6 male albino rabbits, weighing 2.5 kg,
with or without rinsing 60 sec. after instillation. Observations for
conjunctival lesions, chemosis, discharge, conjunctival enanthema,
opacity and affected cornea were made 1 h, 24h, 2,3,4 and 7 days
following instillation. Deltamethrin showed, both with and without
rinsing, transient irritating effects (Coquet 1976b).
Special studies on sensitization
Deltamethrin (0.5 g/animal) was applied topically to the skin of
albino guinea pigs with a 2-day interval for 3 weeks, and once at the
start of the 4th week. The preparation was covered with an occlusive
patch for 48h. On days 1 and 10 the guinea pigs received an
intradermal injection of 0.1 ml of Freund's adjuvant. The animals were
challenged 12 days after the last application with 0.5 g deltamethrin.
The macroscopic and histological examination did not reveal evidence
of sensitization (Guillot and Guilaine 1977).
Special studies on reproduction
Rat
Groups of 10 female and 10 male Charles River rats were fed
deltamethrin in the diet at 0, 2, 20 and 50 mg/kg and mated to begin a
three-generation, 2 litter (first generation, 3 litter) standard
reproduction study. Parental body weights and food consumption were
recorded during the study. After weaning of the second litter, the
survival parental rats were sacrificed and necropsied. Five male and
5 female pups of the F3b were necropsied. No changes in general
behaviour or survival of parental rats or pups relevant to the test
material were observed. The body weight of F0 males of the 50 mg/kg
group was decreased from week eleven onwards. There was some slight
decreases in mean food consumption of the parental F1 male rats in
the 50 mg/kg feed group.
The basic reproduction indices (fertility, gestation, lactation,
viability and litter size) were not affected by the treatment.
However, the mean pup weight was affected in some litters; especially
that of the 50 mg/kg group was slightly decreased in comparison to the
controls. Gross external examination revealed no abnormalities. No
gross or microscopic lesions of treatment-related significance or
significant effects on the organ weights of the F3b generation were
observed (Wrenn et al 1980).
Special studies on teratogenicity
Mouse
Mated female Swiss CDI.SPF mice (24/group) were given orally
deltamethrin dissolved in sesame oil at dose levels of 0, 0.1, 1 and
10 mg/kg bw/day during days 6 to 17 of pregnancy. The animals were
necropsied on day 18 of gestation. No teratogenic effects could be
detected. Total implantation sites, foetal losses, living foetuses and
examinations of skeletal tissue were normal. Minor embryotoxic effects
were observed, e.g. dose-dependent decrease of average foetal weight
and delayed ossifications at all dose levels tested (Glomot and
Vannier 1977).
Technical deltamethrin (Roussel UCLAF), dissolved in corn oil was
administered to CD-1 mice by gastric intubation at doses of 12.0, 6.0,
3.0 or 0 mg/kg during days 7 to 16 of gestation (Kavlock et al
1979). Maternal weight on day 6 was used for calculation of doses and
intubation was 0.2 ml; control animals received vehicle alone. Animals
were sacrificed on day 16 of gestation. Administration of deltamethrin
to pregnant mice resulted in a dose-related (p < 0.001) reduction in
maternal weight gain during pregnancy. Pregnant dams in the high
dosage group (12.0 mg/kg/day) gained 58% less weight than did those in
the control group. No dose-related occurrences of maternal mortality
were observed, but most animals in the high dosage group and some in
the middle dosage group became convulsive soon after dosing. No
effects were observed on the number of implantation sites, foetal
mortality, or foetal weights, or in the number of sternal and caudal
ossification centers. A significant (p < 0.01) dose-related increase
in the occurrence of supernumerary ribs was observed. No other dose-
related skeletal or visceral anomalies were observed in deltamethrin-
treated mouse foetuses. No evidence of teratogenic activity was found
in mice at dose levels that produced maternal toxicity (Kavlock
et al 1979).
Rat
Mated female Sprague-Dawley rats (24/group) were administered
orally 0, 0.1, 1 and 10 mg deltamethrin/kg bw/day during days 6 to 18
of pregnancy. Examination occurred on day 21 of gestation. Twelve
females in the control and 10 mg/kg bw groups were allowed to deliver.
There were no effects on the reproduction or teratogenicity parameters
examined, with the exception of a slight delayed ossification in the
highest dose level (Glomot and Vannier 1977).
Technical deltamethrin (Roussel UCLAF) dissolved in maize oil was
administered to Sprague-Dawley rats by gastric intubation at doses of
5.0, 2.5, 1.25 or 0 mg/kg during days 7 to 20 of gestation (Kavlock
et al 1979), Maternal weights on day 6 were used for the calculation
of doses and intubation volume was 0.2 ml; control animals received
the vehicle alone. Rats were sacrificed on day 21 of gestation.
Administration of deltamethrin to pregnant rats resulted in a dose-
related reduction (p > 0.01) in maternal weight gain during
pregnancy, with animals in the high dosage group (5.0 mg/kg) gaining
only 80% of the control value. This dose produced a mild salivation
for up to 4 h after dosing in approximately 50% of the animals. No
effects were observed on the number of implantation sites, foetal
mortality, foetal weight or number of sternal and caudal ossification
centres. For post-natal studies, an additional group of pregnant rats
was housed individually and intubated from day 7 of gestation to day
15 of lactation with dosages of either 5.0, 2.5 or 0 mg/kg
deltamethrin dissolved in maize oil. No persistent toxicity was
observed in neonatal rats that received perinatal exposure to
deltamethrin (Kavlock et al 1979).
Rabbit
Groups of fifteen mated females received deltamethrin dissolved
in sesame oil at levels of 0, 1, 4 or 16 mg/kg bw/day from days 6 to
19 of pregnancy. Examination was carried out on day 28 of gestation.
The average foetal losses were not dose-related and increased at all
doses tested. This effect was mainly caused by a higher rate of
expelling traces. The average foetal weight in the highest dose group
was decreased. Some malformations (one animal with hydrocephalia, and
one with exencephalia and thoracogastroschisis) were observed in
2 animals of the highest dose level. A complementary study with
15 mg/kg bw/day was performed. In this study one foetus with spina
bifida and shortened tail was detected among 69 parent normal
foetuses. It was concluded that the malformations were within the
normal limits of the strain used and were not related to the
treatment, despite the occurrence at the highest dose level only
(Glomot and Vannier 1977, 1978).
Special studies on neurotoxicity
Adult hens (10/group) were gavaged with a single dose of 0, 500,
1 250 or 5 000 mg/kg bw deltamethrin suspended in maize oil or 0 and
1 000 mg/kg bw dissolved in sesame oil. Tri-o-cresylphosphate (TOCP)
(500 mg/kg bw) was used as positive control for delayed neurotoxicity.
During 21 days, observations were made on mortality, health,
neurotoxic signs and body weight.
In the TOCP group, 8 out of 10 animals died whereas only
2 mortalities were observed in the group dosed at 1 000 mg
deltamethrin/kg with sesame oil as the vehicle. Deltamethrin
induced no clinical, macroscopic or histological signs of delayed
neurotoxicity. TOCP-treated hens showed severe ataxia and degenerative
changes in the spinal cord and, occasionally, in the sciatic nerve
(Ross et al 1978).
Special studies on potentiation
Mice
Deltamethrin is hydrolysed in vitro by esterases in blood and in
brain, kidney, liver and stomach preparations of mice. Pre-treatment
of mice with oxidase inhibitor, piperonyl butoxide (PB), or esterase
inhibitor, S, S, S-tributylphosphorotrithioate (DEF), delayed
metabolism of i.p. administered deltamethrin. Using oxidase or
esterase inhibitor, different vehicles and different administration
routes, it was possible to induce similar toxic effects with a wide
range of deltamethrin doses (6 to 191 mg/kg bw). The different
treatments showed that PB or DEF made mice more sensitive to
deltamethrin (Ruzo et al 1978b).
Special studies in mutagenicity
Bacteria and yeast
In a growth inhibition test with Escherichia coli, deltamethrin
at levels of 1 250, 2 500 and 5 000 µg/ml DMSO (0.1 ml per plate) had
the same marginal inhibitor effect on the mother strains (W 3110 and
WP2) as on their mutants (p 3478 and CM 611). Chloramphenicol and
N-methyl N'-nitro-N-nitroguanidine (MNNG) were used as positive
controls and induced clear inhibition (Peyre et al 1980).
Deltamethrin was compared with MNNG, 9-aminoacridine,
2-nitrofluorene and 2-amino-anthracene for mutagenic activity in the
Ames test with Salmonella typhimurium, strains TA 1535, 100, 1537,
1528 and 98. The concentrations of deltamethrin used were 2, 10, 50,
200, 500, 1 000 and 5 000 µg/plate. Deltamethrin began to precipitate
at 200 µg/plate. The mean number of revertants was not influenced by
any concentration of deltamethrin in any strain with or without S9-mix
(metabolic activation), whereas the positive control mutagens produced
an increase of the number of spontaneous revertants (Peyre et al
1980).
In a similar experiment, deltamethrin (0.2, 2, 20, 200 and
400 µg/plate dissolved in DMSO) in the presence of activated
microsomal enzymes did not influence the number of revertants of
S. typhimurium strains TA 1535, 1537, 1538, 98 and 100.
2-Aminoanthracene, 3-methylcholanthrene, benzo(a)pyrene and acridine
orange showed mutagenic activity, whereas thio-TEPA was negative
(Fouillet 1976).
Deltamethrin at levels of 10, 50, 100, 500 and 1 000 µg/plate was
not mutagenic in assays with S. typhimurium strains TA 1535, 1537,
1538, 98 or 100 either in the presence or absence of a rat-liver
homogenate metabolic activation system (the positive controls were
2-anthramine and 9-aminoacridine) (Kavlock et al 1979).
Deltamethrin was not mutagenic in assays with E. coli WP2 when
tested at levels of 10, 50, 100, 500 and 1 000 µg/plate with or
without metabolic activation (the positive control was 2-anthramine)
(Kavlock et al 1979).
Deltamethrin was not mutagenic at levels of 1 to 5% when tested
with and without metabolic activation with Saccharomyces cerevisiae
(the positive control was 1, 2,3,4-diepoxybutane) (Kavlock et al
1979).
Mammalian cells
Deltamethrin dissolved in Cremophor oil (0, 0.4, 0.2, 1 and 5 mM,
10, 0.08, 0.4, 2 and 10% v/v respectively) in the presence of a
metabolic activation system increased the incidence of chromosome and
chromatid aberrations and SCEs, after incubation with Chinese hamster
ovary cells at 1 mMol. In the absence of S9-mix (metabolic
activation), no higher rate of aberration was observed.
It was shown that this increased incidence was due to a subtoxic
effect of some reaction product of Cremaphor oil and S9-mix.
Deltamethrin in Cremaphor oil without S9-mix and dissolved in DMSO
(1%) at levels of 0, 0.001, 0.01, 0.1 and 0.2 mM with or without
metabolic activation had no effect on the number of aberrations and
SCEs. Due to insolubility, no higher concentrations were tested
(Sobels et al 1978).
Animals
Mice, 3 males and 3 females per dose, were gavaged for two
consecutive days with 5 or 10 mg deltamethrin dissolved in sesame
oil/kg bw. Control mice were gavaged with 0.3 ml sesame oil. The
incidence of chromatid aberrations in bone marrow cells or of
micronuclei in polychromic erythrocytes did not show any significant
statistical difference in treated and control groups (Sobels et al
1978).
A single oral administration of 15 mg/kg deltamethrin was given
to Swiss mice. Groups of 2 animals were sacrificed every 3 h during a
24-h period. Several animals died after treatment. The incidence of
chromatid aberrations in the femoral bone marrow was low. There was no
consistent time-related trend in the distribution of these aberrations
(Sobels et al 1978).
Deltamethrin dissolved in sesame oil in groups of 9 to 13 male
mice dosed orally with 0 or 3 mg/kg bw for 7 days and 6 or 15 mg/kg bw
in a single dose showed no effect on the rate of pre- or post-
implantation losses after mating with 6 to 18 non-treated females.
The highest dose tested was toxic to the males, e.g. 7 out of 20
animals died shortly after treatment. Histological examination of
the testes of all animals revealed no abnormalities. Triethylene
thiophosphoramide (10 mg/kg bw), used as a positive control, reduced
considerably the rate of pregnancies in the second and third week and
increased the number of embryonal losses (Vannier and Glomot 1977).
Special studies on humans
Cutaneo-mucuous manifestations have been observed among plant
workers dermally exposed to technical deltamethrin or its
formulations. Initial lesions were tenacious and painful pruritus
(pricking sensation), especially observed after exposure to hot water
or on perspiration, followed by a blotchy local burning sensation with
blotchy erythema for about 2 days. Thereafter, slight and regular
desquamation, restricted to the contaminated area, occurred. The
cutaneous signs are sometimes accompanied by itching of the face
(mainly around the mouth) and/or rhinorrhoea or lacrimation. No other
symptoms related to exposure were observed (Husson 1978).
The effects described, which were observed before 1978 in a few
plant workers, have not been seen since a new plant was specifically
built for the entire automatization of deltamethrin production, which
does not involve workers being in direct contact with intermediates
and the final product. The only workers exposed are those involved in
the packaging of the technical product (>98% active ingredient) and
they are fully protected by special hermetic clothing (Glomot 1981).
At the level of formulation plants, steps have been taken to
avoid direct contact by workers involved in introducing technical
powder in the basic solvent. No toxic effects have been reported in
the past several years in these formulation plants, although a very
limited number of workers have complained about some irritating
effects. However, these were always transient and without any further
consequences (Glomot 1981).
The formulation which has been most frequently used for
deltamethrin application is the EC. The use of both EC and ULV
formulations corresponds in 1981 to a treated area covering several
million hectares in the world without any case of intoxication. The
majority of users are plant growers who apply the formulations in the
fields and are not in contact with the technical product per se
(Glomot 1981). A few cases of irritation problems have been reported
in some workers, due primarily to the use of the EC formulation. This
has been observed when treatments were made in orchards with high
trees needing high volumes of mixture (e.g. in South Africa) or under
greenhouses in confined spaces. In all cases, the reported problems
were not severe and could be treated using antihistaminic syrups or
with pomades based on cocaine derivatives. The irritation is most
probably enhanced by the presence of aromatic solvent, e.g. xylene, in
the EC formulation. Flowable experimental formulations without any
solvent did not cause irritating effects in human volunteers (Glomot
1981). No cases of neurological effects have been reported with
formulation workers nor have fatal intoxications been reported (Glomot
1981).
RESIDUES IN FOOD
USE PATTERN
Pre-harvest treatments
Information was received from The Netherlands on pre-harvest
treatment of several crops with deltamethrin, which is summarized in
Table 5.
RESIDUES ARISING FROM SUPERVISED TRIALS
Results of supervised trials with deltamethrin on various crops
are summarized in Table 6.
Assorted fruit, inedible peel
Kiwi fruit
Data from two new field trials conducted in France show that
residue levels for the whole fruit are below 0.05 mg/kg, the residue
being entirely in the peel.
TABLE 5. Deltamethrin use in The Netherlands
Application Rate
Crop g/100 l g/ha P.H.I.
Apple 0.5 (from before blossom to
Pear 0.5 (to 14 days P.H.
Grape 0.5 7
Cherry 0.5 7
Plum 0.5 7
Strawberry 0.5 7
Currant 0.5 7
Gooseberry 0.5 7
Raspberry 0.5 7
Cucumber 1.25 3
Melon 25 14
Bell pepper 25 14
Eggplant 25 14
Lettuce 1.25 14
Endive 25 14
Radish 7.5 7
Turnip 7.5 7
Rutabaga 7.5 7
Brussels sprouts 7.5 7
Cauliflower 7.5 7
Broccoli 7.5 7
Kohlrabi 7.5 7
Leek 7.5 7
Onion 7.5 7
Peas 7.5 7
Dwarf french bean 7.5 7
Sugarbeet 7.5 7
Mushroom 0.75-1.5 75/100 2
Hops
Several trials conducted in the UK and the Federal Republic of
Germany show a high level of variation between samples, but relatively
little decline with time over the period of 7 to 14 days following
treatment. No information was provided on the fate of such residues
when the hops were used in brewing.
TABLE 6. Supervised trials with deltamethrin in various crops
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) Formulation of sample 0 1 2/3 4 7 14
Kiwi fruit France 1980 2 12.5 peel 0.05 0.06 0.06 0.08 0.07
whole fruit 0.01 0.01 0.01 0.01 0.01
pulp ND ND ND ND ND
12.5 peel 0.4 0.22 0.23 0.25 0.1
whole fruit 0.06 0.04 0.04 0.04 0.01
pulp ND ND ND ND ND
Hops England 1978 7 12.5 0.02
W. Germany 1978 1 25 0.01
3 62.5 0.1 0.3 1.4 1.1 1.9
3 62.5 16 5.5 0.1 1.8 1.9
3 62.5 3.3 1.3 0.4 2.0 2.5
1980 5 6.25-37.5 1.3 0.2 0.4 0.3 0.7
5 " 0.2 0.7 0.3 0.7 1.6
5 " 1.0 0.7 0.2 0.6 2.7
5 " 0.5 0.3 0.2 0.7 1.1
Mushrooms1 Netherlands 1978 2 6.25 0.0025% whole 0.003 0.002 0.001
1978 2 25 0.01% " 0.009 0.004 0.003
(DAYS)
0 1 3 5 7 14
Black currant Finland 1979 1 6.3mg/bush. 0.1 0.1
W. Germany 1979 2 18.5g/ha 0.3 0.2 0.2 0.1
" 0.3 0.3 0.3 0.3
" 0.3 0.2 0.2 0.2
2 11.25g/ha 0.2 0.2 0.1 0.1
0.3 0.4 0.3 0.2
TABLE 6. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) of sample 0 1 2 4 8 14 21
Spinach France 1978 1 17.5g/ha Fresh leaf 0.48 0.33 0.22
Cooked leaf 0.27 0.21
Cooking water 0.0001 0.00009
17.5g/ha Fresh leaf 0.52 0.30 0.155
Cooked leaf 0.28 0.12
Cooking water 0.00015 0.000,05
1979 1 12.5g/ha Fresh leaf 0.4 0.2 0.14 0.12
Cooked leaf 0.12 0.10
Cooking water 0.0001 ND
12.5g/ha Fresh leaf 0.4 0.2 0.17 0.15
Cooked leaf 0.15 0.12
Cooking water 0.00015 0.0001
1980 1 17.5g/ha Fresh leaf 0.18 0.22 0.35
Cooked leaf 0.16 0.20 0.28
Cooking water ND ND ND
W.Germany 1980 3 12.5g/ha Fresh leaf 0.3 0.7 0.2 0.09
0.7 0.3 0.3 0.1
14 21 28/39 49 61 74 80
Wheat straw France 1978 1 7.5 EC 0.015
1 15 EC 0.05
1 7.5 EC 0.025
1 15 EC 0.025
1 7.5 EC 0.025
1 15 EC 0.025
1 7.5 EC ND
1 7.5 EC ND
1 12.5 EC 0.15
1 12.5 EC 0.06
1 12.5 EC 0.2
1 12.5 EC 0.1
TABLE 6. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) of sample 14 21 28/39 49 61 74 80
Oat Straw W.Germany 1978 1 12.5 EC 0.06
1 12.5 EC 0.06
1 12.5 EC ND
1 12.5 EC 0.08
1979 1 12.5 EC 0.6
1 12.5 0.2
1 12.5 0.2
1 12.5 0.5
Rice straw Surinam 1977 1 6.25 ND
1 12.5 0.010
1 18.75 0.01
1 25 0.08
Phillipines 1978 1 17.5 0.2
25 0.1
1 After oven drying at 7 days.
Small fruits and berries
Black currant
From trials conducted in the Federal Republic of Germany, it
would appear that residues on these berries can range up to 0.3 to
0.4 mg/kg, but as yet, there is no registered use of deltamethrin on
black currants.
Leafy vegetables
Spinach
Additional data from trials carried out in France and the Federal
Republic of Germany indicate that residues range around 0.3 to
0.4 mg/kg, 7 or 8 days after the last treatment. These levels do not
decline significantly during cooking.
Fodder and cereal straws
Additional data were available to indicate the level of
deltamethrin on wheat straw, oat straw and rice straw. Though the bulk
of the values were below 0.2 mg/kg, oat straw sampled 28 to 39 days
after treatment showed residues ranging up to 0.6 mg/kg. The amount of
data is, however, limited and in view of the potential for the wide
application of deltamethrin to cereal crops further data are required.
Cereal grains
A report was received on trials to determine the fate of
deltamethrin on wheat during storage and after milling and baking
(Halls and Periam 1980).
Batches of an English hard wheat were sprayed on a conveyor with
diluted deltamethrin/piperonyl butoxide liquid grain protectant to
give 250 kg at each of two deltamethrin treatment levels. These 250 kg
batches were then placed in 1 ton capacity metal silos. Target
treatment levels for the two batches of grain were 1 mg/kg
deltamethrin + 10 mg/kg piperonyl butoxide and 2 mg/kg deltamethrin +
10 mg/kg piperonyl butoxide respectively, but chemical analysis
revealed that the actual treatment levels achieved were only about 40
to 75% of these.
Samples of the treated wheat were taken after spraying and
thereafter at monthly intervals for nine months and analysed for
deltamethrin content. Replicate samples were taken from each silo,
analysed separately and the result expressed as the mean. Wheat from
the initial sampling and from samplings after 3, 6 and 9 months was
submitted to the Flour Milling and Baking Research Association,
Chorleywood, Buckinghamshire to be milled and baked.
At Chorleywood the wheat was first cleaned of extraneous
material, then divided into two samples and each subjected to a
different milling procedure. The first produced whole-meal flour, the
second produced white flour, bran and fine offal.
Two different samples of white flour were taken from this
procedure, the first reduction flour (the cleanest flour mainly from
the centre of the grain and used in the baking of cakes) and total
white flour (straight-run flour plus flour from the bran and fine
offal fractions). These samples, together with the wholemeal flour,
bran and fine offal were analysed for total deltamethrin content for
both batches of treated wheat. In addition, bread was baked from both
wholemeal and total white flours to produce wholemeal and white bread,
respectively, and these loaves were also analysed for deltamethrin
content.
Results for residues of deltamethrin on wheat, milling data and
residues of NRDC 161 on flour fractions and bread are given in Tables
7, 8 and 9, respectively.
In both the deltamethrin treatments, levels were initially found
by analysis to be only about 40% of the expected application. This
could be due predominantly to inter alia incorrect spraying rates,
inadequate extraction, or loss of compound at some stage between the
nozzle and the grain.
The results given in Table 7 show that for both application rates
there was no detectable breakdown of deltamethrin on wheat over a
nine-month storage period. The fluctuation in levels between different
samplings was within the inherent error of the analytical method
demonstrating that deltamethrin is very stable on wheat. In this
respect deltamethrin differs from many organophosphate grain
protectants such as fenitrothion (Desmarchelier 1978).
The moisture content of the wheat remained fairly consistent over
the storage period of nine months. Deltamethrin analysis data for the
milling fractions and bread derived from treated wheat are given in
Tables 8 and 9.
For all sampling times at both treatment levels there was no
significant change in deltamethrin residues when wheat was milled to
produce wholemeal flour. When this flour was baked, the wholemeal
bread produced contained lower, or identical, deltamethrin residues
but this can be explained by the greater moisture content (up to 45%)
present in bread.
TABLE 7. Deltamethrin residues on wheat after indicated periods of storage in the UK1
Residue analysis (mg/kg deltamethrin)
Target
Application Initial2 1 month 2 months 3 months 4 months 5 months 6 months 7 months 8 months 9 months
rate (mg/kg) (15.11.79) (13.12.79) (17.1.80) (11.2.80) (12.3.80) (21.4.80) (12.5.80) (10.6.80) (10.7.80) (6.8.80)
1.0 0.44 0.53 0.48 0.50 0.50 0.41 0.48 0.49 0.42 0.41
2.0 0.80 1.25 1.33 1.46 1.21 1.01 0.96 1.12 0.94 1.08
1 Analytical results are subject to overall confidence limits of 30%
2 Dates refer to day of sampling.
TABLE 8. Milling data for wheat freshly treated with deltamethrin and from wheat after 3, 6 and 9 months storage1
Target application Sampling Clean wheat Bran Fine Offal Total white flour
rate period
(mg/kg) (months) Weight Prop. of Weight Prop. of Weight Prop. of Weight Prop. of
(kg) total(%) (kg) total (%) (kg) total (%) (kg) total (%)
0 3.835 100 0.596 15.54 0.306 7.98 2.849 74.29
3 4.921 100 0.779 15.83 0.519 10.55 3.502 71.16
1.0
6 3.700 100 0.621 16.78 0.340 9.19 2.670 72.16
9 3.700 100 0.626 16.92 0.288 7.78 2.746 74.22
0 3.786 100 0.590 15.58 0.292 7.71 2.809 74.19
3 4.884 100 0.769 15.75 0.514 10.52 3.489 71.44
2.0
6 3.700 100 0.630 17.03 0.306 8.27 2.688 72.65
9 3.700 100 0.650 17.57 0.275 7.43 2.692 72.76
0 (Control) 0 2.010 100 0.312 15.52 0.175 8.71 1.525 75.87
1 There is a loss of up to 3% in the laboratory milling process
TABLE 9. Deltamethrin residues on milling fractions and bread made from freshly treated wheat and from wheat after 3, 6 and
9 months storage1
Target application Sampling First Total
rate period Wholemeal Wholemeal Bran Fine reduction white White
(mg/kg) (months) Wheat flour bread offal flour flour bread
0 0.44 0.42 0.26 3.00 nd2 nd 0.09 0.10
3 0.50 0.43 0.27 1.80 approx. 0.5 nd approx. 0.05 nd
1.0
6 0.48 0.42 0.26 1.70 0.35 0.05 0.09 0.10
9 0.41 0.36 0.36 1.90 0.46 0.03 0.03 0.05
0 0.80 0.73 0.63 5.40 nd nd 0.20 0.29
3 1.46 0.80 0.57 4.70 0.75 nd 0.20 0.15
2.0
6 0.96 0.83 0.66 3.60 0.75 0.10 0.16 0.20
9 1.08 0.95 0.70 4.40 1.36 0.05 0.17 0.11
1 Analytical results are subject to confidence limits of ± 30%
2 nd = not detectable (<0.03 mg/kg).
When the treated wheats were milled to produce white flour,
relatively high deltamethrin residues were found on the bran fraction,
which is derived from the superficial layers of the grain. Initially,
deltamethrin residues on the bran were 6 to 7 times higher than those
on the wheat at both treatment rates. The levels declined to be about
4 to 5 times higher on the bran than on the wheat after 9 months'
storage.
This observation, together with the appearance of deltamethrin on
the fine offal fractions after 3 months' storage, and on the first-
reduction flours after 6 months' storage, suggests that although there
is no change in the total deltamethrin level on the wheat over 9
months, there is a subtle change in its distribution. Thus, initially,
as might be expected, the insecticide residues were found
predominantly in the outer layers of the grain, mainly in the bran
fraction, but also to a lesser extent in the total white flour
fraction. The first-reduction flour, which tends not to come from the
superficial layers of the grain, and the fine offal, consisting
largely of wheatgerm, is uncontaminated initially but residues move
into these areas as the period of storage is increased.
The deltamethrin levels on total white flour milled from treated
wheat after different storage periods remained quite constant at about
one sixth of the applied rates. Some fluctuation in this proportion
was detectable at the lower application rate, but this is probably
because in these cases the deltamethrin levels on the flour were close
to the limits of detection. When baked in white bread, there is no
significant change in residue levels, showing that no breakdown of
deltamethrin occurred during baking.
Consideration of the levels found on each fraction, and the
weights of each fraction produced by unit weight of wheat, leads to
the conclusion that there is no breakdown of deltamethrin during the
break, reduction and finishing systems of the milling process that
produces white flour.
The following conclusions may be drawn from the results of this
trial:
Deltamethrin is not degraded on wheat over a nine-month storage
period.
There is no degradation of deltamethrin when treated wheat is milled
to produce either wholemeal or white flour.
When wholemeal flour made from deltamethrin treated wheat is baked,
there is an apparent reduction in deltamethrin residues, but this is
due to the greater moisture content of the bread.
In the production of white flour and bread from deltamethrin treated
wheat, there is a reduction in the deltamethrin level to about 10 to
20% of the level applied to the wheat. This is because the
deltamethrin is predominantly found on the bran fraction with a
concentration increase of 4 to 7 fold. There is no evidence for
deltamethrin degradation when white flour is baked to produce white
bread.
Over a 9-month storage period, there appears to be a migration of
deltamethrin to the fine offal fraction (including the wheat germ
which is used in health foods and "Hovis" bread) and the first-
reduction flour (used in the production of cakes) presumably from the
bran fraction (predominantly used for animal feed).
NATIONAL MAXIMUM RESIDUE LIMITS
The Meeting was advised of the following limits established by The
Netherlands:
Strawberry 0.2 mg/kg
Other fruit 0.1 mg/kg
Leafy vegetables 0.2 mg/kg
Other vegetables 0.05 mg/kg
EVALUATION
COMMENTS AND APPRAISAL
Deltamethrin was first evaluated in 1980 but, in the absence of
some essential toxicological information, it was not possible to
establish an ADI. The Meeting proposed a series of guideline levels
reflecting residues likely to occur on various food commodities
following the use of deltamethrin with present good agricultural
practice. A small amount of additional information was available for
consideration at the Meeting.
Residue data from trials on kiwi fruit were considered suitable
to propose guideline levels, but further data are needed to confirm
whether these levels are typical of those occurring in other regions
where these crops are grown. In the case of kiwi fruit, the residues
are entirely confined to the inedible peel. Data were received from
residue trials on black currants, but as there was evidence that
deltamethrin was not yet approved for use on currants, no guideline
levels were proposed.
Results of several trials on spinach indicate that cooking has
little or no effect on the level of deltamethrin residues on such
leafy vegetables. These studies do not, however, provide an adequate
basis for determining whether the guidelines proposed in 1980 for
spinach should be amended.
There is a suggestion that residues of deltamethrin on some
cereal straws used for animal feed could be higher than the 0.5 mg/kg
limit proposed for legume animal feeds, but the amount of information
is inadequate to provide a basis for amending the recommendation.
A report on one further study of the level and fat of
deltamethrin on wheat, milled products and bread confirm that
deltamethrin is not degraded significantly during storage, nor is it
destroyed in cooking. A substantial proportion is, however, removed on
bran during the milling of white flour. In view of the vast experience
with other grain protectant insecticides and the considerations
discussed in 1980, the Meeting deemed it necessary to revise the
recommended guideline levels for deltamethrin on raw cereals and
milled cereal products to accommodate, realistically, all the
situations likely to arise from normal applications under good storage
practice, although in most cases, levels would be considerably lower.
Such provisions are also intended to accommodate the variations due to
sampling difficulties and analytical error.
The Meeting, however, expressed the view that considerably more
information was required about the level and fate of deltamethrin on
different grains under different storage conditions and especially the
effect of processing and cooking of all cereal grains.
RECOMMENDATIONS OF GUIDELINE LEVELS
As no ADI has been allocated, the Meeting proposed the following
guideline levels determined and expressed as deltamethrin.
Commodity Guideline level (mg/kg)
Kiwi fruit 0.05
Hops (dry) 5
Cereal straw (animal feed) 0.5
Cereal grains 2
Wheat bran (unprocessed) 5
Wheat flour (wholemeal) 2
FURTHER WORK OR INFORMATION
Required (by 1982 and before an acceptable daily intake can be
allocated) (as listed in the 1980 JMPR Report).
1. Results of the two-year feeding study in dogs currently in
progress.
2. Clarification of possible embryotoxic effects in animals.
3. Further observations on reported effects in man.
4. Studies on the significance of neurological effects observed in
several animal species.
5. Results of supervised trials on residues in meat, milk, and eggs
arising from the use of deltamethrin for ectoparasite control, in
feeding studies and in stable treatments. Also required before MRLs
can be recommended:
6. Considerably more information on the level and fate of
deltamethrin on various cereal grains treated under different storage
conditions, and especially the effect of processing and cooking of all
cereal grains.
7. Additional residue data from supervised trials on kiwi fruit,
currants and leafy vegetables.
8. Information on the level and fate of deltamethrin residues in
foods of animal origin following the feeding of cattle, pigs and
poultry with rations containing deltamethrin at levels of the order
likely to be encountered in practice.
9. Further information on the level of deltamethrin on cereal grains
following treatment of crops in conformity with approved or proposed
use patterns.
REFERENCES
Barnes, J.M. and Verschoyle, R.D. Toxicity of a new pyrethroid
1974 insecticide. Nature, 248:711 1974
Beavers, J.B. and Fink, R. Eight-day dietary LC50-mallard duck,
1977a technical DECIS. Final report W1-77.18.05/A, Wildlife
International, USA, submitted by Roussel Uclaf to WHO.
(Unpublished)
Beavers, J.B. and Fink, R. Eight-day dietary LC50-bobwhite quail,
1977b technical DECIS. Final report. W1-77-19.05/A, Wildlife
International, USA, submitted by Roussel Uclaf to WHO.
(Unpublished)
Cabral, J.R. In: IARC Annual Report - 1981. International Agency for
1981 Research on Cancer, Lyon, in press.
Chesterman, H,, Heywood, R., Perkin, C.J., Beard, D., Street, E. and
1977 Prentice, D.E. RU 22974. Oral toxicity study in beagle dogs.
Report RSL 253/7751/A 3, Huntingdon Research Centre,
Huntingdon, England, submitted by Roussel Uclaf to WHO.
(Unpublished)
Clair, M. RU 22974 - DECIS. Acute toxicity in the rabbit by
1977 percutaneous administration. Report IFREB-R 770256.1/A,
Institut Francais de Recherches et Essais Biologiques,
Joinville-le-Pont, France, submitted by Roussel Uclaf to
WHO. (Unpublished)
Coombs, D.W. and Clark, G.C. RU 22974. Acute inhalation toxicity in
1978 rats, 6 hour LC50. Report RLS 310/78453/A, Huntingdon
Research Centre, Huntingdon, England, submitted by Roussel
Uclaf to WHO. (Unpublished)
Coquet, B. RU 22974. Test to determine primary cutaneous irritation in
1976a the rabbit. Report no. IFREB-R 761157/A, Institut Francais
de Recherches et Essais Biologiques, Joinville-le-Pont,
France, submitted by Roussel Uclaf to WHO. (Unpublished)
1976b RU 22974. Test to evaluate ocular irritation in the rabbit.
Report no. IFREB-R 761158/A, Institut Francais de Recherches
et Essais Biologiques, Joinville-le-Pont France, submitted
by Roussel Uclaf to WHO. (Unpublished)
Desmarchelier, J., Bengston, M., Connell, M., Minett, W., Moore, B.,
1977 Phillips, M., Snelson, J., Sticka, R. and Tucker, K. A
collaborative study of residues on wheat of
chlorpyrifosmethyl, fenitrothion, malathion, methacrifos and
pirimiphos-methyl. 1. Method Development. Pesticide Science,
8: 473-483.
Desmarchelier, J. Loss of fenitrothion on grains in storage. Pesticide
1978 Science, 9:33-38.
Elliott, M. Synthetic pyrethroids, American Chemical Society
1977 Symposium, 42:1-29.
Fouillet, X. RU 22974. Mutagenicity study of various preparations.
1976 Salmonella microsome test. Report no. IFREB-R 761153/A,
Institut Francais de Recherches et Essais Biologiques,
Joinville-de-Pont, France, submitted by Roussel Uclaf to
WHO. (Unpublished)
Gaughan, L.C., Unai, T. and Casida, J.E. Permethrin metabolism in
1977a rats. Journal of Agricultural and Food Chemistry, 25:9-17
1977b Permethrin metabolism in rats and cows and in bean and
cotton plants. American Chemical Society Symposium,
42:186-193.
Glomot, R. and Chevalier, B. RU 22974. Acute toxicity study, mouse and
1976a rat by oral route. Report Tox 76810/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
1976b RU 22974. Acute toxicity study mouse and rat by
intraperitoneal route. Report Tox 76811/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
1976c RU 22974. Acute toxicity study, mouse and rat by intravenous
route. Report Tox 76812/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
Glomot, R., Chevalier, B., Collas, E. and Audegond, L. RU 22974. Acute
1977 toxicity study by oral route in male and female beagle dogs.
Report Toxico 77804/JL-5, submitted by Roussel Uclaf to WHO.
(Unpublished)
Glomot, R. and Vannier, B. RU 22974. Teratological study in mouse, rat
1977 and rabbit. Report no. Tox 76534-76536/2/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
Glomot, R. and Vannier, B. RU 22974. Teratological study in mouse.
1978 Complementary Information. Report no. Tox. 76534.76536/A3,
submitted by Roussel Uclaf to WHO. (Unpublished)
Glomot, R. Personal communication to WHO. Roussel, Uclaf, Paris,
1981 France.
Goldenthal, E.I., Blair, M., Jefferson, N.D., Spicer, E.J.F., Arceo,
1980a R.J. and Clark Kahn III, A., RU 22974. Two-year toxicity and
carcinogenicity study in mice. Report IRDC 406-001/A/4,
International Research and Development Corp., Mattawan,
Michigan, submitted by Roussel Uclaf to WHO. (Unpublished)
Goldenthal, E.I., Jefferson, N.D., Blair, M., Thorstenson, J.H.,
1980b Spicer, E., J.F. Arceo, and Kahn, III, A. RU 22974. Two year
oral toxicity and carcinogenicity study in rats. Report IRDC
406-002/A 4, International research and Development Corp.,
Mattawan, Michigan, submitted by Roussel Uclaf to WHO.
(Unpublished)
Gray, A.J. and Rickard, J. Distribution of radiolabel in rats after
1981 intravenous injection of a toxic dose of 14C-acid-,
14C-alcohol- or 14C-cyano-labelled deltamethrin. Pesticide
Biochemistry and Physiology, 16:79-85
Guillot, J.P. and Guilaine, J. RU 22974 - Decamethrine. Decis
1977 technical Roussel Uclaf. Sensitization test in the guinea
pig. Report no. IFREB-R 709241/A, Institut Francais de
Recherches et Essais Biologiques, Joinville-le-Pont, France,
submitted by Roussel Uclaf to WHO. (Unpublished)
Halls, G.R.H., and Periam, A.W.. The fate of residues of NRDC 161 on
1980 wheat during storage and after milling and baking - Report
after 9 months storage. Wellcome Research Laboratories
Report HEFH 80-4, November 1980. (Unpublished)
Husson, J.M. Medical observations made on people working on the
1978 manufacture and formulation of the pyrethroid insecticide
decamethrin. Report no. RU 78.25.08/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
Hunter, B., Jordan, J., Heywood, R., Hepworth, P., Street, A.E. and
1977 Prentice, D. RU 22974. Assessment of toxicity to rats by
oral administration for 13 weeks (followed by a 4 week
withdrawal period). Report RSL 254/76938/A3, Huntingdon
Research Centre, Huntingdon, England, submitted by Roussel
Uclaf to WHO. (Unpublished)
IRDC Laboratories. Two year chronic dog feeding study. Report IRDC
1980 406-004/A1, International Research and Development
Corporation, Mattawan, Michigan, submitted by Roussel Uclaf
to WHO. (Unpublished)
Kavlock, R., Chernoff, N., Baron, R., Linder, R., Rogers, E. and
1979 Carver, B. Toxicity studies with decamethrin, a synthetic
pyrethroid insecticide. Journal of Environmental Pathology
and Toxicology, 2:751-765.
Kynoch, S.R., Lloyd, G.K. and Andrews, C.D. Acute percutaneous
1979 toxicity to rats of decamethrin. Report RSL-10098/D
147/79/A, Huntingdon Research Centre, Huntingdon England,
submitted by Roussel Uclaf to WHO. (Unpublished)
Peyre, M., Chantot, J.F., Glomot, R. and Penasse, L. Detection of a
1982 mutagenic potency of decamethrin (RU 22974). Bacterial
tests. Report no. RU/TOX/80.21.01/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
Ray, D.E. and Cremer, J.E. The action of decamethrin (a synthetic
1979 pyrethroid) on the rat. Pesticide Biochemistry and
Physiology, 10:33.
Ray, D.E. An EEG investigation of decamethrin induced choreoathetosis
1980 in the rat. Experimental Brain Research, 38:221.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E., Cooke, L.
1978 and Gibson, W.A. RU 22974 (Decamethrin) LD50 determination
and assessment of neurotoxicity in the domestic hen. Report
no. RSL 293-NI/830/A. Huntingdon Research Centre,
Huntingdon, England, submitted by Roussel Uclaf to WHO.
(Unpublished)
Roussel Uclaf. Test to determine the toxicity in the chicken by oral
1976a route. Report RU-76.05.05/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
1976b Toxicity of decamethrin or DECIS in single ingestion in
game ducks, Anas platyrhynchos L. Report INRA-76.21.12/A.
Institut National de la Recherche Agronomique. Jony-En-
Josas, France, submitted by Roussel Uclaf to WHO.
(Unpublished)
Roussel Uclaf. Toxicity of decamethrin or DECIS by single ingestion
1976c in grey partridge, Perdix perdix l., and Red Partridge
Alectous rufa l. Report INRA-76.28.09/A. Institut National
de la Recherche Agronomique. Joney-en-Josas, France,
submitted by Roussel Uclaf to WHO. (Unpublished)
Ruzo, L.O., Unai, T. and Casida, J.E. decamethrin metabolism in rats.
1977 Report UC-77.06.12/A, submitted by Roussel Uclaf to WHO.
(Unpublished)
1978a Decamethrin metabolism in rats. Journal of Agriculture and
Food Chemistry, 26:918-925.
Ruzo, L.O., Engel, J.L. and Casida, J.E. Oxidative, hydrolytic and
1978b conjugative reactions in the metabolism of decamethrin in
mice. Report UC 78.10.11/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
1979 Decamethrin metabolism from oxidative, hydrolytic and
conjugative reactions in mice. Journal of Agricultural Food
and Chemistry, 27:725-731.
Shono, T., Oshawa, K. and Casida, J.E. Metabolism of trans- and cis
1978 permethrin, trans- and cis-cypermethrin and decamethrin by
microsomal enzymes. Report UC 78.18.05/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
Sobels, F.H., Tates, A.D. and Vannier, B. Cytogenetic study with RU
1978 22974. Detection of a mutagenic potency in mammalian cells.
Report no. ULN-782211/A, State university of Leiden, The
Netherlands, submitted by Roussel Uclaf to WHO.
(Unpublished)
Soderland, D.M. and Casida, J.E. Substrate specificity of mouse liver
1977 microsomal enzymes in pyrethroid metabolism. In: Synthetic
Pyrethroids, American Chemical Society Symposium,
42:152-162.
Vannier, B. and Glomot, R. RU 22974. Mutagenic study. Dominant lethal
1977 assay in the male mouse. Report no. Toxico 76533/DB9/A,
submitted by Roussel Uclaf to WHO. (Unpublished)
Wrenn, J.M., Rodwell, D.E., Goldenthal, E.I., Spider, E.J.C. and
1980 Rajasekaran, B. Three-generation reproduction study in rats.
Report no. IRDC-406-003/A4, International Research and
Development Corporation, Michigan, USA, submitted by Roussel
Uclaf to WHO. (Unpublished)
 phenolic hydroxyl and carboxylic acid groups. The acid moiety is
rapidly excreted as the glucuronide, with smaller amounts as free and
as the glycine conjugate. The trans-hydroxymethyl derivative is also
excreted both free and as the glucuronide.
All major metabolites of the aromatic portion of the alcohol
moiety are rapidly excreted and probably arise from ester cleavage of
deltamethrin or its ester metabolites, conversion of the released
cyanohydrins to the aldehydes which rapidly yield the corresponding
acids and conjugates of these acids. Table 1 illustrates the
radiocarbon in the urine, faeces, carbon dioxide and tissues of rats
up to 8 days after oral administration of labelled deltamethrin.
TABLE 1. Radiocarbon in the urine, faeces, carbon dioxide, and
tissues of rats up to 8 days after oral administration
of 14Cv, 14Cai.e, alpha &: 14CN deltamethrin.
Labeling position
Sample analyzed 14Cva,b 14Calpha 14CN
Administered Dose, mg/kg
0.90 1.60 0.64
% of Administered Dose
Urine
0-1 day 45.1 67.6 10.4
1-2 days 5.6 3.0 8.1
2-4 days 2.7 2.1 11.4
4-8 days 1.0 1.0 13.0
Feces
Methanol extract
0-1 day 35.6 22.7 12.1
1-2 days 2.0 1.8 2.3
2-4 days 1.7 0.3 3.3
4-8 days 0.4 0.0 3.3
Unextractable, 0-8 days 4.4 0.4 14.7
14CO2, 0-2 days 0.0 0.0 0.0
Carcass and tissues, 8 days 1.5 1.1 21.4
TABLE 1. (con't)
Labeling position
Sample analyzed 14Cva,b 14Calpha 14CN
Tissue Residue at 8 Days, ppb of Decamethrin Equiv
Blood 12 89 103
Bone 12 11 57
Brain 4 20 5
Fat 59 182 94
Heart 8 9 41
Intestine
Large 9 5 77
Small 10 5 129
Kidney 8 10 66
Liver 12 38 66
Lung 4 5 73
Muscle 5 5 57
Skin 16 16 603
Spleen 2 5 49
Stomach 8 3 654
Testes 5 3 54
a With (1RS)-trans-[14Cv]decamethrin administered orally at
0.94 mg/kg, the 14C balance sheet at 8 days as percent of dose is
72.6% in urine (48.6% at 1 day, 62.3% at 2 days, and 71.2% at
4 days), 23.2% in the methanol extract of feces (12.3% at 1 day,
18.6% at 2 days, and 21.7% at 4 days), 3.0% in the unextractable
portion of feces (0-8 days), and 1.2% in carcass and tissues (all
individual tissues as above < 10 ppb decamethrin equivalents),
b With [14Cv]Br2CA administered orally at 3.74 mg/kg, the 14C
balance sheet at 8 days as percent of dose is 94.0% in urine (70% at
1 day, 82% at 2 days, and 91% at 3 days), 5.8% in feees (5.4% in methanol
extract), 0.0% 14CO2, and 0.2% in carcass and tissues. The tissue
residues are < 3 ppb decamethrin equiv except for liver which is
27 ppb.
The pathways involved in rat metabolism of deltamethrin isomers
are similar to those utilized for other pyrethroids in many segments
of the ecosystem (Elliott 1977; Gaughan et al 1977a, b).
The tissue distribution of toxic doses of 14C-acid,
14C-alcohol-, and 14C-cyano labelled deltamethrin after i.v.
administration to rats has been studied (Gray and Richard 1981). All
three radiolabelled preparations were found in every tissue examined
1 min. after injection. Peak CNS levels were achieved within 1 to
5 min. but did not correspond to the onset of choreoathetosis. In the
majority of tissues examined, the levels of deltamethrin label were
similar, proportionately to those found after administration of
alcohol labelled cismethrin. A major exception was the CNS, which
contained approximately 20% of the anticipated level of radiolabel,
suggesting that the CNS threshold for deltamethrin was approximately
0.5 to 1.0 nmol/g. Progressive accumulation of radioactivity in the
erythrocytes fraction of the blood was observed following
administration of cyano-labelled deltamethrin; it was suggested that
this may contribute to the selective retention of 14C with
radiolabelled preparations.
Mouse
Studies on mice to determine oxidative, hydrolytic and
conjugative reactions were conducted with (14Calpha) and (14Cv)
labelled deltamethrin (Ruzo et al 1979). The labelled deltamethrins
were orally administered to two mice each at 3.1 and 3.6 mg/kg
respectively and the animals sacrificed 6 h later. The 14C label was
rapidly and almost completely excreted, with little tissue retention
after 8 days (Table 2).
Deltamethrin metabolism in mice involved four sites of oxidative
attack (trans-methyl group of the acid moiety and 2', 4' and 5
positions of the alcohol moiety, hydrolysis and a variety of
conjugation process) (Figure 2). Mice excrete less unmetabolized
deltamethrin than rats (Ruzo et al 1978) suggesting more efficient
absorption and/or metabolism. Mice produce considerable amounts of the
trans-, 2'- and 5'-hydroxy derivatives, whereas rats hydroxylate
deltamethrin predominantly at the 4'-position.
The acid moiety is rapidly excreted as the glucuronide with
smaller amounts free and as the glycine conjugate. The trans hydroxy
methyl derivative is also excreted free, as the glucuronide and as the
sulphate conjugate; the latter was not detected in rats.
Metabolites in mouse, but not rat, excreta include: 3-2(2,2-
dibromovinyl)-2-trans-hydroxymethyl-2-methylcyclopropanecarboxylic
acid sulphate; 3-phenoxybenzaldehyde; 3-phenoxybenzyl alcohol and its
glucuronide; glucuronides of 4'-hydroxy-3-phenoxybenzyl alcohol and
5-hydroxy-3-phenoxybenzoic acid and 3-phenoxybenzoyltaurine.
Intraperitoneal (i.p.) administration of deltamethrin to the mouse
yielded the same metabolites, but in different ratios. Deltamethrin is
detoxified in mice by both oxidative and hydrolytic processes.
TABLE 2. Radiocarbon in the urine, faeces, and tissues of mice up
to 8 days after oral administration of 14Cv, 14Ca
i.e, alpha & 14C deltamethrin.
labeling position
sample analyzed 14Cv 14Calpha 14CN
Administered Dose, mg/kg
4.4 1.7 2.2
% of Administered Doseb
urine
0-1 day 28.9 40.5 13.1
1-2 days 13.8 5.0 8.5
2-5 days 12.0 15.7 10.3
5-8 days 2.6 3.9 3.6
feces
methanol extract
0-1 day 19.0 18.6 20.8
1-2 days 9.5 4.0 16.4
2-5 days 72 5.1 4.5
5-8 days 0.9 0.4 2.2
unextractable, 0-8 days 5.1 5.8 13.8
carcass and tissues, 8 days 1.0 1.0 6.8
Tissue Residue at 8 Days, ppb of Decamethrin Equivc
blood 4 5 54
brain 17 0 4
fat 273 115 79
kidney 39 27 20
liver 47 20 19
skin 77 3 778
stomach 28 9 175
a Values at 3 days are given in Table 1 of the microfiche
supplement to this report.
b 14CO2 values are <0.1% for 0-2-day samples with each labeled
preparation.
c Comparable values for bone, heart, intestine, lung, muscle,
spleen, and testes are <60 ppb.
phenolic hydroxyl and carboxylic acid groups. The acid moiety is
rapidly excreted as the glucuronide, with smaller amounts as free and
as the glycine conjugate. The trans-hydroxymethyl derivative is also
excreted both free and as the glucuronide.
All major metabolites of the aromatic portion of the alcohol
moiety are rapidly excreted and probably arise from ester cleavage of
deltamethrin or its ester metabolites, conversion of the released
cyanohydrins to the aldehydes which rapidly yield the corresponding
acids and conjugates of these acids. Table 1 illustrates the
radiocarbon in the urine, faeces, carbon dioxide and tissues of rats
up to 8 days after oral administration of labelled deltamethrin.
TABLE 1. Radiocarbon in the urine, faeces, carbon dioxide, and
tissues of rats up to 8 days after oral administration
of 14Cv, 14Cai.e, alpha &: 14CN deltamethrin.
Labeling position
Sample analyzed 14Cva,b 14Calpha 14CN
Administered Dose, mg/kg
0.90 1.60 0.64
% of Administered Dose
Urine
0-1 day 45.1 67.6 10.4
1-2 days 5.6 3.0 8.1
2-4 days 2.7 2.1 11.4
4-8 days 1.0 1.0 13.0
Feces
Methanol extract
0-1 day 35.6 22.7 12.1
1-2 days 2.0 1.8 2.3
2-4 days 1.7 0.3 3.3
4-8 days 0.4 0.0 3.3
Unextractable, 0-8 days 4.4 0.4 14.7
14CO2, 0-2 days 0.0 0.0 0.0
Carcass and tissues, 8 days 1.5 1.1 21.4
TABLE 1. (con't)
Labeling position
Sample analyzed 14Cva,b 14Calpha 14CN
Tissue Residue at 8 Days, ppb of Decamethrin Equiv
Blood 12 89 103
Bone 12 11 57
Brain 4 20 5
Fat 59 182 94
Heart 8 9 41
Intestine
Large 9 5 77
Small 10 5 129
Kidney 8 10 66
Liver 12 38 66
Lung 4 5 73
Muscle 5 5 57
Skin 16 16 603
Spleen 2 5 49
Stomach 8 3 654
Testes 5 3 54
a With (1RS)-trans-[14Cv]decamethrin administered orally at
0.94 mg/kg, the 14C balance sheet at 8 days as percent of dose is
72.6% in urine (48.6% at 1 day, 62.3% at 2 days, and 71.2% at
4 days), 23.2% in the methanol extract of feces (12.3% at 1 day,
18.6% at 2 days, and 21.7% at 4 days), 3.0% in the unextractable
portion of feces (0-8 days), and 1.2% in carcass and tissues (all
individual tissues as above < 10 ppb decamethrin equivalents),
b With [14Cv]Br2CA administered orally at 3.74 mg/kg, the 14C
balance sheet at 8 days as percent of dose is 94.0% in urine (70% at
1 day, 82% at 2 days, and 91% at 3 days), 5.8% in feees (5.4% in methanol
extract), 0.0% 14CO2, and 0.2% in carcass and tissues. The tissue
residues are < 3 ppb decamethrin equiv except for liver which is
27 ppb.
The pathways involved in rat metabolism of deltamethrin isomers
are similar to those utilized for other pyrethroids in many segments
of the ecosystem (Elliott 1977; Gaughan et al 1977a, b).
The tissue distribution of toxic doses of 14C-acid,
14C-alcohol-, and 14C-cyano labelled deltamethrin after i.v.
administration to rats has been studied (Gray and Richard 1981). All
three radiolabelled preparations were found in every tissue examined
1 min. after injection. Peak CNS levels were achieved within 1 to
5 min. but did not correspond to the onset of choreoathetosis. In the
majority of tissues examined, the levels of deltamethrin label were
similar, proportionately to those found after administration of
alcohol labelled cismethrin. A major exception was the CNS, which
contained approximately 20% of the anticipated level of radiolabel,
suggesting that the CNS threshold for deltamethrin was approximately
0.5 to 1.0 nmol/g. Progressive accumulation of radioactivity in the
erythrocytes fraction of the blood was observed following
administration of cyano-labelled deltamethrin; it was suggested that
this may contribute to the selective retention of 14C with
radiolabelled preparations.
Mouse
Studies on mice to determine oxidative, hydrolytic and
conjugative reactions were conducted with (14Calpha) and (14Cv)
labelled deltamethrin (Ruzo et al 1979). The labelled deltamethrins
were orally administered to two mice each at 3.1 and 3.6 mg/kg
respectively and the animals sacrificed 6 h later. The 14C label was
rapidly and almost completely excreted, with little tissue retention
after 8 days (Table 2).
Deltamethrin metabolism in mice involved four sites of oxidative
attack (trans-methyl group of the acid moiety and 2', 4' and 5
positions of the alcohol moiety, hydrolysis and a variety of
conjugation process) (Figure 2). Mice excrete less unmetabolized
deltamethrin than rats (Ruzo et al 1978) suggesting more efficient
absorption and/or metabolism. Mice produce considerable amounts of the
trans-, 2'- and 5'-hydroxy derivatives, whereas rats hydroxylate
deltamethrin predominantly at the 4'-position.
The acid moiety is rapidly excreted as the glucuronide with
smaller amounts free and as the glycine conjugate. The trans hydroxy
methyl derivative is also excreted free, as the glucuronide and as the
sulphate conjugate; the latter was not detected in rats.
Metabolites in mouse, but not rat, excreta include: 3-2(2,2-
dibromovinyl)-2-trans-hydroxymethyl-2-methylcyclopropanecarboxylic
acid sulphate; 3-phenoxybenzaldehyde; 3-phenoxybenzyl alcohol and its
glucuronide; glucuronides of 4'-hydroxy-3-phenoxybenzyl alcohol and
5-hydroxy-3-phenoxybenzoic acid and 3-phenoxybenzoyltaurine.
Intraperitoneal (i.p.) administration of deltamethrin to the mouse
yielded the same metabolites, but in different ratios. Deltamethrin is
detoxified in mice by both oxidative and hydrolytic processes.
TABLE 2. Radiocarbon in the urine, faeces, and tissues of mice up
to 8 days after oral administration of 14Cv, 14Ca
i.e, alpha & 14C deltamethrin.
labeling position
sample analyzed 14Cv 14Calpha 14CN
Administered Dose, mg/kg
4.4 1.7 2.2
% of Administered Doseb
urine
0-1 day 28.9 40.5 13.1
1-2 days 13.8 5.0 8.5
2-5 days 12.0 15.7 10.3
5-8 days 2.6 3.9 3.6
feces
methanol extract
0-1 day 19.0 18.6 20.8
1-2 days 9.5 4.0 16.4
2-5 days 72 5.1 4.5
5-8 days 0.9 0.4 2.2
unextractable, 0-8 days 5.1 5.8 13.8
carcass and tissues, 8 days 1.0 1.0 6.8
Tissue Residue at 8 Days, ppb of Decamethrin Equivc
blood 4 5 54
brain 17 0 4
fat 273 115 79
kidney 39 27 20
liver 47 20 19
skin 77 3 778
stomach 28 9 175
a Values at 3 days are given in Table 1 of the microfiche
supplement to this report.
b 14CO2 values are <0.1% for 0-2-day samples with each labeled
preparation.
c Comparable values for bone, heart, intestine, lung, muscle,
spleen, and testes are <60 ppb.
 TABLE 3. Acute toxicity of deltamethrin
LD50
Species Sex Route (mg/kg bw) References
Mouse M+F iv 41 Glomot and Chevalier 1976c
M ip 181 ibid 1976b
M ip 1712 ibid 1976b
M oral 211 ibid 1976a
M oral 332 ibid 1976a
F io 121 ibid 1976b
F ip 1662 ibid 1976b
F oral 191 ibid 1976a
F oral 342 ibid 1976a
Rat M+F iv 311 ibid 1976c
M ip 241 ibid 1976b
M ip 2092 ibid 1976b
M oral 671 ibid 1976a
M oral 1282 ibid 1976a
M+F inhal. 0.63 Coombs and Clark 1978
M+F dermal <29404 Kynoch et al 1979
F ip 251 Glomot and Chevalier 1976b
F ip 1862 ibid 1976b
F oral 861 ibid 1976a
F oral 1392 ibid 1976a
Rabbit M+F dermal >20005 Clair 1977
Dog (beagle) M+F oral >3001 Glomot et al 1977
M+F oral >3006 ibid
Chicken oral ca 10002 Roussel Uclaf 1976a
Hen (adult) F oral >25002 Ross et al 1978
F oral >50007 ibid
Mallard duck oral >46407 Beavers and Fink 1977a
Game duck oral >40006 Roussel Uclaf 1976b
Grey Partridge M+F oral >18007 ibid
Red Partridge M+F oral >30007 ibid
1 Suspended in polyethylene glycol 200; 2 dissolved in sesame oil;
3 expressed for LC50 in mg dust/m3 air; 4 60% w/v suspension in aqueous
methyl-cellulose in occlusion; 5 as paste in PEG 400 on occlusion;
6 in capsules or cachets; 7 dissolved in maize oil.
Pretreatment with either the oxidase inhibitor piperonyl butoxide or
the esterase inhibitor S,S,S-tributyl phosphorotrithioate (DEF)
further increased deltamethrin in mice, reduced its rate of in vivo
metabolism by hydroxylation and hydrolysis, reduced the rate of
product excretion and elevated deltamethrin levels in fat and brain
(Ruzo et al. 1979; Soderlund and Casida 1977).
Deltamethrin-hydrolysing esterases are generally sensitive to
organo-phosphate inhibition (e.g. TEPP inhibition of hydrolysis by
all tissue preparations except stomach). The two major factors
contributing to the rapid detoxification of decamethrin are: a) the
relevant esterases present in many tissues and the oxidases in, at
least, liver microsomes, and b) many molecular sites that are
susceptible to metabolic attack (Ruzo et al 1979).
There are large differences in the toxicity of deltamethrin to
mice, depending on the route of administration, the carrier vehicle
and previous exposure to piperonyl butoxide or DEF. Irrespective of
these factors, there appears to be a critical concentration of
deltamethrin in brain that correlated with the onset of tremors or the
time of death. Additionally, direct intracerebral administration of
deltamethrin at approximately this brain level gave a similar
poisoning effect. The brain appears to be a primary target in
deltamethrin poisoning of mice (Ruzo et al 1979).
Rat
The signs of toxicity observed in rats after deltamethrin
administration included salivation and choreoathetosis (a writhing
type of toxicity) (Barnes and Verschoyle 1974; Ray 1980; Ray and
Cremer 1979; Verschoyle and Aldridge 1980) and have been designated as
the CS syndrome of toxicity (Verschoyle and Aldridge 1980).
Rats injected i.v. with deltamethrin showed muscular
contractions, piloerection, respiratory defects, convulsions and
paresis of the hind quarters immediately following treatment. Death
occurred within 10 min. After 24 h only piloerection was visible;
after 48 h surviving animals showed normal behaviour. After i.p.
injection immediate tremors, convulsions, prostration and cyanosis
were observed. After 48 h surviving animals showed normal behaviour.
Gavage with deltamethrin shortly after dosing induced motor
incoordination, convulsions and respiratory defects. After 24 and
48 h, hypomotility and convulsions were still observed. After 3 days
surviving animals showed normal behaviour (Glomot and Chevalier
1976a,b,c).
In an earlier study, the acute i.v. lethal dose in young adult
female rats was 2 to 2.5 mg/kg when administered as a solution in
glycerol formal (Barnes and Verschoyle 1974). Signs of poisoning
included excessive salivation without lacrimation, rapidly followed by
continuous jerking movements of the limbs, occasionally progressing to
convulsions. The toxic effects were rapid in onset and brief in
duration. Death occurred in some animals within 12 min of dosing while
survivors were generally symptom-free within 6 h. In a more recent
study (Kavlock et al 1979, see Table 4), the acute LD50 for adult
female rats was 31 mg/kg by the oral route and 4 mg/kg by the i.v.
route. The LD50 was observed to be sex and age dependent, with higher
values found for weanlings and males. Initial signs of deltamethrin
poisoning included profuse salivation and convulsive movements;
weakness, dyspnoea, anorexia and staining of the fur were observed
beyond the first day following compound administration.
Rats (7 males and 7 females/group) were exposed (whole body)
during 6 h to aerosols of a.i., aerosol concentration being 0.049,
0.43, 0.54 and 0.72 g/m3. The aerosol contained 66 to 86% of
particles < 5.5 µ. During exposure, hyperactivity and dose-dependent
increase in grooming and irritation were observed. The animals were
hypersensitive to touch and noise and showed uncoordinated movements.
During the observation period of 14 days following exposure, all
animals except those from the lowest dose group developed poor motor
coordination and hypersensitivity. At the end of the period all
animals were recovered to normal. In these groups the body weight gain
and food intake was depressed during 3 days following exposure. In
rats (4 of control and of highest dose group) killed immediately after
exposure, the stomach and small intestine were gas filled. In treated
rats, as result of exposure, massive haemorrhage and oedema in lungs
were observed. Stomachs were filled with gas, blood and mucus. White
deposits were visible in the trachea. In animals killed after the
observation period, a dose-dependent increase of lung degeneration
(coloured spots to congestion) was observed (Coombs and Clark 1978).
Mouse
Mice injected i.v. with deltamethrin showed intense tremors,
convulsions and ataxia immediately after administration. Also,
tachycardia and respiratory defects were observed at higher doses.
Surviving animals showed normal appearance after 4 to 5 h. Immediately
after i.p. injection, jumping movements, slight convulsions and
prostration, ptosis, tail hypertonicity and cyanosis were observed.
Surviving animals appeared normal after 72 h. Animals gavaged with
deltamethrin showed muscular stiffening and convulsions 1 h after
dosing. After 24 h hypermotility, stereotype movements of the head,
tachycardia, hypertonicity of the tail and a few convulsions were
noted. Normal behaviour and appearance were seen after 48 h (Glomot
and Chevalier 1976a,b,c).
TABLE 4. Acute toxicity of deltamethrin to rats1
95 % Confidence Lowest dose Minimum Toxic
Route Strain Sex Age LD50 limit of tested Dose
mg/kg LD50 (mg/kg) (mg/kg) (mg/kg)
Oral2 Sherman M Adult 52 46 - 58 30.00 306
Oral2 Sherman F Adult 31 29 - 34 5.00 107
Oral2 Sherman F Weanling 50 42- 60 7.50 157
Intravenous3 Sherman F Adult 4 2.9-5.3 1.57 1.576
Intravenous3 Sherman F Weanling 1.8 1.5-2.1 0.78 0.76
Dermal4 Sherman F Adult >800 --- 800:0 --- 8
Inhalation5 Sprague-Dawley M Adult 940 mg/m3 --- ---
Inhalation5 Sprague-Dawley F Adult 785 mg/m3 --- ---
1 Reference-Kavlock et al 1979;
2 Dissolved in peanut oil and administered via gavage at a rate of 5 ml/kg bw;
3 Dissolved in acetone and administered via a single injection into tail vein of rat at 0.313 ml/kg bw;
4 Dissolved in xylene and administered at a rate of 3.2 ml/kg bw;
5 Aerosols generated from 10% DMSO solution. Based on exposure time of 120 to 150 min.;
6 Moderate to severe salivation and convulsions;
7 Mild salivation;
8 No signs of toxicity at 800 mg/kg.
Rabbit
Rabbits (10 males and 10 females) were treated with 2 g
deltamethrin in 2 ml PEG 400/kg bw on 80 cm3 shaven skin for 24 h on
occlusion. The animals were observed for 14 days. One animal showed
obvious erythema and another congested skin. No weight changes or
abnormal behaviour were observed. On histological observation of
liver, kidneys and skin, small changes were observed that were common
for this strain of rabbits and not related to treatment (Clair 1977).
Dog
Dogs showed, at non-lethal doses, transient hyperexcitability,
akynesia, vomiting and stiffness of the hind legs (Glomot et al
1977).
Birds
Oral administration of a.i. to hen, game duck or partridge
produced no distinct symptoms except a small initial weight loss
occasionally. In chickens diarrhoea, convulsions and jerky movements
of the head were observed. Mallard ducks, at lethal doses, exhibited
signs of neurotoxic effects, which included ataxia, loss of
equilibrium and loss of coordination. The effect was dose-related: at
lower dose levels only some hyperexcitability and imbalance were
observed (Beavers and Fink 1977a).
Short-term studies
Rat
Male and female weanling Sprague-Dawley rats (20/sex/group)
were daily dosed by oral gavage with 0, 0.1, 1.0, 2.5 or 10.0 mg
deltamethrin in PEG 200/kg bw/day for 13 weeks (Hunter et al 1977).
No treatment-related effects on food and water consumption, mortality,
urinalysis and haematology were observed. Neurological examinations
and ophthalmoscopy revealed no abnormalities. In the 10 mg/kg bw
group, some hypersensitivity was observed in week 6 in males. Body
weight gain among males receiving deltamethrin was significantly lower
at 2.5 and 10 mg/kg/day. The body weight of the females was not
affected by the treatment. The male animals of the 1 mg/kg group
showed a tendency to a reduced body weight gain. In the females, blood
glucose and urea were significantly increased in week 6, but no
significant changes occurred in week 12 or with other blood chemistry
parameters. No clear effects were noted on the weights of the organs.
Gross and microscopic examination of a variety of tissues and organs
showed no treatment-related alterations.
Following the 13-week dosage period, 5 males and 5 females per
group were allowed to recover for 4 weeks. Autopsy was performed at
the end of this recovery period. The body weights of all previously
treated animals appeared not to be different from controls. Thyroid
weights were not dose-related but were increased in males. This
increase was significantly higher in the 1.0 mg/kg and 10.0 mg/kg
group. Marginal no-effect level was 1 mg/kg bw (Hunter et al 1977).
Dog
Male and female beagle dogs, 3-5/sex/group, 25 weeks of age, were
daily dosed orally with 0, 0.1, 1.0, 2.5 and 10.0 mg deltamethrin in
PEG 200/kg bw/day in gelatin capsules. Dosage was continued for 13
weeks, followed by a recovery period of 20 weeks for 2 dogs/sex from
the groups receiving 1.0, 2.5 and 10.0 mg/kg bw/day (Chesterman
et al 1977). Observations were made on behaviour, mortality, body
weight and food and water consumption. Haematology, blood chemistry,
urinalysis and six channel EEG-analysis were performed at week 0, 6
and 1, and ophthalmoscopy at week 0, 5 and 12. Special attention was
paid to the muscular and nervous systems.
Liquid faeces were associated with all groups of treated dogs
throughout the dosing period. All groups of animals receiving
deltamethrin gained less weight than the controls. The effects were
not strictly dose related. The dogs from the control group left
smaller quantities of the offered food than those of the treated
groups, whereas the water consumption was not dose-relatedly decreased
in all treated groups.
Dilation of the pupils was seen to occur in the dogs receiving
2.5 and 10.0 mg/kg/day. The sign was first seen 4 to 7 h after dosing
and persisted throughout the day. They reacted normally prior to
dosing on the following day. The incidence of vomiting was dose-
related increased in all treated groups, except the 0.1 mg dose level.
The incidence decreased in all the animals affected as the dosing
period progressed. In the highest dose group, unsteadiness, body
tremors and jerking movements were seen, particularly in males in
weeks 2, 3 and 4. These effects were reduced during week 5 to 9 and
were seen only in one dog in week 13. Excessive salivation was seen
initially but diminished during dosing period. After 5 and 12 weeks,
depression of the gag reflex was noted in a proportion of animals in
all treated groups. Depression of the patellar reflex was observed in
all treated groups except the dogs administered 0.1 mg/kg. In the
animals given 1 or 2.5 mg/kg/day, exaggeration of the patellar reflex
was noted only after 5 weeks. Some animals of all treated groups
showed variations in the flexor reflex. A high proportion of the
animals had depression of the hind limb tactile placing reaction.
At dosage levels of 2.5 and 10 mg/kg/day, deltamethrin caused
modification of the EEG pattern in some animals following 12 weeks
administration. Histopathological evaluations of tissues and organs,
including nervous system and muscle, did not reveal abnormalities that
could be related to dosage with the test compound. During recovery the
gag reflex continued to be depressed, whereas exaggeration of the
patellar reflex was still seen in some dogs that had previously
received 1.0 mg/kg/day. One animal continued to show an abnormal EEG
pattern (Chesterman et al 1977).
Quail and duck
Deltamethrin was given in the diet to 14-day old mallard ducks
for 5 days, at doses of 0, 464, 1 000, 2 150, 4 640 and 10 000 mg/kg
feed. The number of animals per group was 10. There was some mortality
in the two highest dose groups. Birds of the highest dose group showed
ataxia and loss of coordination. There were dose-related decreased
weight gain and food consumption (Beavers and Fink 1977a).
An identical experiment was performed with 14-day old bobwhite
quails (10 animals/group). The effects were the same as in the mallard
ducks, except there was no mortality (Beavers and Fink 1977b).
Long-term studies
Rat
Male and female Charles River CD rats (90/sex/group) were dietary
fed with 0 (control), 2, 20 and 50 mg deltamethrin/kg in the diet for
two years. Sixty males and 60 females were used in a second control
group. After 6, 12 and 18 months of compound administration, 10
animals/sex/group were sacrificed, except for the second control group
(Goldenthal et al 1980b).
No changes in general behaviour and appearance in relation to
compound treatment were recorded. Survival was similar in control and
treated rats (50 to 67%). Rats at 50 mg/kg feed gained slightly less
weight than control rats, whereas the food consumption was essentially
the same. Ophthalmoscopic findings generally were similar for control
and treated rats. No haematological and biochemical parameters were
changed in a biologically significant way in relation to treatment at
any time, except for a decreased SGPT activity at 6 months in the mid-
and high-dose groups. No treatment-related effects were observed on
organ weights. The macroscopy and microscopy findings were common for
the animals of the species and strain, except for a slightly increased
incidence of axonal degenerations in sciatic, tibial and/or plantar
nerves at 18 months in the 20 and 50 mg/kg groups. Evaluation of
incidence and/or severity of these degenerations at termination of the
study was obscured by the age of the animals.
Seven interstitial cell adenomas were observed in the testes of
the 50 mg/kg feed group compared to 0 and 4 in the two control groups.
Only from some animals of the 2 and 20 mg/kg groups were some organs
and tissues, including the testes, studied histopathologically.
Evaluation of a possible dose-response effect on the testes was
therefore not possible. The no-effect level was suggested to be
2 mg/kg (Goldenthal et al 1980b).
Mouse
Male and female Charles River CD-1 mice (80/sex/group) were fed
dosage levels of 0(control), 1, 5, 25 and 100 mg deltamethrin/kg in
the diet for 24 months. In a second control group 60 mice/sex were
used. After 12 and 18 months 10 mice/sex/group, except for two only in
the control group, were sacrificed.
There were no clear effects related to the administration of
deltamethrin on general behaviour, mortality, body weight and food
consumption. Blood chemistry, haematology and urine analysis
parameters were normal after 12, 18 and 24 months. Increases or
decreases in absolute and/or relative organ weights occurred in a few
organs at each dosage level at any time of sacrifice. Microscopic
examination of tissues did not reveal any lesions indicative of a
compound-related effect. The tumour incidence was unaffected by
deltamethrin administration. The no-effect level was suggested to be
100 mg/kg (Goldenthal et al 1980a).
Studies designed to investigate the potential carcinogenic
effects of deltamethrin have been initiated (Cabral 1981). Groups of
C57B1 mice have been administered orally 0-8 mg/kg bw deltamethrin in
arachis oil.
Dog
Deltamethrin (RU 22974) dissolved in maize oil was administered
in the diet to 64 beagle dogs at dosage levels of 1, 10 and 40 ppm for
24 months. This corresponds to 0.025, 0.25 and 1 mg/kg bw respectively
(IRDC 1980). Eight males and eight females were used at each dosage
level and in a control group. Individual body weights and food
consumption values were determined weekly. Ophthalmic, haematologic,
biochemical and urinalysis examinations were conducted during the pre-
test period at 6, 12, 18 and 24 months of the study. Neurological
exams were conducted at approximately 1 year and before termination.
These later parameters included: for cranial nerves and segmental
nerves, tests for postural reactions, placing reactions and hopping
reactions.
No signs of overt toxicity were observed in any of the dogs. Body
weights and food consumption values were similar for control and
treated dogs. No compound-related effects were observed during the
ophthalmoscopic and physical examinations. Although there were some
randomly statistically significant differences between the control and
other dose groups in the haematologic and biochemical tests, there
were not any physiologically significant changes observed at any
interval in the study. Two treated and two control animals died during
the study.
No compound-related gross or microscopic changes were observed in
the surviving dogs that were sacrificed and necropsied. Inflammatory,
degenerative and proliferative changes described were spontaneous in
nature or related to theoestrous phase of the menstrual cycle and
unrelated to compound administration.
On the basis of the results from this study, it was concluded
that the no-effect level is 40 ppm in the diet (equivalent to
1 mg/kg bw/day) administered RU 22974 over a 2-year period (IRDC
1980).
Special studies on primary irritation
Cutaneous irritation
Male albino rabbits (12/group) weighing 2.5 to 3.5 kg were
administered 0.5 g deltamethrin to either shaven intact or abraded
skin. The occlusive patch was fixed on the skin for 23h. Scoring for
erythematous and oedematous lesions occurred 1 h and 49h after removal
of the patches. Technical deltamethrin, 98% a.i., showed no irritant
effect (Coquet 1976a).
Ocular irritation
Deltamethrin (0.1 g/animal) was administered into the
conjunctival sac of the eye of 6 male albino rabbits, weighing 2.5 kg,
with or without rinsing 60 sec. after instillation. Observations for
conjunctival lesions, chemosis, discharge, conjunctival enanthema,
opacity and affected cornea were made 1 h, 24h, 2,3,4 and 7 days
following instillation. Deltamethrin showed, both with and without
rinsing, transient irritating effects (Coquet 1976b).
Special studies on sensitization
Deltamethrin (0.5 g/animal) was applied topically to the skin of
albino guinea pigs with a 2-day interval for 3 weeks, and once at the
start of the 4th week. The preparation was covered with an occlusive
patch for 48h. On days 1 and 10 the guinea pigs received an
intradermal injection of 0.1 ml of Freund's adjuvant. The animals were
challenged 12 days after the last application with 0.5 g deltamethrin.
The macroscopic and histological examination did not reveal evidence
of sensitization (Guillot and Guilaine 1977).
Special studies on reproduction
Rat
Groups of 10 female and 10 male Charles River rats were fed
deltamethrin in the diet at 0, 2, 20 and 50 mg/kg and mated to begin a
three-generation, 2 litter (first generation, 3 litter) standard
reproduction study. Parental body weights and food consumption were
recorded during the study. After weaning of the second litter, the
survival parental rats were sacrificed and necropsied. Five male and
5 female pups of the F3b were necropsied. No changes in general
behaviour or survival of parental rats or pups relevant to the test
material were observed. The body weight of F0 males of the 50 mg/kg
group was decreased from week eleven onwards. There was some slight
decreases in mean food consumption of the parental F1 male rats in
the 50 mg/kg feed group.
The basic reproduction indices (fertility, gestation, lactation,
viability and litter size) were not affected by the treatment.
However, the mean pup weight was affected in some litters; especially
that of the 50 mg/kg group was slightly decreased in comparison to the
controls. Gross external examination revealed no abnormalities. No
gross or microscopic lesions of treatment-related significance or
significant effects on the organ weights of the F3b generation were
observed (Wrenn et al 1980).
Special studies on teratogenicity
Mouse
Mated female Swiss CDI.SPF mice (24/group) were given orally
deltamethrin dissolved in sesame oil at dose levels of 0, 0.1, 1 and
10 mg/kg bw/day during days 6 to 17 of pregnancy. The animals were
necropsied on day 18 of gestation. No teratogenic effects could be
detected. Total implantation sites, foetal losses, living foetuses and
examinations of skeletal tissue were normal. Minor embryotoxic effects
were observed, e.g. dose-dependent decrease of average foetal weight
and delayed ossifications at all dose levels tested (Glomot and
Vannier 1977).
Technical deltamethrin (Roussel UCLAF), dissolved in corn oil was
administered to CD-1 mice by gastric intubation at doses of 12.0, 6.0,
3.0 or 0 mg/kg during days 7 to 16 of gestation (Kavlock et al
1979). Maternal weight on day 6 was used for calculation of doses and
intubation was 0.2 ml; control animals received vehicle alone. Animals
were sacrificed on day 16 of gestation. Administration of deltamethrin
to pregnant mice resulted in a dose-related (p < 0.001) reduction in
maternal weight gain during pregnancy. Pregnant dams in the high
dosage group (12.0 mg/kg/day) gained 58% less weight than did those in
the control group. No dose-related occurrences of maternal mortality
were observed, but most animals in the high dosage group and some in
the middle dosage group became convulsive soon after dosing. No
effects were observed on the number of implantation sites, foetal
mortality, or foetal weights, or in the number of sternal and caudal
ossification centers. A significant (p < 0.01) dose-related increase
in the occurrence of supernumerary ribs was observed. No other dose-
related skeletal or visceral anomalies were observed in deltamethrin-
treated mouse foetuses. No evidence of teratogenic activity was found
in mice at dose levels that produced maternal toxicity (Kavlock
et al 1979).
Rat
Mated female Sprague-Dawley rats (24/group) were administered
orally 0, 0.1, 1 and 10 mg deltamethrin/kg bw/day during days 6 to 18
of pregnancy. Examination occurred on day 21 of gestation. Twelve
females in the control and 10 mg/kg bw groups were allowed to deliver.
There were no effects on the reproduction or teratogenicity parameters
examined, with the exception of a slight delayed ossification in the
highest dose level (Glomot and Vannier 1977).
Technical deltamethrin (Roussel UCLAF) dissolved in maize oil was
administered to Sprague-Dawley rats by gastric intubation at doses of
5.0, 2.5, 1.25 or 0 mg/kg during days 7 to 20 of gestation (Kavlock
et al 1979), Maternal weights on day 6 were used for the calculation
of doses and intubation volume was 0.2 ml; control animals received
the vehicle alone. Rats were sacrificed on day 21 of gestation.
Administration of deltamethrin to pregnant rats resulted in a dose-
related reduction (p > 0.01) in maternal weight gain during
pregnancy, with animals in the high dosage group (5.0 mg/kg) gaining
only 80% of the control value. This dose produced a mild salivation
for up to 4 h after dosing in approximately 50% of the animals. No
effects were observed on the number of implantation sites, foetal
mortality, foetal weight or number of sternal and caudal ossification
centres. For post-natal studies, an additional group of pregnant rats
was housed individually and intubated from day 7 of gestation to day
15 of lactation with dosages of either 5.0, 2.5 or 0 mg/kg
deltamethrin dissolved in maize oil. No persistent toxicity was
observed in neonatal rats that received perinatal exposure to
deltamethrin (Kavlock et al 1979).
Rabbit
Groups of fifteen mated females received deltamethrin dissolved
in sesame oil at levels of 0, 1, 4 or 16 mg/kg bw/day from days 6 to
19 of pregnancy. Examination was carried out on day 28 of gestation.
The average foetal losses were not dose-related and increased at all
doses tested. This effect was mainly caused by a higher rate of
expelling traces. The average foetal weight in the highest dose group
was decreased. Some malformations (one animal with hydrocephalia, and
one with exencephalia and thoracogastroschisis) were observed in
2 animals of the highest dose level. A complementary study with
15 mg/kg bw/day was performed. In this study one foetus with spina
bifida and shortened tail was detected among 69 parent normal
foetuses. It was concluded that the malformations were within the
normal limits of the strain used and were not related to the
treatment, despite the occurrence at the highest dose level only
(Glomot and Vannier 1977, 1978).
Special studies on neurotoxicity
Adult hens (10/group) were gavaged with a single dose of 0, 500,
1 250 or 5 000 mg/kg bw deltamethrin suspended in maize oil or 0 and
1 000 mg/kg bw dissolved in sesame oil. Tri-o-cresylphosphate (TOCP)
(500 mg/kg bw) was used as positive control for delayed neurotoxicity.
During 21 days, observations were made on mortality, health,
neurotoxic signs and body weight.
In the TOCP group, 8 out of 10 animals died whereas only
2 mortalities were observed in the group dosed at 1 000 mg
deltamethrin/kg with sesame oil as the vehicle. Deltamethrin
induced no clinical, macroscopic or histological signs of delayed
neurotoxicity. TOCP-treated hens showed severe ataxia and degenerative
changes in the spinal cord and, occasionally, in the sciatic nerve
(Ross et al 1978).
Special studies on potentiation
Mice
Deltamethrin is hydrolysed in vitro by esterases in blood and in
brain, kidney, liver and stomach preparations of mice. Pre-treatment
of mice with oxidase inhibitor, piperonyl butoxide (PB), or esterase
inhibitor, S, S, S-tributylphosphorotrithioate (DEF), delayed
metabolism of i.p. administered deltamethrin. Using oxidase or
esterase inhibitor, different vehicles and different administration
routes, it was possible to induce similar toxic effects with a wide
range of deltamethrin doses (6 to 191 mg/kg bw). The different
treatments showed that PB or DEF made mice more sensitive to
deltamethrin (Ruzo et al 1978b).
Special studies in mutagenicity
Bacteria and yeast
In a growth inhibition test with Escherichia coli, deltamethrin
at levels of 1 250, 2 500 and 5 000 µg/ml DMSO (0.1 ml per plate) had
the same marginal inhibitor effect on the mother strains (W 3110 and
WP2) as on their mutants (p 3478 and CM 611). Chloramphenicol and
N-methyl N'-nitro-N-nitroguanidine (MNNG) were used as positive
controls and induced clear inhibition (Peyre et al 1980).
Deltamethrin was compared with MNNG, 9-aminoacridine,
2-nitrofluorene and 2-amino-anthracene for mutagenic activity in the
Ames test with Salmonella typhimurium, strains TA 1535, 100, 1537,
1528 and 98. The concentrations of deltamethrin used were 2, 10, 50,
200, 500, 1 000 and 5 000 µg/plate. Deltamethrin began to precipitate
at 200 µg/plate. The mean number of revertants was not influenced by
any concentration of deltamethrin in any strain with or without S9-mix
(metabolic activation), whereas the positive control mutagens produced
an increase of the number of spontaneous revertants (Peyre et al
1980).
In a similar experiment, deltamethrin (0.2, 2, 20, 200 and
400 µg/plate dissolved in DMSO) in the presence of activated
microsomal enzymes did not influence the number of revertants of
S. typhimurium strains TA 1535, 1537, 1538, 98 and 100.
2-Aminoanthracene, 3-methylcholanthrene, benzo(a)pyrene and acridine
orange showed mutagenic activity, whereas thio-TEPA was negative
(Fouillet 1976).
Deltamethrin at levels of 10, 50, 100, 500 and 1 000 µg/plate was
not mutagenic in assays with S. typhimurium strains TA 1535, 1537,
1538, 98 or 100 either in the presence or absence of a rat-liver
homogenate metabolic activation system (the positive controls were
2-anthramine and 9-aminoacridine) (Kavlock et al 1979).
Deltamethrin was not mutagenic in assays with E. coli WP2 when
tested at levels of 10, 50, 100, 500 and 1 000 µg/plate with or
without metabolic activation (the positive control was 2-anthramine)
(Kavlock et al 1979).
Deltamethrin was not mutagenic at levels of 1 to 5% when tested
with and without metabolic activation with Saccharomyces cerevisiae
(the positive control was 1, 2,3,4-diepoxybutane) (Kavlock et al
1979).
Mammalian cells
Deltamethrin dissolved in Cremophor oil (0, 0.4, 0.2, 1 and 5 mM,
10, 0.08, 0.4, 2 and 10% v/v respectively) in the presence of a
metabolic activation system increased the incidence of chromosome and
chromatid aberrations and SCEs, after incubation with Chinese hamster
ovary cells at 1 mMol. In the absence of S9-mix (metabolic
activation), no higher rate of aberration was observed.
It was shown that this increased incidence was due to a subtoxic
effect of some reaction product of Cremaphor oil and S9-mix.
Deltamethrin in Cremaphor oil without S9-mix and dissolved in DMSO
(1%) at levels of 0, 0.001, 0.01, 0.1 and 0.2 mM with or without
metabolic activation had no effect on the number of aberrations and
SCEs. Due to insolubility, no higher concentrations were tested
(Sobels et al 1978).
Animals
Mice, 3 males and 3 females per dose, were gavaged for two
consecutive days with 5 or 10 mg deltamethrin dissolved in sesame
oil/kg bw. Control mice were gavaged with 0.3 ml sesame oil. The
incidence of chromatid aberrations in bone marrow cells or of
micronuclei in polychromic erythrocytes did not show any significant
statistical difference in treated and control groups (Sobels et al
1978).
A single oral administration of 15 mg/kg deltamethrin was given
to Swiss mice. Groups of 2 animals were sacrificed every 3 h during a
24-h period. Several animals died after treatment. The incidence of
chromatid aberrations in the femoral bone marrow was low. There was no
consistent time-related trend in the distribution of these aberrations
(Sobels et al 1978).
Deltamethrin dissolved in sesame oil in groups of 9 to 13 male
mice dosed orally with 0 or 3 mg/kg bw for 7 days and 6 or 15 mg/kg bw
in a single dose showed no effect on the rate of pre- or post-
implantation losses after mating with 6 to 18 non-treated females.
The highest dose tested was toxic to the males, e.g. 7 out of 20
animals died shortly after treatment. Histological examination of
the testes of all animals revealed no abnormalities. Triethylene
thiophosphoramide (10 mg/kg bw), used as a positive control, reduced
considerably the rate of pregnancies in the second and third week and
increased the number of embryonal losses (Vannier and Glomot 1977).
Special studies on humans
Cutaneo-mucuous manifestations have been observed among plant
workers dermally exposed to technical deltamethrin or its
formulations. Initial lesions were tenacious and painful pruritus
(pricking sensation), especially observed after exposure to hot water
or on perspiration, followed by a blotchy local burning sensation with
blotchy erythema for about 2 days. Thereafter, slight and regular
desquamation, restricted to the contaminated area, occurred. The
cutaneous signs are sometimes accompanied by itching of the face
(mainly around the mouth) and/or rhinorrhoea or lacrimation. No other
symptoms related to exposure were observed (Husson 1978).
The effects described, which were observed before 1978 in a few
plant workers, have not been seen since a new plant was specifically
built for the entire automatization of deltamethrin production, which
does not involve workers being in direct contact with intermediates
and the final product. The only workers exposed are those involved in
the packaging of the technical product (>98% active ingredient) and
they are fully protected by special hermetic clothing (Glomot 1981).
At the level of formulation plants, steps have been taken to
avoid direct contact by workers involved in introducing technical
powder in the basic solvent. No toxic effects have been reported in
the past several years in these formulation plants, although a very
limited number of workers have complained about some irritating
effects. However, these were always transient and without any further
consequences (Glomot 1981).
The formulation which has been most frequently used for
deltamethrin application is the EC. The use of both EC and ULV
formulations corresponds in 1981 to a treated area covering several
million hectares in the world without any case of intoxication. The
majority of users are plant growers who apply the formulations in the
fields and are not in contact with the technical product per se
(Glomot 1981). A few cases of irritation problems have been reported
in some workers, due primarily to the use of the EC formulation. This
has been observed when treatments were made in orchards with high
trees needing high volumes of mixture (e.g. in South Africa) or under
greenhouses in confined spaces. In all cases, the reported problems
were not severe and could be treated using antihistaminic syrups or
with pomades based on cocaine derivatives. The irritation is most
probably enhanced by the presence of aromatic solvent, e.g. xylene, in
the EC formulation. Flowable experimental formulations without any
solvent did not cause irritating effects in human volunteers (Glomot
1981). No cases of neurological effects have been reported with
formulation workers nor have fatal intoxications been reported (Glomot
1981).
RESIDUES IN FOOD
USE PATTERN
Pre-harvest treatments
Information was received from The Netherlands on pre-harvest
treatment of several crops with deltamethrin, which is summarized in
Table 5.
RESIDUES ARISING FROM SUPERVISED TRIALS
Results of supervised trials with deltamethrin on various crops
are summarized in Table 6.
Assorted fruit, inedible peel
Kiwi fruit
Data from two new field trials conducted in France show that
residue levels for the whole fruit are below 0.05 mg/kg, the residue
being entirely in the peel.
TABLE 5. Deltamethrin use in The Netherlands
Application Rate
Crop g/100 l g/ha P.H.I.
Apple 0.5 (from before blossom to
Pear 0.5 (to 14 days P.H.
Grape 0.5 7
Cherry 0.5 7
Plum 0.5 7
Strawberry 0.5 7
Currant 0.5 7
Gooseberry 0.5 7
Raspberry 0.5 7
Cucumber 1.25 3
Melon 25 14
Bell pepper 25 14
Eggplant 25 14
Lettuce 1.25 14
Endive 25 14
Radish 7.5 7
Turnip 7.5 7
Rutabaga 7.5 7
Brussels sprouts 7.5 7
Cauliflower 7.5 7
Broccoli 7.5 7
Kohlrabi 7.5 7
Leek 7.5 7
Onion 7.5 7
Peas 7.5 7
Dwarf french bean 7.5 7
Sugarbeet 7.5 7
Mushroom 0.75-1.5 75/100 2
Hops
Several trials conducted in the UK and the Federal Republic of
Germany show a high level of variation between samples, but relatively
little decline with time over the period of 7 to 14 days following
treatment. No information was provided on the fate of such residues
when the hops were used in brewing.
TABLE 6. Supervised trials with deltamethrin in various crops
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) Formulation of sample 0 1 2/3 4 7 14
Kiwi fruit France 1980 2 12.5 peel 0.05 0.06 0.06 0.08 0.07
whole fruit 0.01 0.01 0.01 0.01 0.01
pulp ND ND ND ND ND
12.5 peel 0.4 0.22 0.23 0.25 0.1
whole fruit 0.06 0.04 0.04 0.04 0.01
pulp ND ND ND ND ND
Hops England 1978 7 12.5 0.02
W. Germany 1978 1 25 0.01
3 62.5 0.1 0.3 1.4 1.1 1.9
3 62.5 16 5.5 0.1 1.8 1.9
3 62.5 3.3 1.3 0.4 2.0 2.5
1980 5 6.25-37.5 1.3 0.2 0.4 0.3 0.7
5 " 0.2 0.7 0.3 0.7 1.6
5 " 1.0 0.7 0.2 0.6 2.7
5 " 0.5 0.3 0.2 0.7 1.1
Mushrooms1 Netherlands 1978 2 6.25 0.0025% whole 0.003 0.002 0.001
1978 2 25 0.01% " 0.009 0.004 0.003
(DAYS)
0 1 3 5 7 14
Black currant Finland 1979 1 6.3mg/bush. 0.1 0.1
W. Germany 1979 2 18.5g/ha 0.3 0.2 0.2 0.1
" 0.3 0.3 0.3 0.3
" 0.3 0.2 0.2 0.2
2 11.25g/ha 0.2 0.2 0.1 0.1
0.3 0.4 0.3 0.2
TABLE 6. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) of sample 0 1 2 4 8 14 21
Spinach France 1978 1 17.5g/ha Fresh leaf 0.48 0.33 0.22
Cooked leaf 0.27 0.21
Cooking water 0.0001 0.00009
17.5g/ha Fresh leaf 0.52 0.30 0.155
Cooked leaf 0.28 0.12
Cooking water 0.00015 0.000,05
1979 1 12.5g/ha Fresh leaf 0.4 0.2 0.14 0.12
Cooked leaf 0.12 0.10
Cooking water 0.0001 ND
12.5g/ha Fresh leaf 0.4 0.2 0.17 0.15
Cooked leaf 0.15 0.12
Cooking water 0.00015 0.0001
1980 1 17.5g/ha Fresh leaf 0.18 0.22 0.35
Cooked leaf 0.16 0.20 0.28
Cooking water ND ND ND
W.Germany 1980 3 12.5g/ha Fresh leaf 0.3 0.7 0.2 0.09
0.7 0.3 0.3 0.1
14 21 28/39 49 61 74 80
Wheat straw France 1978 1 7.5 EC 0.015
1 15 EC 0.05
1 7.5 EC 0.025
1 15 EC 0.025
1 7.5 EC 0.025
1 15 EC 0.025
1 7.5 EC ND
1 7.5 EC ND
1 12.5 EC 0.15
1 12.5 EC 0.06
1 12.5 EC 0.2
1 12.5 EC 0.1
TABLE 6. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) of sample 14 21 28/39 49 61 74 80
Oat Straw W.Germany 1978 1 12.5 EC 0.06
1 12.5 EC 0.06
1 12.5 EC ND
1 12.5 EC 0.08
1979 1 12.5 EC 0.6
1 12.5 0.2
1 12.5 0.2
1 12.5 0.5
Rice straw Surinam 1977 1 6.25 ND
1 12.5 0.010
1 18.75 0.01
1 25 0.08
Phillipines 1978 1 17.5 0.2
25 0.1
1 After oven drying at 7 days.
Small fruits and berries
Black currant
From trials conducted in the Federal Republic of Germany, it
would appear that residues on these berries can range up to 0.3 to
0.4 mg/kg, but as yet, there is no registered use of deltamethrin on
black currants.
Leafy vegetables
Spinach
Additional data from trials carried out in France and the Federal
Republic of Germany indicate that residues range around 0.3 to
0.4 mg/kg, 7 or 8 days after the last treatment. These levels do not
decline significantly during cooking.
Fodder and cereal straws
Additional data were available to indicate the level of
deltamethrin on wheat straw, oat straw and rice straw. Though the bulk
of the values were below 0.2 mg/kg, oat straw sampled 28 to 39 days
after treatment showed residues ranging up to 0.6 mg/kg. The amount of
data is, however, limited and in view of the potential for the wide
application of deltamethrin to cereal crops further data are required.
Cereal grains
A report was received on trials to determine the fate of
deltamethrin on wheat during storage and after milling and baking
(Halls and Periam 1980).
Batches of an English hard wheat were sprayed on a conveyor with
diluted deltamethrin/piperonyl butoxide liquid grain protectant to
give 250 kg at each of two deltamethrin treatment levels. These 250 kg
batches were then placed in 1 ton capacity metal silos. Target
treatment levels for the two batches of grain were 1 mg/kg
deltamethrin + 10 mg/kg piperonyl butoxide and 2 mg/kg deltamethrin +
10 mg/kg piperonyl butoxide respectively, but chemical analysis
revealed that the actual treatment levels achieved were only about 40
to 75% of these.
Samples of the treated wheat were taken after spraying and
thereafter at monthly intervals for nine months and analysed for
deltamethrin content. Replicate samples were taken from each silo,
analysed separately and the result expressed as the mean. Wheat from
the initial sampling and from samplings after 3, 6 and 9 months was
submitted to the Flour Milling and Baking Research Association,
Chorleywood, Buckinghamshire to be milled and baked.
At Chorleywood the wheat was first cleaned of extraneous
material, then divided into two samples and each subjected to a
different milling procedure. The first produced whole-meal flour, the
second produced white flour, bran and fine offal.
Two different samples of white flour were taken from this
procedure, the first reduction flour (the cleanest flour mainly from
the centre of the grain and used in the baking of cakes) and total
white flour (straight-run flour plus flour from the bran and fine
offal fractions). These samples, together with the wholemeal flour,
bran and fine offal were analysed for total deltamethrin content for
both batches of treated wheat. In addition, bread was baked from both
wholemeal and total white flours to produce wholemeal and white bread,
respectively, and these loaves were also analysed for deltamethrin
content.
Results for residues of deltamethrin on wheat, milling data and
residues of NRDC 161 on flour fractions and bread are given in Tables
7, 8 and 9, respectively.
In both the deltamethrin treatments, levels were initially found
by analysis to be only about 40% of the expected application. This
could be due predominantly to inter alia incorrect spraying rates,
inadequate extraction, or loss of compound at some stage between the
nozzle and the grain.
The results given in Table 7 show that for both application rates
there was no detectable breakdown of deltamethrin on wheat over a
nine-month storage period. The fluctuation in levels between different
samplings was within the inherent error of the analytical method
demonstrating that deltamethrin is very stable on wheat. In this
respect deltamethrin differs from many organophosphate grain
protectants such as fenitrothion (Desmarchelier 1978).
The moisture content of the wheat remained fairly consistent over
the storage period of nine months. Deltamethrin analysis data for the
milling fractions and bread derived from treated wheat are given in
Tables 8 and 9.
For all sampling times at both treatment levels there was no
significant change in deltamethrin residues when wheat was milled to
produce wholemeal flour. When this flour was baked, the wholemeal
bread produced contained lower, or identical, deltamethrin residues
but this can be explained by the greater moisture content (up to 45%)
present in bread.
TABLE 7. Deltamethrin residues on wheat after indicated periods of storage in the UK1
Residue analysis (mg/kg deltamethrin)
Target
Application Initial2 1 month 2 months 3 months 4 months 5 months 6 months 7 months 8 months 9 months
rate (mg/kg) (15.11.79) (13.12.79) (17.1.80) (11.2.80) (12.3.80) (21.4.80) (12.5.80) (10.6.80) (10.7.80) (6.8.80)
1.0 0.44 0.53 0.48 0.50 0.50 0.41 0.48 0.49 0.42 0.41
2.0 0.80 1.25 1.33 1.46 1.21 1.01 0.96 1.12 0.94 1.08
1 Analytical results are subject to overall confidence limits of 30%
2 Dates refer to day of sampling.
TABLE 8. Milling data for wheat freshly treated with deltamethrin and from wheat after 3, 6 and 9 months storage1
Target application Sampling Clean wheat Bran Fine Offal Total white flour
rate period
(mg/kg) (months) Weight Prop. of Weight Prop. of Weight Prop. of Weight Prop. of
(kg) total(%) (kg) total (%) (kg) total (%) (kg) total (%)
0 3.835 100 0.596 15.54 0.306 7.98 2.849 74.29
3 4.921 100 0.779 15.83 0.519 10.55 3.502 71.16
1.0
6 3.700 100 0.621 16.78 0.340 9.19 2.670 72.16
9 3.700 100 0.626 16.92 0.288 7.78 2.746 74.22
0 3.786 100 0.590 15.58 0.292 7.71 2.809 74.19
3 4.884 100 0.769 15.75 0.514 10.52 3.489 71.44
2.0
6 3.700 100 0.630 17.03 0.306 8.27 2.688 72.65
9 3.700 100 0.650 17.57 0.275 7.43 2.692 72.76
0 (Control) 0 2.010 100 0.312 15.52 0.175 8.71 1.525 75.87
1 There is a loss of up to 3% in the laboratory milling process
TABLE 9. Deltamethrin residues on milling fractions and bread made from freshly treated wheat and from wheat after 3, 6 and
9 months storage1
Target application Sampling First Total
rate period Wholemeal Wholemeal Bran Fine reduction white White
(mg/kg) (months) Wheat flour bread offal flour flour bread
0 0.44 0.42 0.26 3.00 nd2 nd 0.09 0.10
3 0.50 0.43 0.27 1.80 approx. 0.5 nd approx. 0.05 nd
1.0
6 0.48 0.42 0.26 1.70 0.35 0.05 0.09 0.10
9 0.41 0.36 0.36 1.90 0.46 0.03 0.03 0.05
0 0.80 0.73 0.63 5.40 nd nd 0.20 0.29
3 1.46 0.80 0.57 4.70 0.75 nd 0.20 0.15
2.0
6 0.96 0.83 0.66 3.60 0.75 0.10 0.16 0.20
9 1.08 0.95 0.70 4.40 1.36 0.05 0.17 0.11
1 Analytical results are subject to confidence limits of ± 30%
2 nd = not detectable (<0.03 mg/kg).
When the treated wheats were milled to produce white flour,
relatively high deltamethrin residues were found on the bran fraction,
which is derived from the superficial layers of the grain. Initially,
deltamethrin residues on the bran were 6 to 7 times higher than those
on the wheat at both treatment rates. The levels declined to be about
4 to 5 times higher on the bran than on the wheat after 9 months'
storage.
This observation, together with the appearance of deltamethrin on
the fine offal fractions after 3 months' storage, and on the first-
reduction flours after 6 months' storage, suggests that although there
is no change in the total deltamethrin level on the wheat over 9
months, there is a subtle change in its distribution. Thus, initially,
as might be expected, the insecticide residues were found
predominantly in the outer layers of the grain, mainly in the bran
fraction, but also to a lesser extent in the total white flour
fraction. The first-reduction flour, which tends not to come from the
superficial layers of the grain, and the fine offal, consisting
largely of wheatgerm, is uncontaminated initially but residues move
into these areas as the period of storage is increased.
The deltamethrin levels on total white flour milled from treated
wheat after different storage periods remained quite constant at about
one sixth of the applied rates. Some fluctuation in this proportion
was detectable at the lower application rate, but this is probably
because in these cases the deltamethrin levels on the flour were close
to the limits of detection. When baked in white bread, there is no
significant change in residue levels, showing that no breakdown of
deltamethrin occurred during baking.
Consideration of the levels found on each fraction, and the
weights of each fraction produced by unit weight of wheat, leads to
the conclusion that there is no breakdown of deltamethrin during the
break, reduction and finishing systems of the milling process that
produces white flour.
The following conclusions may be drawn from the results of this
trial:
Deltamethrin is not degraded on wheat over a nine-month storage
period.
There is no degradation of deltamethrin when treated wheat is milled
to produce either wholemeal or white flour.
When wholemeal flour made from deltamethrin treated wheat is baked,
there is an apparent reduction in deltamethrin residues, but this is
due to the greater moisture content of the bread.
In the production of white flour and bread from deltamethrin treated
wheat, there is a reduction in the deltamethrin level to about 10 to
20% of the level applied to the wheat. This is because the
deltamethrin is predominantly found on the bran fraction with a
concentration increase of 4 to 7 fold. There is no evidence for
deltamethrin degradation when white flour is baked to produce white
bread.
Over a 9-month storage period, there appears to be a migration of
deltamethrin to the fine offal fraction (including the wheat germ
which is used in health foods and "Hovis" bread) and the first-
reduction flour (used in the production of cakes) presumably from the
bran fraction (predominantly used for animal feed).
NATIONAL MAXIMUM RESIDUE LIMITS
The Meeting was advised of the following limits established by The
Netherlands:
Strawberry 0.2 mg/kg
Other fruit 0.1 mg/kg
Leafy vegetables 0.2 mg/kg
Other vegetables 0.05 mg/kg
EVALUATION
COMMENTS AND APPRAISAL
Deltamethrin was first evaluated in 1980 but, in the absence of
some essential toxicological information, it was not possible to
establish an ADI. The Meeting proposed a series of guideline levels
reflecting residues likely to occur on various food commodities
following the use of deltamethrin with present good agricultural
practice. A small amount of additional information was available for
consideration at the Meeting.
Residue data from trials on kiwi fruit were considered suitable
to propose guideline levels, but further data are needed to confirm
whether these levels are typical of those occurring in other regions
where these crops are grown. In the case of kiwi fruit, the residues
are entirely confined to the inedible peel. Data were received from
residue trials on black currants, but as there was evidence that
deltamethrin was not yet approved for use on currants, no guideline
levels were proposed.
Results of several trials on spinach indicate that cooking has
little or no effect on the level of deltamethrin residues on such
leafy vegetables. These studies do not, however, provide an adequate
basis for determining whether the guidelines proposed in 1980 for
spinach should be amended.
There is a suggestion that residues of deltamethrin on some
cereal straws used for animal feed could be higher than the 0.5 mg/kg
limit proposed for legume animal feeds, but the amount of information
is inadequate to provide a basis for amending the recommendation.
A report on one further study of the level and fat of
deltamethrin on wheat, milled products and bread confirm that
deltamethrin is not degraded significantly during storage, nor is it
destroyed in cooking. A substantial proportion is, however, removed on
bran during the milling of white flour. In view of the vast experience
with other grain protectant insecticides and the considerations
discussed in 1980, the Meeting deemed it necessary to revise the
recommended guideline levels for deltamethrin on raw cereals and
milled cereal products to accommodate, realistically, all the
situations likely to arise from normal applications under good storage
practice, although in most cases, levels would be considerably lower.
Such provisions are also intended to accommodate the variations due to
sampling difficulties and analytical error.
The Meeting, however, expressed the view that considerably more
information was required about the level and fate of deltamethrin on
different grains under different storage conditions and especially the
effect of processing and cooking of all cereal grains.
RECOMMENDATIONS OF GUIDELINE LEVELS
As no ADI has been allocated, the Meeting proposed the following
guideline levels determined and expressed as deltamethrin.
Commodity Guideline level (mg/kg)
Kiwi fruit 0.05
Hops (dry) 5
Cereal straw (animal feed) 0.5
Cereal grains 2
Wheat bran (unprocessed) 5
Wheat flour (wholemeal) 2
FURTHER WORK OR INFORMATION
Required (by 1982 and before an acceptable daily intake can be
allocated) (as listed in the 1980 JMPR Report).
1. Results of the two-year feeding study in dogs currently in
progress.
2. Clarification of possible embryotoxic effects in animals.
3. Further observations on reported effects in man.
4. Studies on the significance of neurological effects observed in
several animal species.
5. Results of supervised trials on residues in meat, milk, and eggs
arising from the use of deltamethrin for ectoparasite control, in
feeding studies and in stable treatments. Also required before MRLs
can be recommended:
6. Considerably more information on the level and fate of
deltamethrin on various cereal grains treated under different storage
conditions, and especially the effect of processing and cooking of all
cereal grains.
7. Additional residue data from supervised trials on kiwi fruit,
currants and leafy vegetables.
8. Information on the level and fate of deltamethrin residues in
foods of animal origin following the feeding of cattle, pigs and
poultry with rations containing deltamethrin at levels of the order
likely to be encountered in practice.
9. Further information on the level of deltamethrin on cereal grains
following treatment of crops in conformity with approved or proposed
use patterns.
REFERENCES
Barnes, J.M. and Verschoyle, R.D. Toxicity of a new pyrethroid
1974 insecticide. Nature, 248:711 1974
Beavers, J.B. and Fink, R. Eight-day dietary LC50-mallard duck,
1977a technical DECIS. Final report W1-77.18.05/A, Wildlife
International, USA, submitted by Roussel Uclaf to WHO.
(Unpublished)
Beavers, J.B. and Fink, R. Eight-day dietary LC50-bobwhite quail,
1977b technical DECIS. Final report. W1-77-19.05/A, Wildlife
International, USA, submitted by Roussel Uclaf to WHO.
(Unpublished)
Cabral, J.R. In: IARC Annual Report - 1981. International Agency for
1981 Research on Cancer, Lyon, in press.
Chesterman, H,, Heywood, R., Perkin, C.J., Beard, D., Street, E. and
1977 Prentice, D.E. RU 22974. Oral toxicity study in beagle dogs.
Report RSL 253/7751/A 3, Huntingdon Research Centre,
Huntingdon, England, submitted by Roussel Uclaf to WHO.
(Unpublished)
Clair, M. RU 22974 - DECIS. Acute toxicity in the rabbit by
1977 percutaneous administration. Report IFREB-R 770256.1/A,
Institut Francais de Recherches et Essais Biologiques,
Joinville-le-Pont, France, submitted by Roussel Uclaf to
WHO. (Unpublished)
Coombs, D.W. and Clark, G.C. RU 22974. Acute inhalation toxicity in
1978 rats, 6 hour LC50. Report RLS 310/78453/A, Huntingdon
Research Centre, Huntingdon, England, submitted by Roussel
Uclaf to WHO. (Unpublished)
Coquet, B. RU 22974. Test to determine primary cutaneous irritation in
1976a the rabbit. Report no. IFREB-R 761157/A, Institut Francais
de Recherches et Essais Biologiques, Joinville-le-Pont,
France, submitted by Roussel Uclaf to WHO. (Unpublished)
1976b RU 22974. Test to evaluate ocular irritation in the rabbit.
Report no. IFREB-R 761158/A, Institut Francais de Recherches
et Essais Biologiques, Joinville-le-Pont France, submitted
by Roussel Uclaf to WHO. (Unpublished)
Desmarchelier, J., Bengston, M., Connell, M., Minett, W., Moore, B.,
1977 Phillips, M., Snelson, J., Sticka, R. and Tucker, K. A
collaborative study of residues on wheat of
chlorpyrifosmethyl, fenitrothion, malathion, methacrifos and
pirimiphos-methyl. 1. Method Development. Pesticide Science,
8: 473-483.
Desmarchelier, J. Loss of fenitrothion on grains in storage. Pesticide
1978 Science, 9:33-38.
Elliott, M. Synthetic pyrethroids, American Chemical Society
1977 Symposium, 42:1-29.
Fouillet, X. RU 22974. Mutagenicity study of various preparations.
1976 Salmonella microsome test. Report no. IFREB-R 761153/A,
Institut Francais de Recherches et Essais Biologiques,
Joinville-de-Pont, France, submitted by Roussel Uclaf to
WHO. (Unpublished)
Gaughan, L.C., Unai, T. and Casida, J.E. Permethrin metabolism in
1977a rats. Journal of Agricultural and Food Chemistry, 25:9-17
1977b Permethrin metabolism in rats and cows and in bean and
cotton plants. American Chemical Society Symposium,
42:186-193.
Glomot, R. and Chevalier, B. RU 22974. Acute toxicity study, mouse and
1976a rat by oral route. Report Tox 76810/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
1976b RU 22974. Acute toxicity study mouse and rat by
intraperitoneal route. Report Tox 76811/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
1976c RU 22974. Acute toxicity study, mouse and rat by intravenous
route. Report Tox 76812/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
Glomot, R., Chevalier, B., Collas, E. and Audegond, L. RU 22974. Acute
1977 toxicity study by oral route in male and female beagle dogs.
Report Toxico 77804/JL-5, submitted by Roussel Uclaf to WHO.
(Unpublished)
Glomot, R. and Vannier, B. RU 22974. Teratological study in mouse, rat
1977 and rabbit. Report no. Tox 76534-76536/2/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
Glomot, R. and Vannier, B. RU 22974. Teratological study in mouse.
1978 Complementary Information. Report no. Tox. 76534.76536/A3,
submitted by Roussel Uclaf to WHO. (Unpublished)
Glomot, R. Personal communication to WHO. Roussel, Uclaf, Paris,
1981 France.
Goldenthal, E.I., Blair, M., Jefferson, N.D., Spicer, E.J.F., Arceo,
1980a R.J. and Clark Kahn III, A., RU 22974. Two-year toxicity and
carcinogenicity study in mice. Report IRDC 406-001/A/4,
International Research and Development Corp., Mattawan,
Michigan, submitted by Roussel Uclaf to WHO. (Unpublished)
Goldenthal, E.I., Jefferson, N.D., Blair, M., Thorstenson, J.H.,
1980b Spicer, E., J.F. Arceo, and Kahn, III, A. RU 22974. Two year
oral toxicity and carcinogenicity study in rats. Report IRDC
406-002/A 4, International research and Development Corp.,
Mattawan, Michigan, submitted by Roussel Uclaf to WHO.
(Unpublished)
Gray, A.J. and Rickard, J. Distribution of radiolabel in rats after
1981 intravenous injection of a toxic dose of 14C-acid-,
14C-alcohol- or 14C-cyano-labelled deltamethrin. Pesticide
Biochemistry and Physiology, 16:79-85
Guillot, J.P. and Guilaine, J. RU 22974 - Decamethrine. Decis
1977 technical Roussel Uclaf. Sensitization test in the guinea
pig. Report no. IFREB-R 709241/A, Institut Francais de
Recherches et Essais Biologiques, Joinville-le-Pont, France,
submitted by Roussel Uclaf to WHO. (Unpublished)
Halls, G.R.H., and Periam, A.W.. The fate of residues of NRDC 161 on
1980 wheat during storage and after milling and baking - Report
after 9 months storage. Wellcome Research Laboratories
Report HEFH 80-4, November 1980. (Unpublished)
Husson, J.M. Medical observations made on people working on the
1978 manufacture and formulation of the pyrethroid insecticide
decamethrin. Report no. RU 78.25.08/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
Hunter, B., Jordan, J., Heywood, R., Hepworth, P., Street, A.E. and
1977 Prentice, D. RU 22974. Assessment of toxicity to rats by
oral administration for 13 weeks (followed by a 4 week
withdrawal period). Report RSL 254/76938/A3, Huntingdon
Research Centre, Huntingdon, England, submitted by Roussel
Uclaf to WHO. (Unpublished)
IRDC Laboratories. Two year chronic dog feeding study. Report IRDC
1980 406-004/A1, International Research and Development
Corporation, Mattawan, Michigan, submitted by Roussel Uclaf
to WHO. (Unpublished)
Kavlock, R., Chernoff, N., Baron, R., Linder, R., Rogers, E. and
1979 Carver, B. Toxicity studies with decamethrin, a synthetic
pyrethroid insecticide. Journal of Environmental Pathology
and Toxicology, 2:751-765.
Kynoch, S.R., Lloyd, G.K. and Andrews, C.D. Acute percutaneous
1979 toxicity to rats of decamethrin. Report RSL-10098/D
147/79/A, Huntingdon Research Centre, Huntingdon England,
submitted by Roussel Uclaf to WHO. (Unpublished)
Peyre, M., Chantot, J.F., Glomot, R. and Penasse, L. Detection of a
1982 mutagenic potency of decamethrin (RU 22974). Bacterial
tests. Report no. RU/TOX/80.21.01/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
Ray, D.E. and Cremer, J.E. The action of decamethrin (a synthetic
1979 pyrethroid) on the rat. Pesticide Biochemistry and
Physiology, 10:33.
Ray, D.E. An EEG investigation of decamethrin induced choreoathetosis
1980 in the rat. Experimental Brain Research, 38:221.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E., Cooke, L.
1978 and Gibson, W.A. RU 22974 (Decamethrin) LD50 determination
and assessment of neurotoxicity in the domestic hen. Report
no. RSL 293-NI/830/A. Huntingdon Research Centre,
Huntingdon, England, submitted by Roussel Uclaf to WHO.
(Unpublished)
Roussel Uclaf. Test to determine the toxicity in the chicken by oral
1976a route. Report RU-76.05.05/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
1976b Toxicity of decamethrin or DECIS in single ingestion in
game ducks, Anas platyrhynchos L. Report INRA-76.21.12/A.
Institut National de la Recherche Agronomique. Jony-En-
Josas, France, submitted by Roussel Uclaf to WHO.
(Unpublished)
Roussel Uclaf. Toxicity of decamethrin or DECIS by single ingestion
1976c in grey partridge, Perdix perdix l., and Red Partridge
Alectous rufa l. Report INRA-76.28.09/A. Institut National
de la Recherche Agronomique. Joney-en-Josas, France,
submitted by Roussel Uclaf to WHO. (Unpublished)
Ruzo, L.O., Unai, T. and Casida, J.E. decamethrin metabolism in rats.
1977 Report UC-77.06.12/A, submitted by Roussel Uclaf to WHO.
(Unpublished)
1978a Decamethrin metabolism in rats. Journal of Agriculture and
Food Chemistry, 26:918-925.
Ruzo, L.O., Engel, J.L. and Casida, J.E. Oxidative, hydrolytic and
1978b conjugative reactions in the metabolism of decamethrin in
mice. Report UC 78.10.11/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
1979 Decamethrin metabolism from oxidative, hydrolytic and
conjugative reactions in mice. Journal of Agricultural Food
and Chemistry, 27:725-731.
Shono, T., Oshawa, K. and Casida, J.E. Metabolism of trans- and cis
1978 permethrin, trans- and cis-cypermethrin and decamethrin by
microsomal enzymes. Report UC 78.18.05/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
Sobels, F.H., Tates, A.D. and Vannier, B. Cytogenetic study with RU
1978 22974. Detection of a mutagenic potency in mammalian cells.
Report no. ULN-782211/A, State university of Leiden, The
Netherlands, submitted by Roussel Uclaf to WHO.
(Unpublished)
Soderland, D.M. and Casida, J.E. Substrate specificity of mouse liver
1977 microsomal enzymes in pyrethroid metabolism. In: Synthetic
Pyrethroids, American Chemical Society Symposium,
42:152-162.
Vannier, B. and Glomot, R. RU 22974. Mutagenic study. Dominant lethal
1977 assay in the male mouse. Report no. Toxico 76533/DB9/A,
submitted by Roussel Uclaf to WHO. (Unpublished)
Wrenn, J.M., Rodwell, D.E., Goldenthal, E.I., Spider, E.J.C. and
1980 Rajasekaran, B. Three-generation reproduction study in rats.
Report no. IRDC-406-003/A4, International Research and
Development Corporation, Michigan, USA, submitted by Roussel
Uclaf to WHO. (Unpublished)
TABLE 3. Acute toxicity of deltamethrin
LD50
Species Sex Route (mg/kg bw) References
Mouse M+F iv 41 Glomot and Chevalier 1976c
M ip 181 ibid 1976b
M ip 1712 ibid 1976b
M oral 211 ibid 1976a
M oral 332 ibid 1976a
F io 121 ibid 1976b
F ip 1662 ibid 1976b
F oral 191 ibid 1976a
F oral 342 ibid 1976a
Rat M+F iv 311 ibid 1976c
M ip 241 ibid 1976b
M ip 2092 ibid 1976b
M oral 671 ibid 1976a
M oral 1282 ibid 1976a
M+F inhal. 0.63 Coombs and Clark 1978
M+F dermal <29404 Kynoch et al 1979
F ip 251 Glomot and Chevalier 1976b
F ip 1862 ibid 1976b
F oral 861 ibid 1976a
F oral 1392 ibid 1976a
Rabbit M+F dermal >20005 Clair 1977
Dog (beagle) M+F oral >3001 Glomot et al 1977
M+F oral >3006 ibid
Chicken oral ca 10002 Roussel Uclaf 1976a
Hen (adult) F oral >25002 Ross et al 1978
F oral >50007 ibid
Mallard duck oral >46407 Beavers and Fink 1977a
Game duck oral >40006 Roussel Uclaf 1976b
Grey Partridge M+F oral >18007 ibid
Red Partridge M+F oral >30007 ibid
1 Suspended in polyethylene glycol 200; 2 dissolved in sesame oil;
3 expressed for LC50 in mg dust/m3 air; 4 60% w/v suspension in aqueous
methyl-cellulose in occlusion; 5 as paste in PEG 400 on occlusion;
6 in capsules or cachets; 7 dissolved in maize oil.
Pretreatment with either the oxidase inhibitor piperonyl butoxide or
the esterase inhibitor S,S,S-tributyl phosphorotrithioate (DEF)
further increased deltamethrin in mice, reduced its rate of in vivo
metabolism by hydroxylation and hydrolysis, reduced the rate of
product excretion and elevated deltamethrin levels in fat and brain
(Ruzo et al. 1979; Soderlund and Casida 1977).
Deltamethrin-hydrolysing esterases are generally sensitive to
organo-phosphate inhibition (e.g. TEPP inhibition of hydrolysis by
all tissue preparations except stomach). The two major factors
contributing to the rapid detoxification of decamethrin are: a) the
relevant esterases present in many tissues and the oxidases in, at
least, liver microsomes, and b) many molecular sites that are
susceptible to metabolic attack (Ruzo et al 1979).
There are large differences in the toxicity of deltamethrin to
mice, depending on the route of administration, the carrier vehicle
and previous exposure to piperonyl butoxide or DEF. Irrespective of
these factors, there appears to be a critical concentration of
deltamethrin in brain that correlated with the onset of tremors or the
time of death. Additionally, direct intracerebral administration of
deltamethrin at approximately this brain level gave a similar
poisoning effect. The brain appears to be a primary target in
deltamethrin poisoning of mice (Ruzo et al 1979).
Rat
The signs of toxicity observed in rats after deltamethrin
administration included salivation and choreoathetosis (a writhing
type of toxicity) (Barnes and Verschoyle 1974; Ray 1980; Ray and
Cremer 1979; Verschoyle and Aldridge 1980) and have been designated as
the CS syndrome of toxicity (Verschoyle and Aldridge 1980).
Rats injected i.v. with deltamethrin showed muscular
contractions, piloerection, respiratory defects, convulsions and
paresis of the hind quarters immediately following treatment. Death
occurred within 10 min. After 24 h only piloerection was visible;
after 48 h surviving animals showed normal behaviour. After i.p.
injection immediate tremors, convulsions, prostration and cyanosis
were observed. After 48 h surviving animals showed normal behaviour.
Gavage with deltamethrin shortly after dosing induced motor
incoordination, convulsions and respiratory defects. After 24 and
48 h, hypomotility and convulsions were still observed. After 3 days
surviving animals showed normal behaviour (Glomot and Chevalier
1976a,b,c).
In an earlier study, the acute i.v. lethal dose in young adult
female rats was 2 to 2.5 mg/kg when administered as a solution in
glycerol formal (Barnes and Verschoyle 1974). Signs of poisoning
included excessive salivation without lacrimation, rapidly followed by
continuous jerking movements of the limbs, occasionally progressing to
convulsions. The toxic effects were rapid in onset and brief in
duration. Death occurred in some animals within 12 min of dosing while
survivors were generally symptom-free within 6 h. In a more recent
study (Kavlock et al 1979, see Table 4), the acute LD50 for adult
female rats was 31 mg/kg by the oral route and 4 mg/kg by the i.v.
route. The LD50 was observed to be sex and age dependent, with higher
values found for weanlings and males. Initial signs of deltamethrin
poisoning included profuse salivation and convulsive movements;
weakness, dyspnoea, anorexia and staining of the fur were observed
beyond the first day following compound administration.
Rats (7 males and 7 females/group) were exposed (whole body)
during 6 h to aerosols of a.i., aerosol concentration being 0.049,
0.43, 0.54 and 0.72 g/m3. The aerosol contained 66 to 86% of
particles < 5.5 µ. During exposure, hyperactivity and dose-dependent
increase in grooming and irritation were observed. The animals were
hypersensitive to touch and noise and showed uncoordinated movements.
During the observation period of 14 days following exposure, all
animals except those from the lowest dose group developed poor motor
coordination and hypersensitivity. At the end of the period all
animals were recovered to normal. In these groups the body weight gain
and food intake was depressed during 3 days following exposure. In
rats (4 of control and of highest dose group) killed immediately after
exposure, the stomach and small intestine were gas filled. In treated
rats, as result of exposure, massive haemorrhage and oedema in lungs
were observed. Stomachs were filled with gas, blood and mucus. White
deposits were visible in the trachea. In animals killed after the
observation period, a dose-dependent increase of lung degeneration
(coloured spots to congestion) was observed (Coombs and Clark 1978).
Mouse
Mice injected i.v. with deltamethrin showed intense tremors,
convulsions and ataxia immediately after administration. Also,
tachycardia and respiratory defects were observed at higher doses.
Surviving animals showed normal appearance after 4 to 5 h. Immediately
after i.p. injection, jumping movements, slight convulsions and
prostration, ptosis, tail hypertonicity and cyanosis were observed.
Surviving animals appeared normal after 72 h. Animals gavaged with
deltamethrin showed muscular stiffening and convulsions 1 h after
dosing. After 24 h hypermotility, stereotype movements of the head,
tachycardia, hypertonicity of the tail and a few convulsions were
noted. Normal behaviour and appearance were seen after 48 h (Glomot
and Chevalier 1976a,b,c).
TABLE 4. Acute toxicity of deltamethrin to rats1
95 % Confidence Lowest dose Minimum Toxic
Route Strain Sex Age LD50 limit of tested Dose
mg/kg LD50 (mg/kg) (mg/kg) (mg/kg)
Oral2 Sherman M Adult 52 46 - 58 30.00 306
Oral2 Sherman F Adult 31 29 - 34 5.00 107
Oral2 Sherman F Weanling 50 42- 60 7.50 157
Intravenous3 Sherman F Adult 4 2.9-5.3 1.57 1.576
Intravenous3 Sherman F Weanling 1.8 1.5-2.1 0.78 0.76
Dermal4 Sherman F Adult >800 --- 800:0 --- 8
Inhalation5 Sprague-Dawley M Adult 940 mg/m3 --- ---
Inhalation5 Sprague-Dawley F Adult 785 mg/m3 --- ---
1 Reference-Kavlock et al 1979;
2 Dissolved in peanut oil and administered via gavage at a rate of 5 ml/kg bw;
3 Dissolved in acetone and administered via a single injection into tail vein of rat at 0.313 ml/kg bw;
4 Dissolved in xylene and administered at a rate of 3.2 ml/kg bw;
5 Aerosols generated from 10% DMSO solution. Based on exposure time of 120 to 150 min.;
6 Moderate to severe salivation and convulsions;
7 Mild salivation;
8 No signs of toxicity at 800 mg/kg.
Rabbit
Rabbits (10 males and 10 females) were treated with 2 g
deltamethrin in 2 ml PEG 400/kg bw on 80 cm3 shaven skin for 24 h on
occlusion. The animals were observed for 14 days. One animal showed
obvious erythema and another congested skin. No weight changes or
abnormal behaviour were observed. On histological observation of
liver, kidneys and skin, small changes were observed that were common
for this strain of rabbits and not related to treatment (Clair 1977).
Dog
Dogs showed, at non-lethal doses, transient hyperexcitability,
akynesia, vomiting and stiffness of the hind legs (Glomot et al
1977).
Birds
Oral administration of a.i. to hen, game duck or partridge
produced no distinct symptoms except a small initial weight loss
occasionally. In chickens diarrhoea, convulsions and jerky movements
of the head were observed. Mallard ducks, at lethal doses, exhibited
signs of neurotoxic effects, which included ataxia, loss of
equilibrium and loss of coordination. The effect was dose-related: at
lower dose levels only some hyperexcitability and imbalance were
observed (Beavers and Fink 1977a).
Short-term studies
Rat
Male and female weanling Sprague-Dawley rats (20/sex/group)
were daily dosed by oral gavage with 0, 0.1, 1.0, 2.5 or 10.0 mg
deltamethrin in PEG 200/kg bw/day for 13 weeks (Hunter et al 1977).
No treatment-related effects on food and water consumption, mortality,
urinalysis and haematology were observed. Neurological examinations
and ophthalmoscopy revealed no abnormalities. In the 10 mg/kg bw
group, some hypersensitivity was observed in week 6 in males. Body
weight gain among males receiving deltamethrin was significantly lower
at 2.5 and 10 mg/kg/day. The body weight of the females was not
affected by the treatment. The male animals of the 1 mg/kg group
showed a tendency to a reduced body weight gain. In the females, blood
glucose and urea were significantly increased in week 6, but no
significant changes occurred in week 12 or with other blood chemistry
parameters. No clear effects were noted on the weights of the organs.
Gross and microscopic examination of a variety of tissues and organs
showed no treatment-related alterations.
Following the 13-week dosage period, 5 males and 5 females per
group were allowed to recover for 4 weeks. Autopsy was performed at
the end of this recovery period. The body weights of all previously
treated animals appeared not to be different from controls. Thyroid
weights were not dose-related but were increased in males. This
increase was significantly higher in the 1.0 mg/kg and 10.0 mg/kg
group. Marginal no-effect level was 1 mg/kg bw (Hunter et al 1977).
Dog
Male and female beagle dogs, 3-5/sex/group, 25 weeks of age, were
daily dosed orally with 0, 0.1, 1.0, 2.5 and 10.0 mg deltamethrin in
PEG 200/kg bw/day in gelatin capsules. Dosage was continued for 13
weeks, followed by a recovery period of 20 weeks for 2 dogs/sex from
the groups receiving 1.0, 2.5 and 10.0 mg/kg bw/day (Chesterman
et al 1977). Observations were made on behaviour, mortality, body
weight and food and water consumption. Haematology, blood chemistry,
urinalysis and six channel EEG-analysis were performed at week 0, 6
and 1, and ophthalmoscopy at week 0, 5 and 12. Special attention was
paid to the muscular and nervous systems.
Liquid faeces were associated with all groups of treated dogs
throughout the dosing period. All groups of animals receiving
deltamethrin gained less weight than the controls. The effects were
not strictly dose related. The dogs from the control group left
smaller quantities of the offered food than those of the treated
groups, whereas the water consumption was not dose-relatedly decreased
in all treated groups.
Dilation of the pupils was seen to occur in the dogs receiving
2.5 and 10.0 mg/kg/day. The sign was first seen 4 to 7 h after dosing
and persisted throughout the day. They reacted normally prior to
dosing on the following day. The incidence of vomiting was dose-
related increased in all treated groups, except the 0.1 mg dose level.
The incidence decreased in all the animals affected as the dosing
period progressed. In the highest dose group, unsteadiness, body
tremors and jerking movements were seen, particularly in males in
weeks 2, 3 and 4. These effects were reduced during week 5 to 9 and
were seen only in one dog in week 13. Excessive salivation was seen
initially but diminished during dosing period. After 5 and 12 weeks,
depression of the gag reflex was noted in a proportion of animals in
all treated groups. Depression of the patellar reflex was observed in
all treated groups except the dogs administered 0.1 mg/kg. In the
animals given 1 or 2.5 mg/kg/day, exaggeration of the patellar reflex
was noted only after 5 weeks. Some animals of all treated groups
showed variations in the flexor reflex. A high proportion of the
animals had depression of the hind limb tactile placing reaction.
At dosage levels of 2.5 and 10 mg/kg/day, deltamethrin caused
modification of the EEG pattern in some animals following 12 weeks
administration. Histopathological evaluations of tissues and organs,
including nervous system and muscle, did not reveal abnormalities that
could be related to dosage with the test compound. During recovery the
gag reflex continued to be depressed, whereas exaggeration of the
patellar reflex was still seen in some dogs that had previously
received 1.0 mg/kg/day. One animal continued to show an abnormal EEG
pattern (Chesterman et al 1977).
Quail and duck
Deltamethrin was given in the diet to 14-day old mallard ducks
for 5 days, at doses of 0, 464, 1 000, 2 150, 4 640 and 10 000 mg/kg
feed. The number of animals per group was 10. There was some mortality
in the two highest dose groups. Birds of the highest dose group showed
ataxia and loss of coordination. There were dose-related decreased
weight gain and food consumption (Beavers and Fink 1977a).
An identical experiment was performed with 14-day old bobwhite
quails (10 animals/group). The effects were the same as in the mallard
ducks, except there was no mortality (Beavers and Fink 1977b).
Long-term studies
Rat
Male and female Charles River CD rats (90/sex/group) were dietary
fed with 0 (control), 2, 20 and 50 mg deltamethrin/kg in the diet for
two years. Sixty males and 60 females were used in a second control
group. After 6, 12 and 18 months of compound administration, 10
animals/sex/group were sacrificed, except for the second control group
(Goldenthal et al 1980b).
No changes in general behaviour and appearance in relation to
compound treatment were recorded. Survival was similar in control and
treated rats (50 to 67%). Rats at 50 mg/kg feed gained slightly less
weight than control rats, whereas the food consumption was essentially
the same. Ophthalmoscopic findings generally were similar for control
and treated rats. No haematological and biochemical parameters were
changed in a biologically significant way in relation to treatment at
any time, except for a decreased SGPT activity at 6 months in the mid-
and high-dose groups. No treatment-related effects were observed on
organ weights. The macroscopy and microscopy findings were common for
the animals of the species and strain, except for a slightly increased
incidence of axonal degenerations in sciatic, tibial and/or plantar
nerves at 18 months in the 20 and 50 mg/kg groups. Evaluation of
incidence and/or severity of these degenerations at termination of the
study was obscured by the age of the animals.
Seven interstitial cell adenomas were observed in the testes of
the 50 mg/kg feed group compared to 0 and 4 in the two control groups.
Only from some animals of the 2 and 20 mg/kg groups were some organs
and tissues, including the testes, studied histopathologically.
Evaluation of a possible dose-response effect on the testes was
therefore not possible. The no-effect level was suggested to be
2 mg/kg (Goldenthal et al 1980b).
Mouse
Male and female Charles River CD-1 mice (80/sex/group) were fed
dosage levels of 0(control), 1, 5, 25 and 100 mg deltamethrin/kg in
the diet for 24 months. In a second control group 60 mice/sex were
used. After 12 and 18 months 10 mice/sex/group, except for two only in
the control group, were sacrificed.
There were no clear effects related to the administration of
deltamethrin on general behaviour, mortality, body weight and food
consumption. Blood chemistry, haematology and urine analysis
parameters were normal after 12, 18 and 24 months. Increases or
decreases in absolute and/or relative organ weights occurred in a few
organs at each dosage level at any time of sacrifice. Microscopic
examination of tissues did not reveal any lesions indicative of a
compound-related effect. The tumour incidence was unaffected by
deltamethrin administration. The no-effect level was suggested to be
100 mg/kg (Goldenthal et al 1980a).
Studies designed to investigate the potential carcinogenic
effects of deltamethrin have been initiated (Cabral 1981). Groups of
C57B1 mice have been administered orally 0-8 mg/kg bw deltamethrin in
arachis oil.
Dog
Deltamethrin (RU 22974) dissolved in maize oil was administered
in the diet to 64 beagle dogs at dosage levels of 1, 10 and 40 ppm for
24 months. This corresponds to 0.025, 0.25 and 1 mg/kg bw respectively
(IRDC 1980). Eight males and eight females were used at each dosage
level and in a control group. Individual body weights and food
consumption values were determined weekly. Ophthalmic, haematologic,
biochemical and urinalysis examinations were conducted during the pre-
test period at 6, 12, 18 and 24 months of the study. Neurological
exams were conducted at approximately 1 year and before termination.
These later parameters included: for cranial nerves and segmental
nerves, tests for postural reactions, placing reactions and hopping
reactions.
No signs of overt toxicity were observed in any of the dogs. Body
weights and food consumption values were similar for control and
treated dogs. No compound-related effects were observed during the
ophthalmoscopic and physical examinations. Although there were some
randomly statistically significant differences between the control and
other dose groups in the haematologic and biochemical tests, there
were not any physiologically significant changes observed at any
interval in the study. Two treated and two control animals died during
the study.
No compound-related gross or microscopic changes were observed in
the surviving dogs that were sacrificed and necropsied. Inflammatory,
degenerative and proliferative changes described were spontaneous in
nature or related to theoestrous phase of the menstrual cycle and
unrelated to compound administration.
On the basis of the results from this study, it was concluded
that the no-effect level is 40 ppm in the diet (equivalent to
1 mg/kg bw/day) administered RU 22974 over a 2-year period (IRDC
1980).
Special studies on primary irritation
Cutaneous irritation
Male albino rabbits (12/group) weighing 2.5 to 3.5 kg were
administered 0.5 g deltamethrin to either shaven intact or abraded
skin. The occlusive patch was fixed on the skin for 23h. Scoring for
erythematous and oedematous lesions occurred 1 h and 49h after removal
of the patches. Technical deltamethrin, 98% a.i., showed no irritant
effect (Coquet 1976a).
Ocular irritation
Deltamethrin (0.1 g/animal) was administered into the
conjunctival sac of the eye of 6 male albino rabbits, weighing 2.5 kg,
with or without rinsing 60 sec. after instillation. Observations for
conjunctival lesions, chemosis, discharge, conjunctival enanthema,
opacity and affected cornea were made 1 h, 24h, 2,3,4 and 7 days
following instillation. Deltamethrin showed, both with and without
rinsing, transient irritating effects (Coquet 1976b).
Special studies on sensitization
Deltamethrin (0.5 g/animal) was applied topically to the skin of
albino guinea pigs with a 2-day interval for 3 weeks, and once at the
start of the 4th week. The preparation was covered with an occlusive
patch for 48h. On days 1 and 10 the guinea pigs received an
intradermal injection of 0.1 ml of Freund's adjuvant. The animals were
challenged 12 days after the last application with 0.5 g deltamethrin.
The macroscopic and histological examination did not reveal evidence
of sensitization (Guillot and Guilaine 1977).
Special studies on reproduction
Rat
Groups of 10 female and 10 male Charles River rats were fed
deltamethrin in the diet at 0, 2, 20 and 50 mg/kg and mated to begin a
three-generation, 2 litter (first generation, 3 litter) standard
reproduction study. Parental body weights and food consumption were
recorded during the study. After weaning of the second litter, the
survival parental rats were sacrificed and necropsied. Five male and
5 female pups of the F3b were necropsied. No changes in general
behaviour or survival of parental rats or pups relevant to the test
material were observed. The body weight of F0 males of the 50 mg/kg
group was decreased from week eleven onwards. There was some slight
decreases in mean food consumption of the parental F1 male rats in
the 50 mg/kg feed group.
The basic reproduction indices (fertility, gestation, lactation,
viability and litter size) were not affected by the treatment.
However, the mean pup weight was affected in some litters; especially
that of the 50 mg/kg group was slightly decreased in comparison to the
controls. Gross external examination revealed no abnormalities. No
gross or microscopic lesions of treatment-related significance or
significant effects on the organ weights of the F3b generation were
observed (Wrenn et al 1980).
Special studies on teratogenicity
Mouse
Mated female Swiss CDI.SPF mice (24/group) were given orally
deltamethrin dissolved in sesame oil at dose levels of 0, 0.1, 1 and
10 mg/kg bw/day during days 6 to 17 of pregnancy. The animals were
necropsied on day 18 of gestation. No teratogenic effects could be
detected. Total implantation sites, foetal losses, living foetuses and
examinations of skeletal tissue were normal. Minor embryotoxic effects
were observed, e.g. dose-dependent decrease of average foetal weight
and delayed ossifications at all dose levels tested (Glomot and
Vannier 1977).
Technical deltamethrin (Roussel UCLAF), dissolved in corn oil was
administered to CD-1 mice by gastric intubation at doses of 12.0, 6.0,
3.0 or 0 mg/kg during days 7 to 16 of gestation (Kavlock et al
1979). Maternal weight on day 6 was used for calculation of doses and
intubation was 0.2 ml; control animals received vehicle alone. Animals
were sacrificed on day 16 of gestation. Administration of deltamethrin
to pregnant mice resulted in a dose-related (p < 0.001) reduction in
maternal weight gain during pregnancy. Pregnant dams in the high
dosage group (12.0 mg/kg/day) gained 58% less weight than did those in
the control group. No dose-related occurrences of maternal mortality
were observed, but most animals in the high dosage group and some in
the middle dosage group became convulsive soon after dosing. No
effects were observed on the number of implantation sites, foetal
mortality, or foetal weights, or in the number of sternal and caudal
ossification centers. A significant (p < 0.01) dose-related increase
in the occurrence of supernumerary ribs was observed. No other dose-
related skeletal or visceral anomalies were observed in deltamethrin-
treated mouse foetuses. No evidence of teratogenic activity was found
in mice at dose levels that produced maternal toxicity (Kavlock
et al 1979).
Rat
Mated female Sprague-Dawley rats (24/group) were administered
orally 0, 0.1, 1 and 10 mg deltamethrin/kg bw/day during days 6 to 18
of pregnancy. Examination occurred on day 21 of gestation. Twelve
females in the control and 10 mg/kg bw groups were allowed to deliver.
There were no effects on the reproduction or teratogenicity parameters
examined, with the exception of a slight delayed ossification in the
highest dose level (Glomot and Vannier 1977).
Technical deltamethrin (Roussel UCLAF) dissolved in maize oil was
administered to Sprague-Dawley rats by gastric intubation at doses of
5.0, 2.5, 1.25 or 0 mg/kg during days 7 to 20 of gestation (Kavlock
et al 1979), Maternal weights on day 6 were used for the calculation
of doses and intubation volume was 0.2 ml; control animals received
the vehicle alone. Rats were sacrificed on day 21 of gestation.
Administration of deltamethrin to pregnant rats resulted in a dose-
related reduction (p > 0.01) in maternal weight gain during
pregnancy, with animals in the high dosage group (5.0 mg/kg) gaining
only 80% of the control value. This dose produced a mild salivation
for up to 4 h after dosing in approximately 50% of the animals. No
effects were observed on the number of implantation sites, foetal
mortality, foetal weight or number of sternal and caudal ossification
centres. For post-natal studies, an additional group of pregnant rats
was housed individually and intubated from day 7 of gestation to day
15 of lactation with dosages of either 5.0, 2.5 or 0 mg/kg
deltamethrin dissolved in maize oil. No persistent toxicity was
observed in neonatal rats that received perinatal exposure to
deltamethrin (Kavlock et al 1979).
Rabbit
Groups of fifteen mated females received deltamethrin dissolved
in sesame oil at levels of 0, 1, 4 or 16 mg/kg bw/day from days 6 to
19 of pregnancy. Examination was carried out on day 28 of gestation.
The average foetal losses were not dose-related and increased at all
doses tested. This effect was mainly caused by a higher rate of
expelling traces. The average foetal weight in the highest dose group
was decreased. Some malformations (one animal with hydrocephalia, and
one with exencephalia and thoracogastroschisis) were observed in
2 animals of the highest dose level. A complementary study with
15 mg/kg bw/day was performed. In this study one foetus with spina
bifida and shortened tail was detected among 69 parent normal
foetuses. It was concluded that the malformations were within the
normal limits of the strain used and were not related to the
treatment, despite the occurrence at the highest dose level only
(Glomot and Vannier 1977, 1978).
Special studies on neurotoxicity
Adult hens (10/group) were gavaged with a single dose of 0, 500,
1 250 or 5 000 mg/kg bw deltamethrin suspended in maize oil or 0 and
1 000 mg/kg bw dissolved in sesame oil. Tri-o-cresylphosphate (TOCP)
(500 mg/kg bw) was used as positive control for delayed neurotoxicity.
During 21 days, observations were made on mortality, health,
neurotoxic signs and body weight.
In the TOCP group, 8 out of 10 animals died whereas only
2 mortalities were observed in the group dosed at 1 000 mg
deltamethrin/kg with sesame oil as the vehicle. Deltamethrin
induced no clinical, macroscopic or histological signs of delayed
neurotoxicity. TOCP-treated hens showed severe ataxia and degenerative
changes in the spinal cord and, occasionally, in the sciatic nerve
(Ross et al 1978).
Special studies on potentiation
Mice
Deltamethrin is hydrolysed in vitro by esterases in blood and in
brain, kidney, liver and stomach preparations of mice. Pre-treatment
of mice with oxidase inhibitor, piperonyl butoxide (PB), or esterase
inhibitor, S, S, S-tributylphosphorotrithioate (DEF), delayed
metabolism of i.p. administered deltamethrin. Using oxidase or
esterase inhibitor, different vehicles and different administration
routes, it was possible to induce similar toxic effects with a wide
range of deltamethrin doses (6 to 191 mg/kg bw). The different
treatments showed that PB or DEF made mice more sensitive to
deltamethrin (Ruzo et al 1978b).
Special studies in mutagenicity
Bacteria and yeast
In a growth inhibition test with Escherichia coli, deltamethrin
at levels of 1 250, 2 500 and 5 000 µg/ml DMSO (0.1 ml per plate) had
the same marginal inhibitor effect on the mother strains (W 3110 and
WP2) as on their mutants (p 3478 and CM 611). Chloramphenicol and
N-methyl N'-nitro-N-nitroguanidine (MNNG) were used as positive
controls and induced clear inhibition (Peyre et al 1980).
Deltamethrin was compared with MNNG, 9-aminoacridine,
2-nitrofluorene and 2-amino-anthracene for mutagenic activity in the
Ames test with Salmonella typhimurium, strains TA 1535, 100, 1537,
1528 and 98. The concentrations of deltamethrin used were 2, 10, 50,
200, 500, 1 000 and 5 000 µg/plate. Deltamethrin began to precipitate
at 200 µg/plate. The mean number of revertants was not influenced by
any concentration of deltamethrin in any strain with or without S9-mix
(metabolic activation), whereas the positive control mutagens produced
an increase of the number of spontaneous revertants (Peyre et al
1980).
In a similar experiment, deltamethrin (0.2, 2, 20, 200 and
400 µg/plate dissolved in DMSO) in the presence of activated
microsomal enzymes did not influence the number of revertants of
S. typhimurium strains TA 1535, 1537, 1538, 98 and 100.
2-Aminoanthracene, 3-methylcholanthrene, benzo(a)pyrene and acridine
orange showed mutagenic activity, whereas thio-TEPA was negative
(Fouillet 1976).
Deltamethrin at levels of 10, 50, 100, 500 and 1 000 µg/plate was
not mutagenic in assays with S. typhimurium strains TA 1535, 1537,
1538, 98 or 100 either in the presence or absence of a rat-liver
homogenate metabolic activation system (the positive controls were
2-anthramine and 9-aminoacridine) (Kavlock et al 1979).
Deltamethrin was not mutagenic in assays with E. coli WP2 when
tested at levels of 10, 50, 100, 500 and 1 000 µg/plate with or
without metabolic activation (the positive control was 2-anthramine)
(Kavlock et al 1979).
Deltamethrin was not mutagenic at levels of 1 to 5% when tested
with and without metabolic activation with Saccharomyces cerevisiae
(the positive control was 1, 2,3,4-diepoxybutane) (Kavlock et al
1979).
Mammalian cells
Deltamethrin dissolved in Cremophor oil (0, 0.4, 0.2, 1 and 5 mM,
10, 0.08, 0.4, 2 and 10% v/v respectively) in the presence of a
metabolic activation system increased the incidence of chromosome and
chromatid aberrations and SCEs, after incubation with Chinese hamster
ovary cells at 1 mMol. In the absence of S9-mix (metabolic
activation), no higher rate of aberration was observed.
It was shown that this increased incidence was due to a subtoxic
effect of some reaction product of Cremaphor oil and S9-mix.
Deltamethrin in Cremaphor oil without S9-mix and dissolved in DMSO
(1%) at levels of 0, 0.001, 0.01, 0.1 and 0.2 mM with or without
metabolic activation had no effect on the number of aberrations and
SCEs. Due to insolubility, no higher concentrations were tested
(Sobels et al 1978).
Animals
Mice, 3 males and 3 females per dose, were gavaged for two
consecutive days with 5 or 10 mg deltamethrin dissolved in sesame
oil/kg bw. Control mice were gavaged with 0.3 ml sesame oil. The
incidence of chromatid aberrations in bone marrow cells or of
micronuclei in polychromic erythrocytes did not show any significant
statistical difference in treated and control groups (Sobels et al
1978).
A single oral administration of 15 mg/kg deltamethrin was given
to Swiss mice. Groups of 2 animals were sacrificed every 3 h during a
24-h period. Several animals died after treatment. The incidence of
chromatid aberrations in the femoral bone marrow was low. There was no
consistent time-related trend in the distribution of these aberrations
(Sobels et al 1978).
Deltamethrin dissolved in sesame oil in groups of 9 to 13 male
mice dosed orally with 0 or 3 mg/kg bw for 7 days and 6 or 15 mg/kg bw
in a single dose showed no effect on the rate of pre- or post-
implantation losses after mating with 6 to 18 non-treated females.
The highest dose tested was toxic to the males, e.g. 7 out of 20
animals died shortly after treatment. Histological examination of
the testes of all animals revealed no abnormalities. Triethylene
thiophosphoramide (10 mg/kg bw), used as a positive control, reduced
considerably the rate of pregnancies in the second and third week and
increased the number of embryonal losses (Vannier and Glomot 1977).
Special studies on humans
Cutaneo-mucuous manifestations have been observed among plant
workers dermally exposed to technical deltamethrin or its
formulations. Initial lesions were tenacious and painful pruritus
(pricking sensation), especially observed after exposure to hot water
or on perspiration, followed by a blotchy local burning sensation with
blotchy erythema for about 2 days. Thereafter, slight and regular
desquamation, restricted to the contaminated area, occurred. The
cutaneous signs are sometimes accompanied by itching of the face
(mainly around the mouth) and/or rhinorrhoea or lacrimation. No other
symptoms related to exposure were observed (Husson 1978).
The effects described, which were observed before 1978 in a few
plant workers, have not been seen since a new plant was specifically
built for the entire automatization of deltamethrin production, which
does not involve workers being in direct contact with intermediates
and the final product. The only workers exposed are those involved in
the packaging of the technical product (>98% active ingredient) and
they are fully protected by special hermetic clothing (Glomot 1981).
At the level of formulation plants, steps have been taken to
avoid direct contact by workers involved in introducing technical
powder in the basic solvent. No toxic effects have been reported in
the past several years in these formulation plants, although a very
limited number of workers have complained about some irritating
effects. However, these were always transient and without any further
consequences (Glomot 1981).
The formulation which has been most frequently used for
deltamethrin application is the EC. The use of both EC and ULV
formulations corresponds in 1981 to a treated area covering several
million hectares in the world without any case of intoxication. The
majority of users are plant growers who apply the formulations in the
fields and are not in contact with the technical product per se
(Glomot 1981). A few cases of irritation problems have been reported
in some workers, due primarily to the use of the EC formulation. This
has been observed when treatments were made in orchards with high
trees needing high volumes of mixture (e.g. in South Africa) or under
greenhouses in confined spaces. In all cases, the reported problems
were not severe and could be treated using antihistaminic syrups or
with pomades based on cocaine derivatives. The irritation is most
probably enhanced by the presence of aromatic solvent, e.g. xylene, in
the EC formulation. Flowable experimental formulations without any
solvent did not cause irritating effects in human volunteers (Glomot
1981). No cases of neurological effects have been reported with
formulation workers nor have fatal intoxications been reported (Glomot
1981).
RESIDUES IN FOOD
USE PATTERN
Pre-harvest treatments
Information was received from The Netherlands on pre-harvest
treatment of several crops with deltamethrin, which is summarized in
Table 5.
RESIDUES ARISING FROM SUPERVISED TRIALS
Results of supervised trials with deltamethrin on various crops
are summarized in Table 6.
Assorted fruit, inedible peel
Kiwi fruit
Data from two new field trials conducted in France show that
residue levels for the whole fruit are below 0.05 mg/kg, the residue
being entirely in the peel.
TABLE 5. Deltamethrin use in The Netherlands
Application Rate
Crop g/100 l g/ha P.H.I.
Apple 0.5 (from before blossom to
Pear 0.5 (to 14 days P.H.
Grape 0.5 7
Cherry 0.5 7
Plum 0.5 7
Strawberry 0.5 7
Currant 0.5 7
Gooseberry 0.5 7
Raspberry 0.5 7
Cucumber 1.25 3
Melon 25 14
Bell pepper 25 14
Eggplant 25 14
Lettuce 1.25 14
Endive 25 14
Radish 7.5 7
Turnip 7.5 7
Rutabaga 7.5 7
Brussels sprouts 7.5 7
Cauliflower 7.5 7
Broccoli 7.5 7
Kohlrabi 7.5 7
Leek 7.5 7
Onion 7.5 7
Peas 7.5 7
Dwarf french bean 7.5 7
Sugarbeet 7.5 7
Mushroom 0.75-1.5 75/100 2
Hops
Several trials conducted in the UK and the Federal Republic of
Germany show a high level of variation between samples, but relatively
little decline with time over the period of 7 to 14 days following
treatment. No information was provided on the fate of such residues
when the hops were used in brewing.
TABLE 6. Supervised trials with deltamethrin in various crops
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) Formulation of sample 0 1 2/3 4 7 14
Kiwi fruit France 1980 2 12.5 peel 0.05 0.06 0.06 0.08 0.07
whole fruit 0.01 0.01 0.01 0.01 0.01
pulp ND ND ND ND ND
12.5 peel 0.4 0.22 0.23 0.25 0.1
whole fruit 0.06 0.04 0.04 0.04 0.01
pulp ND ND ND ND ND
Hops England 1978 7 12.5 0.02
W. Germany 1978 1 25 0.01
3 62.5 0.1 0.3 1.4 1.1 1.9
3 62.5 16 5.5 0.1 1.8 1.9
3 62.5 3.3 1.3 0.4 2.0 2.5
1980 5 6.25-37.5 1.3 0.2 0.4 0.3 0.7
5 " 0.2 0.7 0.3 0.7 1.6
5 " 1.0 0.7 0.2 0.6 2.7
5 " 0.5 0.3 0.2 0.7 1.1
Mushrooms1 Netherlands 1978 2 6.25 0.0025% whole 0.003 0.002 0.001
1978 2 25 0.01% " 0.009 0.004 0.003
(DAYS)
0 1 3 5 7 14
Black currant Finland 1979 1 6.3mg/bush. 0.1 0.1
W. Germany 1979 2 18.5g/ha 0.3 0.2 0.2 0.1
" 0.3 0.3 0.3 0.3
" 0.3 0.2 0.2 0.2
2 11.25g/ha 0.2 0.2 0.1 0.1
0.3 0.4 0.3 0.2
TABLE 6. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) of sample 0 1 2 4 8 14 21
Spinach France 1978 1 17.5g/ha Fresh leaf 0.48 0.33 0.22
Cooked leaf 0.27 0.21
Cooking water 0.0001 0.00009
17.5g/ha Fresh leaf 0.52 0.30 0.155
Cooked leaf 0.28 0.12
Cooking water 0.00015 0.000,05
1979 1 12.5g/ha Fresh leaf 0.4 0.2 0.14 0.12
Cooked leaf 0.12 0.10
Cooking water 0.0001 ND
12.5g/ha Fresh leaf 0.4 0.2 0.17 0.15
Cooked leaf 0.15 0.12
Cooking water 0.00015 0.0001
1980 1 17.5g/ha Fresh leaf 0.18 0.22 0.35
Cooked leaf 0.16 0.20 0.28
Cooking water ND ND ND
W.Germany 1980 3 12.5g/ha Fresh leaf 0.3 0.7 0.2 0.09
0.7 0.3 0.3 0.1
14 21 28/39 49 61 74 80
Wheat straw France 1978 1 7.5 EC 0.015
1 15 EC 0.05
1 7.5 EC 0.025
1 15 EC 0.025
1 7.5 EC 0.025
1 15 EC 0.025
1 7.5 EC ND
1 7.5 EC ND
1 12.5 EC 0.15
1 12.5 EC 0.06
1 12.5 EC 0.2
1 12.5 EC 0.1
TABLE 6. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Part
(g a.i./ha) of sample 14 21 28/39 49 61 74 80
Oat Straw W.Germany 1978 1 12.5 EC 0.06
1 12.5 EC 0.06
1 12.5 EC ND
1 12.5 EC 0.08
1979 1 12.5 EC 0.6
1 12.5 0.2
1 12.5 0.2
1 12.5 0.5
Rice straw Surinam 1977 1 6.25 ND
1 12.5 0.010
1 18.75 0.01
1 25 0.08
Phillipines 1978 1 17.5 0.2
25 0.1
1 After oven drying at 7 days.
Small fruits and berries
Black currant
From trials conducted in the Federal Republic of Germany, it
would appear that residues on these berries can range up to 0.3 to
0.4 mg/kg, but as yet, there is no registered use of deltamethrin on
black currants.
Leafy vegetables
Spinach
Additional data from trials carried out in France and the Federal
Republic of Germany indicate that residues range around 0.3 to
0.4 mg/kg, 7 or 8 days after the last treatment. These levels do not
decline significantly during cooking.
Fodder and cereal straws
Additional data were available to indicate the level of
deltamethrin on wheat straw, oat straw and rice straw. Though the bulk
of the values were below 0.2 mg/kg, oat straw sampled 28 to 39 days
after treatment showed residues ranging up to 0.6 mg/kg. The amount of
data is, however, limited and in view of the potential for the wide
application of deltamethrin to cereal crops further data are required.
Cereal grains
A report was received on trials to determine the fate of
deltamethrin on wheat during storage and after milling and baking
(Halls and Periam 1980).
Batches of an English hard wheat were sprayed on a conveyor with
diluted deltamethrin/piperonyl butoxide liquid grain protectant to
give 250 kg at each of two deltamethrin treatment levels. These 250 kg
batches were then placed in 1 ton capacity metal silos. Target
treatment levels for the two batches of grain were 1 mg/kg
deltamethrin + 10 mg/kg piperonyl butoxide and 2 mg/kg deltamethrin +
10 mg/kg piperonyl butoxide respectively, but chemical analysis
revealed that the actual treatment levels achieved were only about 40
to 75% of these.
Samples of the treated wheat were taken after spraying and
thereafter at monthly intervals for nine months and analysed for
deltamethrin content. Replicate samples were taken from each silo,
analysed separately and the result expressed as the mean. Wheat from
the initial sampling and from samplings after 3, 6 and 9 months was
submitted to the Flour Milling and Baking Research Association,
Chorleywood, Buckinghamshire to be milled and baked.
At Chorleywood the wheat was first cleaned of extraneous
material, then divided into two samples and each subjected to a
different milling procedure. The first produced whole-meal flour, the
second produced white flour, bran and fine offal.
Two different samples of white flour were taken from this
procedure, the first reduction flour (the cleanest flour mainly from
the centre of the grain and used in the baking of cakes) and total
white flour (straight-run flour plus flour from the bran and fine
offal fractions). These samples, together with the wholemeal flour,
bran and fine offal were analysed for total deltamethrin content for
both batches of treated wheat. In addition, bread was baked from both
wholemeal and total white flours to produce wholemeal and white bread,
respectively, and these loaves were also analysed for deltamethrin
content.
Results for residues of deltamethrin on wheat, milling data and
residues of NRDC 161 on flour fractions and bread are given in Tables
7, 8 and 9, respectively.
In both the deltamethrin treatments, levels were initially found
by analysis to be only about 40% of the expected application. This
could be due predominantly to inter alia incorrect spraying rates,
inadequate extraction, or loss of compound at some stage between the
nozzle and the grain.
The results given in Table 7 show that for both application rates
there was no detectable breakdown of deltamethrin on wheat over a
nine-month storage period. The fluctuation in levels between different
samplings was within the inherent error of the analytical method
demonstrating that deltamethrin is very stable on wheat. In this
respect deltamethrin differs from many organophosphate grain
protectants such as fenitrothion (Desmarchelier 1978).
The moisture content of the wheat remained fairly consistent over
the storage period of nine months. Deltamethrin analysis data for the
milling fractions and bread derived from treated wheat are given in
Tables 8 and 9.
For all sampling times at both treatment levels there was no
significant change in deltamethrin residues when wheat was milled to
produce wholemeal flour. When this flour was baked, the wholemeal
bread produced contained lower, or identical, deltamethrin residues
but this can be explained by the greater moisture content (up to 45%)
present in bread.
TABLE 7. Deltamethrin residues on wheat after indicated periods of storage in the UK1
Residue analysis (mg/kg deltamethrin)
Target
Application Initial2 1 month 2 months 3 months 4 months 5 months 6 months 7 months 8 months 9 months
rate (mg/kg) (15.11.79) (13.12.79) (17.1.80) (11.2.80) (12.3.80) (21.4.80) (12.5.80) (10.6.80) (10.7.80) (6.8.80)
1.0 0.44 0.53 0.48 0.50 0.50 0.41 0.48 0.49 0.42 0.41
2.0 0.80 1.25 1.33 1.46 1.21 1.01 0.96 1.12 0.94 1.08
1 Analytical results are subject to overall confidence limits of 30%
2 Dates refer to day of sampling.
TABLE 8. Milling data for wheat freshly treated with deltamethrin and from wheat after 3, 6 and 9 months storage1
Target application Sampling Clean wheat Bran Fine Offal Total white flour
rate period
(mg/kg) (months) Weight Prop. of Weight Prop. of Weight Prop. of Weight Prop. of
(kg) total(%) (kg) total (%) (kg) total (%) (kg) total (%)
0 3.835 100 0.596 15.54 0.306 7.98 2.849 74.29
3 4.921 100 0.779 15.83 0.519 10.55 3.502 71.16
1.0
6 3.700 100 0.621 16.78 0.340 9.19 2.670 72.16
9 3.700 100 0.626 16.92 0.288 7.78 2.746 74.22
0 3.786 100 0.590 15.58 0.292 7.71 2.809 74.19
3 4.884 100 0.769 15.75 0.514 10.52 3.489 71.44
2.0
6 3.700 100 0.630 17.03 0.306 8.27 2.688 72.65
9 3.700 100 0.650 17.57 0.275 7.43 2.692 72.76
0 (Control) 0 2.010 100 0.312 15.52 0.175 8.71 1.525 75.87
1 There is a loss of up to 3% in the laboratory milling process
TABLE 9. Deltamethrin residues on milling fractions and bread made from freshly treated wheat and from wheat after 3, 6 and
9 months storage1
Target application Sampling First Total
rate period Wholemeal Wholemeal Bran Fine reduction white White
(mg/kg) (months) Wheat flour bread offal flour flour bread
0 0.44 0.42 0.26 3.00 nd2 nd 0.09 0.10
3 0.50 0.43 0.27 1.80 approx. 0.5 nd approx. 0.05 nd
1.0
6 0.48 0.42 0.26 1.70 0.35 0.05 0.09 0.10
9 0.41 0.36 0.36 1.90 0.46 0.03 0.03 0.05
0 0.80 0.73 0.63 5.40 nd nd 0.20 0.29
3 1.46 0.80 0.57 4.70 0.75 nd 0.20 0.15
2.0
6 0.96 0.83 0.66 3.60 0.75 0.10 0.16 0.20
9 1.08 0.95 0.70 4.40 1.36 0.05 0.17 0.11
1 Analytical results are subject to confidence limits of ± 30%
2 nd = not detectable (<0.03 mg/kg).
When the treated wheats were milled to produce white flour,
relatively high deltamethrin residues were found on the bran fraction,
which is derived from the superficial layers of the grain. Initially,
deltamethrin residues on the bran were 6 to 7 times higher than those
on the wheat at both treatment rates. The levels declined to be about
4 to 5 times higher on the bran than on the wheat after 9 months'
storage.
This observation, together with the appearance of deltamethrin on
the fine offal fractions after 3 months' storage, and on the first-
reduction flours after 6 months' storage, suggests that although there
is no change in the total deltamethrin level on the wheat over 9
months, there is a subtle change in its distribution. Thus, initially,
as might be expected, the insecticide residues were found
predominantly in the outer layers of the grain, mainly in the bran
fraction, but also to a lesser extent in the total white flour
fraction. The first-reduction flour, which tends not to come from the
superficial layers of the grain, and the fine offal, consisting
largely of wheatgerm, is uncontaminated initially but residues move
into these areas as the period of storage is increased.
The deltamethrin levels on total white flour milled from treated
wheat after different storage periods remained quite constant at about
one sixth of the applied rates. Some fluctuation in this proportion
was detectable at the lower application rate, but this is probably
because in these cases the deltamethrin levels on the flour were close
to the limits of detection. When baked in white bread, there is no
significant change in residue levels, showing that no breakdown of
deltamethrin occurred during baking.
Consideration of the levels found on each fraction, and the
weights of each fraction produced by unit weight of wheat, leads to
the conclusion that there is no breakdown of deltamethrin during the
break, reduction and finishing systems of the milling process that
produces white flour.
The following conclusions may be drawn from the results of this
trial:
Deltamethrin is not degraded on wheat over a nine-month storage
period.
There is no degradation of deltamethrin when treated wheat is milled
to produce either wholemeal or white flour.
When wholemeal flour made from deltamethrin treated wheat is baked,
there is an apparent reduction in deltamethrin residues, but this is
due to the greater moisture content of the bread.
In the production of white flour and bread from deltamethrin treated
wheat, there is a reduction in the deltamethrin level to about 10 to
20% of the level applied to the wheat. This is because the
deltamethrin is predominantly found on the bran fraction with a
concentration increase of 4 to 7 fold. There is no evidence for
deltamethrin degradation when white flour is baked to produce white
bread.
Over a 9-month storage period, there appears to be a migration of
deltamethrin to the fine offal fraction (including the wheat germ
which is used in health foods and "Hovis" bread) and the first-
reduction flour (used in the production of cakes) presumably from the
bran fraction (predominantly used for animal feed).
NATIONAL MAXIMUM RESIDUE LIMITS
The Meeting was advised of the following limits established by The
Netherlands:
Strawberry 0.2 mg/kg
Other fruit 0.1 mg/kg
Leafy vegetables 0.2 mg/kg
Other vegetables 0.05 mg/kg
EVALUATION
COMMENTS AND APPRAISAL
Deltamethrin was first evaluated in 1980 but, in the absence of
some essential toxicological information, it was not possible to
establish an ADI. The Meeting proposed a series of guideline levels
reflecting residues likely to occur on various food commodities
following the use of deltamethrin with present good agricultural
practice. A small amount of additional information was available for
consideration at the Meeting.
Residue data from trials on kiwi fruit were considered suitable
to propose guideline levels, but further data are needed to confirm
whether these levels are typical of those occurring in other regions
where these crops are grown. In the case of kiwi fruit, the residues
are entirely confined to the inedible peel. Data were received from
residue trials on black currants, but as there was evidence that
deltamethrin was not yet approved for use on currants, no guideline
levels were proposed.
Results of several trials on spinach indicate that cooking has
little or no effect on the level of deltamethrin residues on such
leafy vegetables. These studies do not, however, provide an adequate
basis for determining whether the guidelines proposed in 1980 for
spinach should be amended.
There is a suggestion that residues of deltamethrin on some
cereal straws used for animal feed could be higher than the 0.5 mg/kg
limit proposed for legume animal feeds, but the amount of information
is inadequate to provide a basis for amending the recommendation.
A report on one further study of the level and fat of
deltamethrin on wheat, milled products and bread confirm that
deltamethrin is not degraded significantly during storage, nor is it
destroyed in cooking. A substantial proportion is, however, removed on
bran during the milling of white flour. In view of the vast experience
with other grain protectant insecticides and the considerations
discussed in 1980, the Meeting deemed it necessary to revise the
recommended guideline levels for deltamethrin on raw cereals and
milled cereal products to accommodate, realistically, all the
situations likely to arise from normal applications under good storage
practice, although in most cases, levels would be considerably lower.
Such provisions are also intended to accommodate the variations due to
sampling difficulties and analytical error.
The Meeting, however, expressed the view that considerably more
information was required about the level and fate of deltamethrin on
different grains under different storage conditions and especially the
effect of processing and cooking of all cereal grains.
RECOMMENDATIONS OF GUIDELINE LEVELS
As no ADI has been allocated, the Meeting proposed the following
guideline levels determined and expressed as deltamethrin.
Commodity Guideline level (mg/kg)
Kiwi fruit 0.05
Hops (dry) 5
Cereal straw (animal feed) 0.5
Cereal grains 2
Wheat bran (unprocessed) 5
Wheat flour (wholemeal) 2
FURTHER WORK OR INFORMATION
Required (by 1982 and before an acceptable daily intake can be
allocated) (as listed in the 1980 JMPR Report).
1. Results of the two-year feeding study in dogs currently in
progress.
2. Clarification of possible embryotoxic effects in animals.
3. Further observations on reported effects in man.
4. Studies on the significance of neurological effects observed in
several animal species.
5. Results of supervised trials on residues in meat, milk, and eggs
arising from the use of deltamethrin for ectoparasite control, in
feeding studies and in stable treatments. Also required before MRLs
can be recommended:
6. Considerably more information on the level and fate of
deltamethrin on various cereal grains treated under different storage
conditions, and especially the effect of processing and cooking of all
cereal grains.
7. Additional residue data from supervised trials on kiwi fruit,
currants and leafy vegetables.
8. Information on the level and fate of deltamethrin residues in
foods of animal origin following the feeding of cattle, pigs and
poultry with rations containing deltamethrin at levels of the order
likely to be encountered in practice.
9. Further information on the level of deltamethrin on cereal grains
following treatment of crops in conformity with approved or proposed
use patterns.
REFERENCES
Barnes, J.M. and Verschoyle, R.D. Toxicity of a new pyrethroid
1974 insecticide. Nature, 248:711 1974
Beavers, J.B. and Fink, R. Eight-day dietary LC50-mallard duck,
1977a technical DECIS. Final report W1-77.18.05/A, Wildlife
International, USA, submitted by Roussel Uclaf to WHO.
(Unpublished)
Beavers, J.B. and Fink, R. Eight-day dietary LC50-bobwhite quail,
1977b technical DECIS. Final report. W1-77-19.05/A, Wildlife
International, USA, submitted by Roussel Uclaf to WHO.
(Unpublished)
Cabral, J.R. In: IARC Annual Report - 1981. International Agency for
1981 Research on Cancer, Lyon, in press.
Chesterman, H,, Heywood, R., Perkin, C.J., Beard, D., Street, E. and
1977 Prentice, D.E. RU 22974. Oral toxicity study in beagle dogs.
Report RSL 253/7751/A 3, Huntingdon Research Centre,
Huntingdon, England, submitted by Roussel Uclaf to WHO.
(Unpublished)
Clair, M. RU 22974 - DECIS. Acute toxicity in the rabbit by
1977 percutaneous administration. Report IFREB-R 770256.1/A,
Institut Francais de Recherches et Essais Biologiques,
Joinville-le-Pont, France, submitted by Roussel Uclaf to
WHO. (Unpublished)
Coombs, D.W. and Clark, G.C. RU 22974. Acute inhalation toxicity in
1978 rats, 6 hour LC50. Report RLS 310/78453/A, Huntingdon
Research Centre, Huntingdon, England, submitted by Roussel
Uclaf to WHO. (Unpublished)
Coquet, B. RU 22974. Test to determine primary cutaneous irritation in
1976a the rabbit. Report no. IFREB-R 761157/A, Institut Francais
de Recherches et Essais Biologiques, Joinville-le-Pont,
France, submitted by Roussel Uclaf to WHO. (Unpublished)
1976b RU 22974. Test to evaluate ocular irritation in the rabbit.
Report no. IFREB-R 761158/A, Institut Francais de Recherches
et Essais Biologiques, Joinville-le-Pont France, submitted
by Roussel Uclaf to WHO. (Unpublished)
Desmarchelier, J., Bengston, M., Connell, M., Minett, W., Moore, B.,
1977 Phillips, M., Snelson, J., Sticka, R. and Tucker, K. A
collaborative study of residues on wheat of
chlorpyrifosmethyl, fenitrothion, malathion, methacrifos and
pirimiphos-methyl. 1. Method Development. Pesticide Science,
8: 473-483.
Desmarchelier, J. Loss of fenitrothion on grains in storage. Pesticide
1978 Science, 9:33-38.
Elliott, M. Synthetic pyrethroids, American Chemical Society
1977 Symposium, 42:1-29.
Fouillet, X. RU 22974. Mutagenicity study of various preparations.
1976 Salmonella microsome test. Report no. IFREB-R 761153/A,
Institut Francais de Recherches et Essais Biologiques,
Joinville-de-Pont, France, submitted by Roussel Uclaf to
WHO. (Unpublished)
Gaughan, L.C., Unai, T. and Casida, J.E. Permethrin metabolism in
1977a rats. Journal of Agricultural and Food Chemistry, 25:9-17
1977b Permethrin metabolism in rats and cows and in bean and
cotton plants. American Chemical Society Symposium,
42:186-193.
Glomot, R. and Chevalier, B. RU 22974. Acute toxicity study, mouse and
1976a rat by oral route. Report Tox 76810/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
1976b RU 22974. Acute toxicity study mouse and rat by
intraperitoneal route. Report Tox 76811/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
1976c RU 22974. Acute toxicity study, mouse and rat by intravenous
route. Report Tox 76812/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
Glomot, R., Chevalier, B., Collas, E. and Audegond, L. RU 22974. Acute
1977 toxicity study by oral route in male and female beagle dogs.
Report Toxico 77804/JL-5, submitted by Roussel Uclaf to WHO.
(Unpublished)
Glomot, R. and Vannier, B. RU 22974. Teratological study in mouse, rat
1977 and rabbit. Report no. Tox 76534-76536/2/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
Glomot, R. and Vannier, B. RU 22974. Teratological study in mouse.
1978 Complementary Information. Report no. Tox. 76534.76536/A3,
submitted by Roussel Uclaf to WHO. (Unpublished)
Glomot, R. Personal communication to WHO. Roussel, Uclaf, Paris,
1981 France.
Goldenthal, E.I., Blair, M., Jefferson, N.D., Spicer, E.J.F., Arceo,
1980a R.J. and Clark Kahn III, A., RU 22974. Two-year toxicity and
carcinogenicity study in mice. Report IRDC 406-001/A/4,
International Research and Development Corp., Mattawan,
Michigan, submitted by Roussel Uclaf to WHO. (Unpublished)
Goldenthal, E.I., Jefferson, N.D., Blair, M., Thorstenson, J.H.,
1980b Spicer, E., J.F. Arceo, and Kahn, III, A. RU 22974. Two year
oral toxicity and carcinogenicity study in rats. Report IRDC
406-002/A 4, International research and Development Corp.,
Mattawan, Michigan, submitted by Roussel Uclaf to WHO.
(Unpublished)
Gray, A.J. and Rickard, J. Distribution of radiolabel in rats after
1981 intravenous injection of a toxic dose of 14C-acid-,
14C-alcohol- or 14C-cyano-labelled deltamethrin. Pesticide
Biochemistry and Physiology, 16:79-85
Guillot, J.P. and Guilaine, J. RU 22974 - Decamethrine. Decis
1977 technical Roussel Uclaf. Sensitization test in the guinea
pig. Report no. IFREB-R 709241/A, Institut Francais de
Recherches et Essais Biologiques, Joinville-le-Pont, France,
submitted by Roussel Uclaf to WHO. (Unpublished)
Halls, G.R.H., and Periam, A.W.. The fate of residues of NRDC 161 on
1980 wheat during storage and after milling and baking - Report
after 9 months storage. Wellcome Research Laboratories
Report HEFH 80-4, November 1980. (Unpublished)
Husson, J.M. Medical observations made on people working on the
1978 manufacture and formulation of the pyrethroid insecticide
decamethrin. Report no. RU 78.25.08/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
Hunter, B., Jordan, J., Heywood, R., Hepworth, P., Street, A.E. and
1977 Prentice, D. RU 22974. Assessment of toxicity to rats by
oral administration for 13 weeks (followed by a 4 week
withdrawal period). Report RSL 254/76938/A3, Huntingdon
Research Centre, Huntingdon, England, submitted by Roussel
Uclaf to WHO. (Unpublished)
IRDC Laboratories. Two year chronic dog feeding study. Report IRDC
1980 406-004/A1, International Research and Development
Corporation, Mattawan, Michigan, submitted by Roussel Uclaf
to WHO. (Unpublished)
Kavlock, R., Chernoff, N., Baron, R., Linder, R., Rogers, E. and
1979 Carver, B. Toxicity studies with decamethrin, a synthetic
pyrethroid insecticide. Journal of Environmental Pathology
and Toxicology, 2:751-765.
Kynoch, S.R., Lloyd, G.K. and Andrews, C.D. Acute percutaneous
1979 toxicity to rats of decamethrin. Report RSL-10098/D
147/79/A, Huntingdon Research Centre, Huntingdon England,
submitted by Roussel Uclaf to WHO. (Unpublished)
Peyre, M., Chantot, J.F., Glomot, R. and Penasse, L. Detection of a
1982 mutagenic potency of decamethrin (RU 22974). Bacterial
tests. Report no. RU/TOX/80.21.01/A, submitted by Roussel
Uclaf to WHO. (Unpublished)
Ray, D.E. and Cremer, J.E. The action of decamethrin (a synthetic
1979 pyrethroid) on the rat. Pesticide Biochemistry and
Physiology, 10:33.
Ray, D.E. An EEG investigation of decamethrin induced choreoathetosis
1980 in the rat. Experimental Brain Research, 38:221.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E., Cooke, L.
1978 and Gibson, W.A. RU 22974 (Decamethrin) LD50 determination
and assessment of neurotoxicity in the domestic hen. Report
no. RSL 293-NI/830/A. Huntingdon Research Centre,
Huntingdon, England, submitted by Roussel Uclaf to WHO.
(Unpublished)
Roussel Uclaf. Test to determine the toxicity in the chicken by oral
1976a route. Report RU-76.05.05/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
1976b Toxicity of decamethrin or DECIS in single ingestion in
game ducks, Anas platyrhynchos L. Report INRA-76.21.12/A.
Institut National de la Recherche Agronomique. Jony-En-
Josas, France, submitted by Roussel Uclaf to WHO.
(Unpublished)
Roussel Uclaf. Toxicity of decamethrin or DECIS by single ingestion
1976c in grey partridge, Perdix perdix l., and Red Partridge
Alectous rufa l. Report INRA-76.28.09/A. Institut National
de la Recherche Agronomique. Joney-en-Josas, France,
submitted by Roussel Uclaf to WHO. (Unpublished)
Ruzo, L.O., Unai, T. and Casida, J.E. decamethrin metabolism in rats.
1977 Report UC-77.06.12/A, submitted by Roussel Uclaf to WHO.
(Unpublished)
1978a Decamethrin metabolism in rats. Journal of Agriculture and
Food Chemistry, 26:918-925.
Ruzo, L.O., Engel, J.L. and Casida, J.E. Oxidative, hydrolytic and
1978b conjugative reactions in the metabolism of decamethrin in
mice. Report UC 78.10.11/A, submitted by Roussel Uclaf to
WHO. (Unpublished)
1979 Decamethrin metabolism from oxidative, hydrolytic and
conjugative reactions in mice. Journal of Agricultural Food
and Chemistry, 27:725-731.
Shono, T., Oshawa, K. and Casida, J.E. Metabolism of trans- and cis
1978 permethrin, trans- and cis-cypermethrin and decamethrin by
microsomal enzymes. Report UC 78.18.05/A, submitted by
Roussel Uclaf to WHO. (Unpublished)
Sobels, F.H., Tates, A.D. and Vannier, B. Cytogenetic study with RU
1978 22974. Detection of a mutagenic potency in mammalian cells.
Report no. ULN-782211/A, State university of Leiden, The
Netherlands, submitted by Roussel Uclaf to WHO.
(Unpublished)
Soderland, D.M. and Casida, J.E. Substrate specificity of mouse liver
1977 microsomal enzymes in pyrethroid metabolism. In: Synthetic
Pyrethroids, American Chemical Society Symposium,
42:152-162.
Vannier, B. and Glomot, R. RU 22974. Mutagenic study. Dominant lethal
1977 assay in the male mouse. Report no. Toxico 76533/DB9/A,
submitted by Roussel Uclaf to WHO. (Unpublished)
Wrenn, J.M., Rodwell, D.E., Goldenthal, E.I., Spider, E.J.C. and
1980 Rajasekaran, B. Three-generation reproduction study in rats.
Report no. IRDC-406-003/A4, International Research and
Development Corporation, Michigan, USA, submitted by Roussel
Uclaf to WHO. (Unpublished)
