
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
CARBARYL
Chemical names
1-naphtyl N-methylcarbamate; N-methyl-1-naphtyl carbamate;
N-methyl-a-naphtyl urethane.
Synonym
Sevin
Empirical formula
C12H11O2N
Structural formula
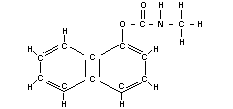 BIOLOGICAL DATA
Biochemical studies
A slight rise in free 1-naphthol and a definite rise in
conjugated 1-naphthol in the urine were observed in the 48 hours
following ingestion of carbaryl by rats (Carpenter et al., 1961).
Workers engaged in bagging carbaryl showed excretion of conjugated
1-naphthol in the urine (Best & Murray, 1962). Metabolism of
14C-carbaryl by rat-liver microsomes and insects leads to the
appearance of at least 8 metabolites, 5 of which are carbamates,
suggesting a non-hydrolytic pathway in the metabolism of carbaryl
(Dorough et al., 1963; Dorough & Casida, 1964). When 14C-carbaryl was
administered to a goat in a dose of 1.3 mg/kg, several of these
metabolites were found in both the milk and the urine. The level of
total 14C-equivalents of carbaryl reached a peak of 0.93 ppm in the
milk at 8 hours and decreased to below 0.003 ppm at 60 hours (Dorough
et al., 1963, Dorough & Casida, 1964). Dairy cattle were fed carbaryl
for 2 weeks, up to 450 ppm, and carbaryl was searched for in the milk
by analytical methods. Carbaryl, if present, was below the sensitivity
of the method (0.01 ppm) (Gyrisco et al., 1960). Similar results were
obtained with the analysis of tissues (Roberts et al., 1960).
Some knowledge has recently been obtained on the changes which
the compound undergoes in plants following injection of 14C-carbaryl
into the stem of garden snapbeans and cotton seedlings. After 28 days
55% of the original 14C was still present but only 5.7% of the
original compound was found. It has been suggested that the
metabolite(s) are water soluble and quite stable in the plant (Dorough
et al., 1963; Dorough & Casida, 1964).
A depression of blood and brain cholinesterase has been reported
following single large doses of carbaryl (Carpenter et al., 1961;
Mellon Institute, 1958b). Approximately the same molar concentrations
are required to produce 50% inhibition in the blood of man, rabbit,
rat and dog (Mellon Institute, 1958b). As a cholinesterase inhibitor,
carbaryl is less active than parathion. Pyridine-2-aldoxime
methiodide, which is a good antidote for organo-phosphorus compounds
is not effective on cholinesterase inhibition by carbaryl (Mellon
Institute, 1958b).
Acute toxicity
Animals Route Solvent LD50 (mg/kg) Reference
Mice I.P. Corn oil 25 Baron et al., 1964
Rats, male P.O. 10% Tween 80 190 Mellon Institute, 1958a
Rat, male P.O. 10% Tween 80 310 " " "
in 0.75% NaCl
Rat, male
and female P.O. 0.25% agar 480-610 " " "
Rat, female P.O. Corn oil 560 " " "
Rat, male P.O. Corn oil 308 " " "
Rats I.V. Propylene glycol 18 " " "
Rats I.V. PEG 400 24 " " "
Rats I.V. Undiluted 93 " " "
(continued)
Animals Route Solvent LD50 (mg/kg) Reference
Guinea-pigs, P.O. 0.25% agar 280 " " "
male
Rabbits, male P.O. 0.25% agar 707 " " "
Dogs P.O. Powder none died " " "
(250-795 mg/kg)
Chicken. Chickens given 2 g/kg showed leg weakness for 1-2 days
but recovered. A nephrotoxic action was observed in fowl receiving 2
g/kg or more. No demyelination was noted (Carpenter et al., 1961).
Man. A 19-month-old child swallowed an unknown amount of
carbaryl. The child developed typical early signs of cholinesterase
inhibition (constricted pupils, salivation, muscular incoordination)
(Best & Murray, 1962).
Short-term studies
Mouse. In one experiment 30 A/Jax and 30 C3H mice at the age of
3 months were started on a treatment of weekly subcutaneous injections
of 0.5 mg of carbaryl. Five months later the survivors (26 A/Jax and
28 C3H) were sacrificed. In neither group did the survival rate and
the tumour incidence differ from those of untreated animals of the
same strains (Mellon Institute, 1958a).
Rat. Groups of 10 rats (5 of each sex) were fed 1500 ppm or
2250 ppm carbaryl in their diets for 96 days. The 2250 ppm level
produced a decrease in body-weight of females, an increase in
liver-weight of males and an increase in kidney-weight of females. At
the 1500 ppm level the only effect was an increase in kidney-weight in
females. A minor histopathological change in the form of diffuse
cloudy swelling of the kidney tubules was noted at the higher
concentrations (Carpenter et al., 1961).
Guinea-pig. Four of a group of 16 male guinea-pigs were
sensitized and after a 3-week incubation period were given a challenge
dose of carbaryl without the development of sensitization (Carpenter
et al., 1961).
Dog. Groups of 3 or 4 dogs distributed randomly by sex and
litter were given by capsule, 5 days per week, dosage levels of
carbaryl approximately equivalent to 25, 100 and 400 ppm in the diet
(0.45, 1.8 and 7.2 mg/kg body-weight) for 1 year. Slight kidney
damage, consisting of diffuse cloudy swelling of the tubules, was
noted in the dogs on 400 ppm. One female dog on 25 ppm showed a
transient hind-leg weakness but no histopathological lesion was noted
in this animal at autopsy (Carpenter et al., 1961).
Long-term studies
Mouse. Groups of 48 male and 48 females Cd-1 mice were given
400, 100 and 0 ppm of carbaryl in the diet. After 80 weeks 12
survivors of each sex from each group were sacrificed for histological
examination. Survival rate, pathology and tumour incidence were
comparable in all groups (Mellon Institute, 1963).
Rat. In a 2-year experiment, groups of 40 rats (20 of each sex)
were given diets containing 50, 100, 200 and 400 ppm carbaryl. After
periods of 6, 9 and 12 months, 4, 6 or 8 rats of each sex were killed
in order to make organ weight comparisons and to provide tissues for
histopathological examinations. The highest dosage level produced
cloudy swelling in the tubules of the kidneys after 1 year and cloudy
swelling of the central hepatic cords and a decreased body-weight gain
(males only) after 2 years. Other dosages showed no effect (Carpenter
et al., 1961).
Special studies
Some studies have been reported describing the effects of a
single administration of carbaryl on discreet avoidance and food
reward behavioural tests in rats. In one report (Goldberg et al.,
1965a) the dose necessary to suppress avoidance response to 50%
efficiency was slightly lower than the dose required to reduce brain
cholinesterase to 50% of control value. The effects of carbaryl on
behaviour are reversed by atropine pre-treatment and the association
with chlorpromazine leads to more than additive effects.
ß-diethylaminoethyl-diphenylpropyl-acetate (SKF-525) increases the
behavioural effects of carbaryl without enhancement of cholinesterase
inhibition (Goldberg & Johnson, 1964a; Goldberg & Johnson, 1964b;
Goldberg et al., 1965a; Goldberg et al., 1965b).
Comments on the experimental studies reported
The short-term and long-term studies in the rat showed that 200
ppm in the diet of the animals did not cause histopathological
lesions. With higher doses, liver and kidney enlargement and kidney
lesions were seen. In dogs, the maximum no-effect level is 100 ppm.
The behavioural studies appear interesting in relation to the
mode of action and as a potential system for detection of toxicity.
However, so far no data are available concerning experiments with
continuous exposure to the compound.
EVALUATION
Level causing no significant toxicological effect
Mouse: 400 ppm in the diet equivalent to 60 mg/kg body-weight
per day
Rat: 200 ppm in the diet equivalent to 10 mg/kg body-weight
per day
Dog: 100 ppm in the diet equivalent to 1.8 mg/kg body-weight
per day
Estimate of acceptable daily intake for man
0-0.02 mg/kg body-weight per day. (This value is based on
experiments carried out with carbaryl, and thus does not take account
of chemical alterations in the pesticide brought about by the plants
to which it has been applied.)
Further work desirable
Determination and evaluation of the toxicity of residues
occurring in the plants. Biochemical studies.
REFERENCES
Baron, R. L., Casterline, J. L. & Fitzhugh, O. G. (1964) Toxicol.
Appl. Pharmacol., 6, 402
Best, E. M. jr & Murray, B. L. (1962) J. Occupational Med., 4, 507
Carpenter, C. P. Weil, C. S., Palm, P. E., Woodside, M. W., Nair, J.
H. & Smyth, H. F. (1961) J. Agr. Food Chem., 9, 30
Dorough, H. W., Leeling, N. C. & Casida, J. E. (1963) Science,
140, 170
Dorough, H. W. & Casida, J. E. (1964) Agr. Food Chem., 12, 294
Goldberg, M. E. & Johnson, H. E. (1964a) J. Pharm. Pharmacol., 16,
60
Goldberg, M. E. & Johnson, H. E. (1964b) J. Pharmacol. exp. Ther.,
145, 367
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965a)
Psychopharmacologia. (In press)
Goldberg, M. E., Johnson. H. E. & Knaak. J. B. (1965b) Biochem.
Pharmacol. (in press)
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, W. E., Fox, F.
H., Holland, R. F. & Trimberger, G. W. (1960) J. Agr. Food Chem.,
8, 409
Mellon Institute of Industrial Research (1958a) 21-90, Unpublished
Report
Mellon Institute of Industrial Research (1958b) 21-94, Unpublished
Report
Mellon Institute of Industrial Research (1963) 26-53, Unpublished
Report
Roberts, R. H., Jackson, J. B., Westlake, W. E., Ackerman, A. J. &
Claborn, H. V. (1960) J. Econ. Ent., 53, 326
BIOLOGICAL DATA
Biochemical studies
A slight rise in free 1-naphthol and a definite rise in
conjugated 1-naphthol in the urine were observed in the 48 hours
following ingestion of carbaryl by rats (Carpenter et al., 1961).
Workers engaged in bagging carbaryl showed excretion of conjugated
1-naphthol in the urine (Best & Murray, 1962). Metabolism of
14C-carbaryl by rat-liver microsomes and insects leads to the
appearance of at least 8 metabolites, 5 of which are carbamates,
suggesting a non-hydrolytic pathway in the metabolism of carbaryl
(Dorough et al., 1963; Dorough & Casida, 1964). When 14C-carbaryl was
administered to a goat in a dose of 1.3 mg/kg, several of these
metabolites were found in both the milk and the urine. The level of
total 14C-equivalents of carbaryl reached a peak of 0.93 ppm in the
milk at 8 hours and decreased to below 0.003 ppm at 60 hours (Dorough
et al., 1963, Dorough & Casida, 1964). Dairy cattle were fed carbaryl
for 2 weeks, up to 450 ppm, and carbaryl was searched for in the milk
by analytical methods. Carbaryl, if present, was below the sensitivity
of the method (0.01 ppm) (Gyrisco et al., 1960). Similar results were
obtained with the analysis of tissues (Roberts et al., 1960).
Some knowledge has recently been obtained on the changes which
the compound undergoes in plants following injection of 14C-carbaryl
into the stem of garden snapbeans and cotton seedlings. After 28 days
55% of the original 14C was still present but only 5.7% of the
original compound was found. It has been suggested that the
metabolite(s) are water soluble and quite stable in the plant (Dorough
et al., 1963; Dorough & Casida, 1964).
A depression of blood and brain cholinesterase has been reported
following single large doses of carbaryl (Carpenter et al., 1961;
Mellon Institute, 1958b). Approximately the same molar concentrations
are required to produce 50% inhibition in the blood of man, rabbit,
rat and dog (Mellon Institute, 1958b). As a cholinesterase inhibitor,
carbaryl is less active than parathion. Pyridine-2-aldoxime
methiodide, which is a good antidote for organo-phosphorus compounds
is not effective on cholinesterase inhibition by carbaryl (Mellon
Institute, 1958b).
Acute toxicity
Animals Route Solvent LD50 (mg/kg) Reference
Mice I.P. Corn oil 25 Baron et al., 1964
Rats, male P.O. 10% Tween 80 190 Mellon Institute, 1958a
Rat, male P.O. 10% Tween 80 310 " " "
in 0.75% NaCl
Rat, male
and female P.O. 0.25% agar 480-610 " " "
Rat, female P.O. Corn oil 560 " " "
Rat, male P.O. Corn oil 308 " " "
Rats I.V. Propylene glycol 18 " " "
Rats I.V. PEG 400 24 " " "
Rats I.V. Undiluted 93 " " "
(continued)
Animals Route Solvent LD50 (mg/kg) Reference
Guinea-pigs, P.O. 0.25% agar 280 " " "
male
Rabbits, male P.O. 0.25% agar 707 " " "
Dogs P.O. Powder none died " " "
(250-795 mg/kg)
Chicken. Chickens given 2 g/kg showed leg weakness for 1-2 days
but recovered. A nephrotoxic action was observed in fowl receiving 2
g/kg or more. No demyelination was noted (Carpenter et al., 1961).
Man. A 19-month-old child swallowed an unknown amount of
carbaryl. The child developed typical early signs of cholinesterase
inhibition (constricted pupils, salivation, muscular incoordination)
(Best & Murray, 1962).
Short-term studies
Mouse. In one experiment 30 A/Jax and 30 C3H mice at the age of
3 months were started on a treatment of weekly subcutaneous injections
of 0.5 mg of carbaryl. Five months later the survivors (26 A/Jax and
28 C3H) were sacrificed. In neither group did the survival rate and
the tumour incidence differ from those of untreated animals of the
same strains (Mellon Institute, 1958a).
Rat. Groups of 10 rats (5 of each sex) were fed 1500 ppm or
2250 ppm carbaryl in their diets for 96 days. The 2250 ppm level
produced a decrease in body-weight of females, an increase in
liver-weight of males and an increase in kidney-weight of females. At
the 1500 ppm level the only effect was an increase in kidney-weight in
females. A minor histopathological change in the form of diffuse
cloudy swelling of the kidney tubules was noted at the higher
concentrations (Carpenter et al., 1961).
Guinea-pig. Four of a group of 16 male guinea-pigs were
sensitized and after a 3-week incubation period were given a challenge
dose of carbaryl without the development of sensitization (Carpenter
et al., 1961).
Dog. Groups of 3 or 4 dogs distributed randomly by sex and
litter were given by capsule, 5 days per week, dosage levels of
carbaryl approximately equivalent to 25, 100 and 400 ppm in the diet
(0.45, 1.8 and 7.2 mg/kg body-weight) for 1 year. Slight kidney
damage, consisting of diffuse cloudy swelling of the tubules, was
noted in the dogs on 400 ppm. One female dog on 25 ppm showed a
transient hind-leg weakness but no histopathological lesion was noted
in this animal at autopsy (Carpenter et al., 1961).
Long-term studies
Mouse. Groups of 48 male and 48 females Cd-1 mice were given
400, 100 and 0 ppm of carbaryl in the diet. After 80 weeks 12
survivors of each sex from each group were sacrificed for histological
examination. Survival rate, pathology and tumour incidence were
comparable in all groups (Mellon Institute, 1963).
Rat. In a 2-year experiment, groups of 40 rats (20 of each sex)
were given diets containing 50, 100, 200 and 400 ppm carbaryl. After
periods of 6, 9 and 12 months, 4, 6 or 8 rats of each sex were killed
in order to make organ weight comparisons and to provide tissues for
histopathological examinations. The highest dosage level produced
cloudy swelling in the tubules of the kidneys after 1 year and cloudy
swelling of the central hepatic cords and a decreased body-weight gain
(males only) after 2 years. Other dosages showed no effect (Carpenter
et al., 1961).
Special studies
Some studies have been reported describing the effects of a
single administration of carbaryl on discreet avoidance and food
reward behavioural tests in rats. In one report (Goldberg et al.,
1965a) the dose necessary to suppress avoidance response to 50%
efficiency was slightly lower than the dose required to reduce brain
cholinesterase to 50% of control value. The effects of carbaryl on
behaviour are reversed by atropine pre-treatment and the association
with chlorpromazine leads to more than additive effects.
ß-diethylaminoethyl-diphenylpropyl-acetate (SKF-525) increases the
behavioural effects of carbaryl without enhancement of cholinesterase
inhibition (Goldberg & Johnson, 1964a; Goldberg & Johnson, 1964b;
Goldberg et al., 1965a; Goldberg et al., 1965b).
Comments on the experimental studies reported
The short-term and long-term studies in the rat showed that 200
ppm in the diet of the animals did not cause histopathological
lesions. With higher doses, liver and kidney enlargement and kidney
lesions were seen. In dogs, the maximum no-effect level is 100 ppm.
The behavioural studies appear interesting in relation to the
mode of action and as a potential system for detection of toxicity.
However, so far no data are available concerning experiments with
continuous exposure to the compound.
EVALUATION
Level causing no significant toxicological effect
Mouse: 400 ppm in the diet equivalent to 60 mg/kg body-weight
per day
Rat: 200 ppm in the diet equivalent to 10 mg/kg body-weight
per day
Dog: 100 ppm in the diet equivalent to 1.8 mg/kg body-weight
per day
Estimate of acceptable daily intake for man
0-0.02 mg/kg body-weight per day. (This value is based on
experiments carried out with carbaryl, and thus does not take account
of chemical alterations in the pesticide brought about by the plants
to which it has been applied.)
Further work desirable
Determination and evaluation of the toxicity of residues
occurring in the plants. Biochemical studies.
REFERENCES
Baron, R. L., Casterline, J. L. & Fitzhugh, O. G. (1964) Toxicol.
Appl. Pharmacol., 6, 402
Best, E. M. jr & Murray, B. L. (1962) J. Occupational Med., 4, 507
Carpenter, C. P. Weil, C. S., Palm, P. E., Woodside, M. W., Nair, J.
H. & Smyth, H. F. (1961) J. Agr. Food Chem., 9, 30
Dorough, H. W., Leeling, N. C. & Casida, J. E. (1963) Science,
140, 170
Dorough, H. W. & Casida, J. E. (1964) Agr. Food Chem., 12, 294
Goldberg, M. E. & Johnson, H. E. (1964a) J. Pharm. Pharmacol., 16,
60
Goldberg, M. E. & Johnson, H. E. (1964b) J. Pharmacol. exp. Ther.,
145, 367
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965a)
Psychopharmacologia. (In press)
Goldberg, M. E., Johnson. H. E. & Knaak. J. B. (1965b) Biochem.
Pharmacol. (in press)
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, W. E., Fox, F.
H., Holland, R. F. & Trimberger, G. W. (1960) J. Agr. Food Chem.,
8, 409
Mellon Institute of Industrial Research (1958a) 21-90, Unpublished
Report
Mellon Institute of Industrial Research (1958b) 21-94, Unpublished
Report
Mellon Institute of Industrial Research (1963) 26-53, Unpublished
Report
Roberts, R. H., Jackson, J. B., Westlake, W. E., Ackerman, A. J. &
Claborn, H. V. (1960) J. Econ. Ent., 53, 326
 BIOLOGICAL DATA
Biochemical studies
A slight rise in free 1-naphthol and a definite rise in
conjugated 1-naphthol in the urine were observed in the 48 hours
following ingestion of carbaryl by rats (Carpenter et al., 1961).
Workers engaged in bagging carbaryl showed excretion of conjugated
1-naphthol in the urine (Best & Murray, 1962). Metabolism of
14C-carbaryl by rat-liver microsomes and insects leads to the
appearance of at least 8 metabolites, 5 of which are carbamates,
suggesting a non-hydrolytic pathway in the metabolism of carbaryl
(Dorough et al., 1963; Dorough & Casida, 1964). When 14C-carbaryl was
administered to a goat in a dose of 1.3 mg/kg, several of these
metabolites were found in both the milk and the urine. The level of
total 14C-equivalents of carbaryl reached a peak of 0.93 ppm in the
milk at 8 hours and decreased to below 0.003 ppm at 60 hours (Dorough
et al., 1963, Dorough & Casida, 1964). Dairy cattle were fed carbaryl
for 2 weeks, up to 450 ppm, and carbaryl was searched for in the milk
by analytical methods. Carbaryl, if present, was below the sensitivity
of the method (0.01 ppm) (Gyrisco et al., 1960). Similar results were
obtained with the analysis of tissues (Roberts et al., 1960).
Some knowledge has recently been obtained on the changes which
the compound undergoes in plants following injection of 14C-carbaryl
into the stem of garden snapbeans and cotton seedlings. After 28 days
55% of the original 14C was still present but only 5.7% of the
original compound was found. It has been suggested that the
metabolite(s) are water soluble and quite stable in the plant (Dorough
et al., 1963; Dorough & Casida, 1964).
A depression of blood and brain cholinesterase has been reported
following single large doses of carbaryl (Carpenter et al., 1961;
Mellon Institute, 1958b). Approximately the same molar concentrations
are required to produce 50% inhibition in the blood of man, rabbit,
rat and dog (Mellon Institute, 1958b). As a cholinesterase inhibitor,
carbaryl is less active than parathion. Pyridine-2-aldoxime
methiodide, which is a good antidote for organo-phosphorus compounds
is not effective on cholinesterase inhibition by carbaryl (Mellon
Institute, 1958b).
Acute toxicity
Animals Route Solvent LD50 (mg/kg) Reference
Mice I.P. Corn oil 25 Baron et al., 1964
Rats, male P.O. 10% Tween 80 190 Mellon Institute, 1958a
Rat, male P.O. 10% Tween 80 310 " " "
in 0.75% NaCl
Rat, male
and female P.O. 0.25% agar 480-610 " " "
Rat, female P.O. Corn oil 560 " " "
Rat, male P.O. Corn oil 308 " " "
Rats I.V. Propylene glycol 18 " " "
Rats I.V. PEG 400 24 " " "
Rats I.V. Undiluted 93 " " "
(continued)
Animals Route Solvent LD50 (mg/kg) Reference
Guinea-pigs, P.O. 0.25% agar 280 " " "
male
Rabbits, male P.O. 0.25% agar 707 " " "
Dogs P.O. Powder none died " " "
(250-795 mg/kg)
Chicken. Chickens given 2 g/kg showed leg weakness for 1-2 days
but recovered. A nephrotoxic action was observed in fowl receiving 2
g/kg or more. No demyelination was noted (Carpenter et al., 1961).
Man. A 19-month-old child swallowed an unknown amount of
carbaryl. The child developed typical early signs of cholinesterase
inhibition (constricted pupils, salivation, muscular incoordination)
(Best & Murray, 1962).
Short-term studies
Mouse. In one experiment 30 A/Jax and 30 C3H mice at the age of
3 months were started on a treatment of weekly subcutaneous injections
of 0.5 mg of carbaryl. Five months later the survivors (26 A/Jax and
28 C3H) were sacrificed. In neither group did the survival rate and
the tumour incidence differ from those of untreated animals of the
same strains (Mellon Institute, 1958a).
Rat. Groups of 10 rats (5 of each sex) were fed 1500 ppm or
2250 ppm carbaryl in their diets for 96 days. The 2250 ppm level
produced a decrease in body-weight of females, an increase in
liver-weight of males and an increase in kidney-weight of females. At
the 1500 ppm level the only effect was an increase in kidney-weight in
females. A minor histopathological change in the form of diffuse
cloudy swelling of the kidney tubules was noted at the higher
concentrations (Carpenter et al., 1961).
Guinea-pig. Four of a group of 16 male guinea-pigs were
sensitized and after a 3-week incubation period were given a challenge
dose of carbaryl without the development of sensitization (Carpenter
et al., 1961).
Dog. Groups of 3 or 4 dogs distributed randomly by sex and
litter were given by capsule, 5 days per week, dosage levels of
carbaryl approximately equivalent to 25, 100 and 400 ppm in the diet
(0.45, 1.8 and 7.2 mg/kg body-weight) for 1 year. Slight kidney
damage, consisting of diffuse cloudy swelling of the tubules, was
noted in the dogs on 400 ppm. One female dog on 25 ppm showed a
transient hind-leg weakness but no histopathological lesion was noted
in this animal at autopsy (Carpenter et al., 1961).
Long-term studies
Mouse. Groups of 48 male and 48 females Cd-1 mice were given
400, 100 and 0 ppm of carbaryl in the diet. After 80 weeks 12
survivors of each sex from each group were sacrificed for histological
examination. Survival rate, pathology and tumour incidence were
comparable in all groups (Mellon Institute, 1963).
Rat. In a 2-year experiment, groups of 40 rats (20 of each sex)
were given diets containing 50, 100, 200 and 400 ppm carbaryl. After
periods of 6, 9 and 12 months, 4, 6 or 8 rats of each sex were killed
in order to make organ weight comparisons and to provide tissues for
histopathological examinations. The highest dosage level produced
cloudy swelling in the tubules of the kidneys after 1 year and cloudy
swelling of the central hepatic cords and a decreased body-weight gain
(males only) after 2 years. Other dosages showed no effect (Carpenter
et al., 1961).
Special studies
Some studies have been reported describing the effects of a
single administration of carbaryl on discreet avoidance and food
reward behavioural tests in rats. In one report (Goldberg et al.,
1965a) the dose necessary to suppress avoidance response to 50%
efficiency was slightly lower than the dose required to reduce brain
cholinesterase to 50% of control value. The effects of carbaryl on
behaviour are reversed by atropine pre-treatment and the association
with chlorpromazine leads to more than additive effects.
ß-diethylaminoethyl-diphenylpropyl-acetate (SKF-525) increases the
behavioural effects of carbaryl without enhancement of cholinesterase
inhibition (Goldberg & Johnson, 1964a; Goldberg & Johnson, 1964b;
Goldberg et al., 1965a; Goldberg et al., 1965b).
Comments on the experimental studies reported
The short-term and long-term studies in the rat showed that 200
ppm in the diet of the animals did not cause histopathological
lesions. With higher doses, liver and kidney enlargement and kidney
lesions were seen. In dogs, the maximum no-effect level is 100 ppm.
The behavioural studies appear interesting in relation to the
mode of action and as a potential system for detection of toxicity.
However, so far no data are available concerning experiments with
continuous exposure to the compound.
EVALUATION
Level causing no significant toxicological effect
Mouse: 400 ppm in the diet equivalent to 60 mg/kg body-weight
per day
Rat: 200 ppm in the diet equivalent to 10 mg/kg body-weight
per day
Dog: 100 ppm in the diet equivalent to 1.8 mg/kg body-weight
per day
Estimate of acceptable daily intake for man
0-0.02 mg/kg body-weight per day. (This value is based on
experiments carried out with carbaryl, and thus does not take account
of chemical alterations in the pesticide brought about by the plants
to which it has been applied.)
Further work desirable
Determination and evaluation of the toxicity of residues
occurring in the plants. Biochemical studies.
REFERENCES
Baron, R. L., Casterline, J. L. & Fitzhugh, O. G. (1964) Toxicol.
Appl. Pharmacol., 6, 402
Best, E. M. jr & Murray, B. L. (1962) J. Occupational Med., 4, 507
Carpenter, C. P. Weil, C. S., Palm, P. E., Woodside, M. W., Nair, J.
H. & Smyth, H. F. (1961) J. Agr. Food Chem., 9, 30
Dorough, H. W., Leeling, N. C. & Casida, J. E. (1963) Science,
140, 170
Dorough, H. W. & Casida, J. E. (1964) Agr. Food Chem., 12, 294
Goldberg, M. E. & Johnson, H. E. (1964a) J. Pharm. Pharmacol., 16,
60
Goldberg, M. E. & Johnson, H. E. (1964b) J. Pharmacol. exp. Ther.,
145, 367
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965a)
Psychopharmacologia. (In press)
Goldberg, M. E., Johnson. H. E. & Knaak. J. B. (1965b) Biochem.
Pharmacol. (in press)
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, W. E., Fox, F.
H., Holland, R. F. & Trimberger, G. W. (1960) J. Agr. Food Chem.,
8, 409
Mellon Institute of Industrial Research (1958a) 21-90, Unpublished
Report
Mellon Institute of Industrial Research (1958b) 21-94, Unpublished
Report
Mellon Institute of Industrial Research (1963) 26-53, Unpublished
Report
Roberts, R. H., Jackson, J. B., Westlake, W. E., Ackerman, A. J. &
Claborn, H. V. (1960) J. Econ. Ent., 53, 326
BIOLOGICAL DATA
Biochemical studies
A slight rise in free 1-naphthol and a definite rise in
conjugated 1-naphthol in the urine were observed in the 48 hours
following ingestion of carbaryl by rats (Carpenter et al., 1961).
Workers engaged in bagging carbaryl showed excretion of conjugated
1-naphthol in the urine (Best & Murray, 1962). Metabolism of
14C-carbaryl by rat-liver microsomes and insects leads to the
appearance of at least 8 metabolites, 5 of which are carbamates,
suggesting a non-hydrolytic pathway in the metabolism of carbaryl
(Dorough et al., 1963; Dorough & Casida, 1964). When 14C-carbaryl was
administered to a goat in a dose of 1.3 mg/kg, several of these
metabolites were found in both the milk and the urine. The level of
total 14C-equivalents of carbaryl reached a peak of 0.93 ppm in the
milk at 8 hours and decreased to below 0.003 ppm at 60 hours (Dorough
et al., 1963, Dorough & Casida, 1964). Dairy cattle were fed carbaryl
for 2 weeks, up to 450 ppm, and carbaryl was searched for in the milk
by analytical methods. Carbaryl, if present, was below the sensitivity
of the method (0.01 ppm) (Gyrisco et al., 1960). Similar results were
obtained with the analysis of tissues (Roberts et al., 1960).
Some knowledge has recently been obtained on the changes which
the compound undergoes in plants following injection of 14C-carbaryl
into the stem of garden snapbeans and cotton seedlings. After 28 days
55% of the original 14C was still present but only 5.7% of the
original compound was found. It has been suggested that the
metabolite(s) are water soluble and quite stable in the plant (Dorough
et al., 1963; Dorough & Casida, 1964).
A depression of blood and brain cholinesterase has been reported
following single large doses of carbaryl (Carpenter et al., 1961;
Mellon Institute, 1958b). Approximately the same molar concentrations
are required to produce 50% inhibition in the blood of man, rabbit,
rat and dog (Mellon Institute, 1958b). As a cholinesterase inhibitor,
carbaryl is less active than parathion. Pyridine-2-aldoxime
methiodide, which is a good antidote for organo-phosphorus compounds
is not effective on cholinesterase inhibition by carbaryl (Mellon
Institute, 1958b).
Acute toxicity
Animals Route Solvent LD50 (mg/kg) Reference
Mice I.P. Corn oil 25 Baron et al., 1964
Rats, male P.O. 10% Tween 80 190 Mellon Institute, 1958a
Rat, male P.O. 10% Tween 80 310 " " "
in 0.75% NaCl
Rat, male
and female P.O. 0.25% agar 480-610 " " "
Rat, female P.O. Corn oil 560 " " "
Rat, male P.O. Corn oil 308 " " "
Rats I.V. Propylene glycol 18 " " "
Rats I.V. PEG 400 24 " " "
Rats I.V. Undiluted 93 " " "
(continued)
Animals Route Solvent LD50 (mg/kg) Reference
Guinea-pigs, P.O. 0.25% agar 280 " " "
male
Rabbits, male P.O. 0.25% agar 707 " " "
Dogs P.O. Powder none died " " "
(250-795 mg/kg)
Chicken. Chickens given 2 g/kg showed leg weakness for 1-2 days
but recovered. A nephrotoxic action was observed in fowl receiving 2
g/kg or more. No demyelination was noted (Carpenter et al., 1961).
Man. A 19-month-old child swallowed an unknown amount of
carbaryl. The child developed typical early signs of cholinesterase
inhibition (constricted pupils, salivation, muscular incoordination)
(Best & Murray, 1962).
Short-term studies
Mouse. In one experiment 30 A/Jax and 30 C3H mice at the age of
3 months were started on a treatment of weekly subcutaneous injections
of 0.5 mg of carbaryl. Five months later the survivors (26 A/Jax and
28 C3H) were sacrificed. In neither group did the survival rate and
the tumour incidence differ from those of untreated animals of the
same strains (Mellon Institute, 1958a).
Rat. Groups of 10 rats (5 of each sex) were fed 1500 ppm or
2250 ppm carbaryl in their diets for 96 days. The 2250 ppm level
produced a decrease in body-weight of females, an increase in
liver-weight of males and an increase in kidney-weight of females. At
the 1500 ppm level the only effect was an increase in kidney-weight in
females. A minor histopathological change in the form of diffuse
cloudy swelling of the kidney tubules was noted at the higher
concentrations (Carpenter et al., 1961).
Guinea-pig. Four of a group of 16 male guinea-pigs were
sensitized and after a 3-week incubation period were given a challenge
dose of carbaryl without the development of sensitization (Carpenter
et al., 1961).
Dog. Groups of 3 or 4 dogs distributed randomly by sex and
litter were given by capsule, 5 days per week, dosage levels of
carbaryl approximately equivalent to 25, 100 and 400 ppm in the diet
(0.45, 1.8 and 7.2 mg/kg body-weight) for 1 year. Slight kidney
damage, consisting of diffuse cloudy swelling of the tubules, was
noted in the dogs on 400 ppm. One female dog on 25 ppm showed a
transient hind-leg weakness but no histopathological lesion was noted
in this animal at autopsy (Carpenter et al., 1961).
Long-term studies
Mouse. Groups of 48 male and 48 females Cd-1 mice were given
400, 100 and 0 ppm of carbaryl in the diet. After 80 weeks 12
survivors of each sex from each group were sacrificed for histological
examination. Survival rate, pathology and tumour incidence were
comparable in all groups (Mellon Institute, 1963).
Rat. In a 2-year experiment, groups of 40 rats (20 of each sex)
were given diets containing 50, 100, 200 and 400 ppm carbaryl. After
periods of 6, 9 and 12 months, 4, 6 or 8 rats of each sex were killed
in order to make organ weight comparisons and to provide tissues for
histopathological examinations. The highest dosage level produced
cloudy swelling in the tubules of the kidneys after 1 year and cloudy
swelling of the central hepatic cords and a decreased body-weight gain
(males only) after 2 years. Other dosages showed no effect (Carpenter
et al., 1961).
Special studies
Some studies have been reported describing the effects of a
single administration of carbaryl on discreet avoidance and food
reward behavioural tests in rats. In one report (Goldberg et al.,
1965a) the dose necessary to suppress avoidance response to 50%
efficiency was slightly lower than the dose required to reduce brain
cholinesterase to 50% of control value. The effects of carbaryl on
behaviour are reversed by atropine pre-treatment and the association
with chlorpromazine leads to more than additive effects.
ß-diethylaminoethyl-diphenylpropyl-acetate (SKF-525) increases the
behavioural effects of carbaryl without enhancement of cholinesterase
inhibition (Goldberg & Johnson, 1964a; Goldberg & Johnson, 1964b;
Goldberg et al., 1965a; Goldberg et al., 1965b).
Comments on the experimental studies reported
The short-term and long-term studies in the rat showed that 200
ppm in the diet of the animals did not cause histopathological
lesions. With higher doses, liver and kidney enlargement and kidney
lesions were seen. In dogs, the maximum no-effect level is 100 ppm.
The behavioural studies appear interesting in relation to the
mode of action and as a potential system for detection of toxicity.
However, so far no data are available concerning experiments with
continuous exposure to the compound.
EVALUATION
Level causing no significant toxicological effect
Mouse: 400 ppm in the diet equivalent to 60 mg/kg body-weight
per day
Rat: 200 ppm in the diet equivalent to 10 mg/kg body-weight
per day
Dog: 100 ppm in the diet equivalent to 1.8 mg/kg body-weight
per day
Estimate of acceptable daily intake for man
0-0.02 mg/kg body-weight per day. (This value is based on
experiments carried out with carbaryl, and thus does not take account
of chemical alterations in the pesticide brought about by the plants
to which it has been applied.)
Further work desirable
Determination and evaluation of the toxicity of residues
occurring in the plants. Biochemical studies.
REFERENCES
Baron, R. L., Casterline, J. L. & Fitzhugh, O. G. (1964) Toxicol.
Appl. Pharmacol., 6, 402
Best, E. M. jr & Murray, B. L. (1962) J. Occupational Med., 4, 507
Carpenter, C. P. Weil, C. S., Palm, P. E., Woodside, M. W., Nair, J.
H. & Smyth, H. F. (1961) J. Agr. Food Chem., 9, 30
Dorough, H. W., Leeling, N. C. & Casida, J. E. (1963) Science,
140, 170
Dorough, H. W. & Casida, J. E. (1964) Agr. Food Chem., 12, 294
Goldberg, M. E. & Johnson, H. E. (1964a) J. Pharm. Pharmacol., 16,
60
Goldberg, M. E. & Johnson, H. E. (1964b) J. Pharmacol. exp. Ther.,
145, 367
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965a)
Psychopharmacologia. (In press)
Goldberg, M. E., Johnson. H. E. & Knaak. J. B. (1965b) Biochem.
Pharmacol. (in press)
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, W. E., Fox, F.
H., Holland, R. F. & Trimberger, G. W. (1960) J. Agr. Food Chem.,
8, 409
Mellon Institute of Industrial Research (1958a) 21-90, Unpublished
Report
Mellon Institute of Industrial Research (1958b) 21-94, Unpublished
Report
Mellon Institute of Industrial Research (1963) 26-53, Unpublished
Report
Roberts, R. H., Jackson, J. B., Westlake, W. E., Ackerman, A. J. &
Claborn, H. V. (1960) J. Econ. Ent., 53, 326
