
CARBARYL JMPR 1973
EXPLANATION
Carbaryl was evaluated by Joint Meetings in 1966, 1967, 1968,
1969, and 1970 (FAO/WHO, 1967b, 1968b, 1969b, 1970b, 1971b). At the
1969 Meeting it was decided that all temporary tolerances for carbaryl
should be reviewed in 1973 and the previously proposed tolerance for
whole milk was withdrawn until data on the levels of water-soluble
metabolites could be evaluated. In the course of the numerous
re-evaluations of carbaryl, many recommendations for tolerances were
altered and in 1969 the original broad crop categories were expanded
into subgroups or individual crops. This has led to some confusion and
in particular certain crops (such as root crops), originally covered
by the broad categories (such as vegetables), were inadvertently
omitted from subsequent recommendations. Therefore, such oversights
are corrected and a complete listing of current tolerance
recommendations is provided in this monograph addendum. Also, the
results of additional experimental work on certain commodities not
previously considered became available and are summarized therein.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
In rats it was observed that absorption of carbaryl probably
occurs in the stomach or anterior portions of the small intestine
(Casper and Pekas, 1971). Carbaryl absorbed through the stomach was
found to be unchanged in the blood (Casper, 1972). Carbaryl absorbed
through the intestine underwent transformation to alpha-naphthol and
was conjugated as a glucuronide (Pekas, 1971; Pekas and Paulson,
1970). In insects, as well as mammals, it was observed that metabolism
occurred during penetration from the gut (Shah and Guthrie, 1971).
Studies on metabolites from the rat have confirmed the identity of
5,6-dihydro-5,6-dihydroxycarbaryl glucuronide (Sullivan et al., 1972;
Richey et al., 1972). Andrawes et al. (1972) found alpha-naphthol
conjugated as a sulfate to be the predominant metabolite in eggs after
continuous dosing of hens. In insects, a new metabolite was isolated,
characterized and suggested to be 2-hydroxy-carbaryl (Moriyama et al.,
1972). Using human lung cell cultures, rat liver and plant cell
cultures, carbaryl was found to be metabolized to hydroxylated
products and subsequently conjugated. The initial report of a new
metabolite, the N-O-conjugate of carbaryl (Locke, 1972a and b), has
not been confirmed by these authors (Personal communication). An in
vitro study using human liver and rat liver preparations suggested
that human liver produced minor carbaryl metabolites, not previously
seen with rat tissue (Strother, 1972). These products were not
identified, although the major metabolites were common to both
systems. Several studies have been reported on the biological
interaction of carbaryl with endogenous materials. Pinolene, a beta
pinene polymer, has been shown to extend the residual life of carbaryl
in certain crops. The probability that this activity is physical
rather than biological is strong (Blazques et al., 1970). Oral
administration of gossypol was found to stimulate liver microsomal
oxidative activity and concomitantly the ability to dealkylate
carbaryl (Abou-Donia and Dieckert, 1971). Similarly, methylmercury and
chlordane were observed to have the same increased effect on the
metabolism of carbaryl (Lucier et al., 1972). Compounds which were
found to decrease MAO activity reduced the excretion of carbaryl from
rats (Dorough et al., 1972). Atropine reduced the acute toxicity of
carbaryl and seven other carbamates administered subcutaneously to
male mice. Oximes were slightly effective in reducing the acute
toxicity of seven carbamates and were slightly therapeutic in
combination with atropine. With carbaryl, obidoxime and P2S were
synergistic and were antagonistic to the therapeutic effects of
atropine. This reverse effect was noted only with carbaryl (Natoff and
Reiff, 1973).
Stenberg (1970) reported that 0.7 mg/kg administered orally for
3-1/2 months induced tension and stimulation of the thyroid activity
(increase of PSI, thyroid weight increase, and proliferation of
colloid content of RNA). The effects diminished at six months.
Rappoport (1969) administered carbaryl for three months at 50
mg/kg/day and found changes in the thyroid gland using the electron
microscope.
Carbaryl at 7.6 and 38 mg/kg administered orally to rabbits for
11 months led to disturbances in carbohydrate and protein metabolism
and other biochemical changes in the liver. When administered at 0.76
and 0.38 mg/kg doses, a retention of bromsulphalein in blood was
observed; a decrease in protein and an increase in alpha and ß
globulin content. Tissue cholinesterase was depressed at all doses
(Kagan et al., 1970). In subacute test, carbaryl decreased the
glycolytic activity in brain (Jakushko, 1971) and other tissues
(Hajkina, 1970).
With the aid of microelectronic techniques, Homenko (1971)
examined the effect of carbaryl on the membrane potential of motor
neurons of the spinal cord in rats. Carbaryl administered orally at
doses of 8.5 mg/kg and above caused increases in the potential,
depending on the dose and duration of treatment.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
The mutagenic potential of carbaryl was demonstrated in tests
with Drosophilia melanogaster. It was suggested that there was a
slight mutagenic tendency in these tests (Hogue, 1972; Brzheskiy,
1972).
Special studies on neurotoxicity
Carbaryl has been reported to exert a possible sympathomimetic
effect in addition to its parasympathomimetic properties (Santolucito
et al., 1972). This property of carbaryl may explain apparent
discrepancies in behavioural studies (Sideroff and Santolucito, 1972).
Special studies on reproduction
Previous work in the Rhesus monkey (FAO/WHO, 1970) indicated that
carbaryl may interfere with reproduction in this species. Eleven
female monkeys were treated with carbaryl at the rate of 0, 2, 6.3,
and 20 mg/kg. One of these monkeys delivered a baby while four
controls conceived and delivered normal babies. In a recent, as yet
uncompleted, experiment, groups of 16 pregnant Rhesus monkeys were
administered carbaryl orally, by stomach tube, twice daily from day 18
to day 40 of gestation at a dose of 0, 0.2, 2 and 20 mg/kg. An interim
report on this study indicates that carbaryl at levels up to and
including 20 mg/kg/day does not have an effect on the reproduction
parameters measured. Of 15 control monkeys, there were 12 live births,
two abortions and one still-born. Of the 11 monkeys receiving the
vehicle as a control, there were 10 live births and one abortion. In
the group receiving the low dose (0.2 mg/kg), there were 11 live
births and two abortions. In the group receiving the intermediate dose
(2.0 mg/kg), there were 11 live births and one abortion, while in the
group receiving the highest dose (2.0 mg/kg) there were 10 live births
and three abortions. Although the study is not complete, the initial
indications of a reproductive hazard in Rhesus monkeys, based upon
previous data, is unfounded. The abortions and still births occurring
in the current study have been reported to be within the normal limits
for the Rhesus colony and the results to date indicate that carbaryl
does not induce abortion in these monkeys (Dougherty et al., 1973).
Weil et al. (1972a and b) reported on a reproduction study in progress
where carbaryl was administered to rats orally by gavage or in the
diet (with and without corn oil as a vehicle). Rats were divided into
three groups; one group (five subgroups, 15 male and 25 female)
received daily intubation (five days per week) of carbaryl suspended
in corn oil at doses of 0, 3, 7, 25 and 100 mg/kg; one group (five
subgroups) received dietary concentrations of 0, 7, 25, 100 and 200
mg/kg/day, five days per week; one group (two subgroups) received
carbaryl in dietary concentrations of 0 and 100 mg/kg/day, five days
per week, with corn oil (4 ml/kg/day) as a carrier. Data were reported
for the F1a generation. There were significant effects on reproduction
in only the intubated groups receiving 100 mg/kg, where fertility was
reduced and mortality was observed. Signs of poisoning were evident at
levels of 7 mg/kg and above. There were some effects reported on the
F1b generation, where a reduction in the number of litters was
reported in the intubated group receiving 100 mg/kg/day. No effects
were noted in rats receiving 200 mg/kg/day in the diet. A similar
study was reported for guinea-pigs. Guinea-pigs receiving 200 mg/kg by
gavage or 300 mg/kg in the diet administered during gestation or
organogenesis showed no effect on reproduction (Weil et al., 1972b).
Benson et al. (1967 report cited in Weil et al., 1972a) gave groups of
20 hybrid female mice diets containing carbaryl at concentrations of
10 and 30 mg/kg/day from day 6 after mating. No effects were noted in
parents and no teratological changes were noted in the pups.
Collins et al. (1970) reported reproduction on a study where
carbaryl was fed to rats at levels of 0, 2000, 5000 and 10 000 ppm for
three generations. Carbaryl at 10 000 ppm inhibited reproduction. At
2000 ppm and above only a dose-related decrease in pup weight was
observed. No effects on reproduction parameters were observed at 2000
ppm. Gerbils fed carbaryl at 0, 2000, 6000 and 10 000 ppm in the diet
for three generations showed no effects on reproduction at 2000 ppm.
At 6000 and 10 000 ppm the survival index and the average number of
young weaned per litter were reduced.
Following i.v. administration of 14C-carbaryl to mature dogs,
small amounts of radio-activity were detected 30 or 60 minutes after
injection in the testes, vas deferens and prostate gland. In mice,
oral administration of carbaryl (0.9 mg/kg) resulted in 14C in
testes, prostate gland, seminal vesicle (seminal plasma) and the
epididymis. Carbaryl administered orally for five days to mice at a
dosage of 38 or 68 mg/kg had little effect on the reproductive organs
in male mice (gonad weight or sex accessory gland weight). The doses
had no effect on testosterone metabolism in the prostate gland,
although androgen hydroxylase activity in liver microsomes was
stimulated (16 alpha-hydroxyl testosterone activity was stimulated).
Carbaryl had a far less affinity for reproductive organs than DDT.
Regardless of species, carbaryl did not seem to possess any particular
affinity for organs of reproduction (Thomas et al., 1973; Dieringer et
al., 1973).
Peroral administration to rats resulted in reproductive effects
on both males and females, impaired oogenesis and spermatogenesis.
When fed to successive generations, carbaryl at 2 mg/kg in the diet
also resulted in ovarian and testicular problems. especially noted in
the second, third and fourth generations (Shtenberg and Orlova, 1970).
Carbaryl was fed for 90 or 138 days to female rats at levels of 5
or 10 mg/kg. The rate of fertilization was reduced at 5 mg/kg with a
normal number of corpora lutea observed. The litters were larger with
no teratogenic effects noted. At 10 mg/kg, a greater rate of reduction
of fecundity was observed (Trifonovia et al., 1970 - Abstract only).
Carbaryl has been reported to interfere with oogenesis and the
oestrous cycle and was said to exert a direct gonadatoxic effect
(Mandzhgaladze and Vashakidze, 1972). Dosing was reported to be at
1/200) -> 1/1000 of the LD50 level for an unspecified time. (Details
of this report were unavailable.)
Carbaryl was fed to hens at 0, 250 and 500 ppm in the diet for 36
weeks and to their progeny for four weeks at 0 and 500 ppm, either
alone or in combination with malathion. Growth was affected in both
parents and chicks, but reproduction and egg characteristics were not
affected. A study at 500 ppm in males showed no effects on fertility
over a four-week period (Lillie, 1973).
In hens fed carbaryl at 500 ppm for 36 weeks, no effects on hens
or progeny were observed, except a slight weight loss and growth
depression. Administration of carbaryl to male leghorns resulted in no
effect on reproduction (Lillie, 1973).
Carbaryl introduced in albino rats perorally at doses of 2 and 5
mg/kg over six months resulted in unfavourable effects on ovaries and
testes and gonadotropic function of the hypophysis. Progressive
atrophic, dystrophic and necrotic changes in the testes and the
ovaries were shown histologically and histochemically (Stenberg and
Otovan, 1971).
Vashakidze (1970) reported on gonadotropic, embryotoxic and
mutagenic effects of carbaryl following oral administration.
Structural changes were reported in the gonads and
spermatogenesis was impaired in the late period of meiosis. These
changes were clearly expressed in subacute tests.
Carbaryl affected reproductive capability of the treated animals
and caused sterility in subthreshold doses - 2 and 1 mg/kg in a six-
month experiment. An increased quantity of undeveloped and dead
embryos was noted in a chronic test at a dose of 1.3 mg/kg.
The results of the cytogenetic investigations show that carbaryl
causes the chromosomes to stick together frequently during a
continuous introduction of small doses (0.5 mg/kg). The effect of
carbaryl is characterized also by changes of the ovogenesis: affected
cycle; and injury of the cells of the follicular apparatus. The effect
on ovogenesis was dependent on the dose and the duration of treatment.
Carbaryl also caused embryo mortality.
A threshold dose of the specific effects of carbaryl on the
gonads of female albino rats is 10 mg/kg, in subacute test - 1 mg/kg,
and in chronic test - 0.5 mg/kg.
Sensitivity of the embryo to carbaryl appeared during the second
half of pregnancy (10-18 days).
In a six-month oral administration study (0.3-10 mg/kg), the
average weight of the semen decreased at doses of 4 and 2 mg/kg, with
changes noted in spermatogenesis. A dose of 0.5 mg/kg causes no effect
on spermatogenesis.
Comments
When carbaryl was evaluated by a previous meeting (FAO/WHO, 1970)
adverse effects on reproductive physiology in several animal species
and an increased urinary amino acid to creatinine ratio in man were
regarded as matters for concern. Several studies were reviewed by the
present Meeting relating to the effect of carbaryl on reproduction. No
effect on reproduction was seen in Rhesus monkeys. Studies in several
species of animals showed that administration by gavage is more likely
to affect reproduction than administration in the diet. Further work
was reported which indicated disturbance in the thyroid gland
following short-term treatment. In longer-term studies, disturbances
of carbohydrate and protein metabolism, liver function and endocrine
function and effects on gonads were observed. Behavioural changes have
been reported indicating possible sympathomimetic effects on
peripheral systems. New data with respect to the effects of carbaryl
on renal function have not been reported.
In the light of all the data available, the Meeting felt
justified in establishing a permanent ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 200 ppm in the diet equivalent to 10 mg/kg bw
Man: 0.06 mg/kg/day
Estimate of acceptable daily intake for man
0-0.01 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Carbaryl has been in general use around the world since 1959 for
control of insect pests which attack agricultural crops as well as
certain other non-agricultural pests. Approximately one-half of the
carbaryl produced annually in the United States of America is used
within the country. The balance is exported and, in 1972, foreign
sales were distributed as follows: cotton 40%, vegetables 150/, rice
10%, potatoes 10%, fruit 5%, livestock 5%, and miscellaneous 15%.
Within the United States of America, the most important uses are (in
decreasing order): soybeans, corn (sweet and field), ornamentals and
turf, forest and shade tree, cotton, deciduous tree, fruit, peanuts,
poultry, and vegetables. The registered uses and recommended rates are
given in more detail in Table 16 under National Tolerances. The
versatility of carbaryl, as indicated by its registration of 85 crops
for control of 160 different insect pests, accounts in part for the
broad usage. Reliable performance against target pests and the low
hazard to man and the environment have also been important factors in
moulding the pattern of use for carbaryl.
The strongest influence on the use patterns of carbaryl in recent
years has been a marked reduction in the general use of certain
low-cost organochlorine insecticides.
Post-harvest treatments
None.
Residues resulting from supervised trials
Root crop vegetables
The available data on residues found when small plots of carrots,
turnips, beets, radishes and parsnips were treated one or more times
at the maximum recommended rate of 2 lb ai/acre is shown in Table 1
(Union Carbide, 1973).
TABLE 1. CARBARYL RESIDUES (ppm) IN ROOT CROP VEGETABLES
Days after last treatment
Crop lb ai/acre Number of 0 3 7
treatments
Carrot 2 11 1.7 - -
2 4 0.1 - 2
2 1 0.5 - -
Turnip 2 1 0.8 - -
2 2 0.6 - -
2 6 10.3 1.3 0.9
2 2 1.2 0.9 0.5
Beet 2 7 6.5 0.3 0.4
2 1 1.1 - -
2 2 0.6 - -
Radish 2 6 11.0 - -
Parsnip 2 3 1.2 - -
The pre-harvest use limitation Is three days except for carrots,
where no limit exists.
Peanuts, soybeans, sorghum grain and cowpeas
The results of supervised trials on both small experimental plots
and large commercial fields are shown in Tables 2, 3, 4 and 5 (Union
Carbide, 1973). Although applications of carbaryl to these crops are
often made close to harvest, an interval of at least one week would
usually precede harvest. There are no pre-harvest limitations on
peanuts, soybeans, or cowpeas; a limit of 21 days is set for sorghum
grain.
TABLE 2. CARBARYL RESIDUES (ppm) IN PEANUTS: NUT PLUS HULL
Number of Days after last application
lb ai/acre Number of
applications 0 41a 48a 73b 80b
1.5 1 0.33 - - - -
1.0 1 - - 0.26 - 0.72
1.0 2 - 0.40 - 0.98 -
2.0 1 - - 0.21 - 0.72
2.0 2 - 0.18 - 1.6 -
1.0 1 - - 0.26 - -
1.0 2 - 0.46 - 2.0 -
2.0 1 - - 0.18 - 0.63
2.0 2 - 0.28 - 0.96 -
a Normal digging time.
b Normal picking time - after drying.
TABLE 3. CARBARYL RESIDUES (ppm) IN SOYBEANS (DRY MATURE SEED)
lb ai/acre Number of Days after last application
applications
38 85 99
1 2 0.96 - -
2 3 - 0.07 -
1.5 1 - - 0.08
2 1 - - 0.05
TABLE 4. CARBARYL RESIDUES (ppm) IN SORGHUM GRAIN
lb ai/acre Number of Days after last application
applications
0 3 7-8 11-16
4 3 40 30 - -
2 4 7.2 4.1 2.9 -
2 3 22 14 15 24
2 2 7.0 1.9 1.1 1.3
2 1 45 - 39 11
2 1 35 - 9.7 -
2 1 20 - 5.0 -
2 1 3.9 - 2.3 1.3
2 1 - - 5.0 1.0
2 1 - - 4.5 0.8
2 1 - - 5.0 -
1.5 2 44 - 39 8.8
1 3 16 1.0 0.6 0.7
TABLE 4. (Cont'd.)
lb ai/acre Number of Days after last application
applications
0 3 7-8 11-16
1 1 11 - 4.1 1.8
1 1 5.9 - 2.0 1.0
1 1 2.9 - 0.6 -
Maximum, ppm 45 30 39 24
Average, ppm 20 10 9 5
TABLE 5. CARBARYL RESIDUES (ppm) IN COWPEA HULLS AND PEAS
lb ai/lacre Number of Days after last application
applications
0 1 3 7
Hull + pea
sample
2.8 3 - 8.1 6.3 4.5
2 8 2.3 - 1.5 1.0
2 2 4.0 - 0.6 1.6
2 2 3.4 - - -
2 1 26 24 4.8 3.1
2 3 12 - 4.5 0.9
1.5 1 2.5 - 1.3 -
1.3 1 3.1 - 1.6 -
Hull only
2 1 4.2
1 1 3.4
Pea only
2.8 3 - 0.7 0.8 0.6
2 1 0.9 - - -
1 1 0.8 - - -
2 2 0.4 - 0.5 0.7
Sugar beets
Good agricultural practice for the use of carbaryl on sugar beets
requires 2 lb ai/acre maximum with one or two applications up to 14
days before harvest. The results from small plot replicated trials are
shown in Table 6 (Union Carbide, 1973).
Forage crops
Forage crops on which carbaryl is used include alfalfa, clovers,
cowpea foliage, corn forage, grasses, peanut hay, sorghum forage and
soybean foliage. A maximum dosage of 1.5 lb ai/acre (2 lb ai/acre on
cowpeas and sorghum) is permitted with no pre-harvest limitation.
Although applications of carbaryl to these forage crops are often made
close to harvest, an interval of one week would precede harvest in
most cases.
The average and maximum residues determined in field trials on
these crops are summarized in Table 7 (Union Carbide, 1973).
Cucurbits
Residues resulting from replicated small plot and field
treatments are given in Table 8 (Union Carbide, 1973). Good
agricultural practice calls for a maximum dosage of 1 lb ai/acre
repeated as needed at 7-10 day intervals with no harvest limitation.
Fate of residues
General comments
The nature of the terminal residues of carbaryl in plants and
animals was summarized in the 1969 review (FAO/WHO, 1970b). Since
then, new information has become available on the distribution of
metabolites in plant tissues and on the transfer of residues to meat,
milk and eggs.
In plants
The metabolism and distribution of 14C-radio-labelled carbaryl
in a variety of plants has been examined (Andrawes and Chancey, 1970;
Chancey and Andrawes, 1971a, 1971b, 1972a, 1972b, 1973). These results
are summarized in Tables 9, 10 and 11. The conditions for extraction
of total 14C-materials and subsequent hydrolysis of the water-soluble
components were not optimized for each individual crop and the methods
described by Wiggins and Weiden (1969a and b) were applied without
modification to determine the metabolic profile of carbaryl in various
plant species. Thus, metabolic data obtained should be regarded as
qualitative. With this limitation, it is concluded that the metabolic
pathway of carbaryl is qualitatively similar in all plant species
studied and that the metabolic profile in foliage is generally similar
to that in fruit (Andrawes, 1973).
TABLE 6. CARBARYL RESIDUES (PPM) IN SUGAR BEETS
Days after last treatment
Number of Tops Roots
lb ai/.cre treatments
0 7 14 21 28 0 7 14 21 28
2 1 17 4.5 1.5 0.6 0.2 0.09 0.03 0.03 0.03 0.03
2 1 6.5 1.3 0.7 - 0.1 0.02 0.02 0.04 - 0.03
2 2 86 2.9 0.3 - - 0.04 0.05 0.03 - -
2 2 7.8 18 0.6 0.1 - 0.02 0.02 0.02 - -
4 1 170 15 3.6 0.7 0.5 0.11 0.03 0.05 0.02 0.03
4 1 26 1.5 0.5 - 0.1 0.03 0.03 0.04 - 0.02
4 2 160 5.8 0.4 - - 0.05 0.11 0.08 - -
4 2 15 8.5 1.4 0.5 - 0.05 0.03 0.03 0.03 -
1 1 12 5 - 0.5 - - - - - -
2 4 8 5 - - - - - - - -
1.5 1 10 - 1.4 - - - - - - -
TABLE 7. CARBARYL RESIDUES (ppm) IN FORAGE CROPS
Days after last application
Forage crop Average Maximum
0 3 7 0 3 7
Alfalfa 40 23 9 190 33 27
Clover 32 24 8 77 60 16
Corn forage 16 6 4 33 9 9
Cowpea foliage 26 14 10 72 42 17
Grass 45 26 12 98 60 26
Peanut hay 24 11 3 63 19 8
Sorghum forage 51 21 13 144 70 62
Soybean foliage 48 20 9 136 38 19
TABLE 8. CARBARYL RESIDUES (ppm) IN CUCURBITS
lb ai/acre Number of Days after last spray
treatments
0 1-2 3 7
Cucumbers 1 2 3.7 - - -
1 6 1.5 - - -
1 5 6.9 - - -
1 4-7 4.6 - 1.6 1.6
2 3-4 1.6 1.7 1.0 0.6
1 1 3.4 2.5 1.9 1.1
Summer squash 1 6 1.2 - - -
1 2 1.7 1.3 - -
2 3-4 1.2 1.3 1.4 0.5
Winter squash 2 7 5.7 - - -
2 6 1.8 - - -
Cantaloupe 1 9 5.4 - - -
Table 8 (cont'd)
lb ai/acre Number of Days after last spray
treatments
0 1-2 3 7
1 7 3.8 - - -
2 3 3.4 - - -
2 6 2.1 - - -
2 9 1.7 - - -
Water melon 2 7 5.7 - - -
2 6 1.8 - - -
TABLE 9. TRANSLOCATION OF 1-NAPHTHYL-14C-CARBARYL FROM FOLIAGE TO FRUIT
Plant Holding Method of Time % of applied
conditions application (days) translocated
Tomato Greenhouse 7 surface treatments 50 0.4
Wheat Greenhouse Leaf blade surface 21 <0.1
Greenhouse Stem injection 21 2.5
Potato Field Stem injection 21 0.6
42 1.4
Peanut Field Stem injection 21 0.5
In animals
Radio-tracer studies have shown that carbaryl is rapidly
metabolized and is generally excreted almost entirely within 24-96
hours after consumption. Elimination takes place mainly through the
urine, faeces, and respiratory gas and, to a lesser extent, through
the milk of dairy animals and eggs of poultry. The faecal route of
elimination is quite minor (<10%) in species other than the dog.
Long-term feeding of l-naphthyl-14C-carbaryl to laying chickens and
lactating cows showed only 0.15% and 0.22% of the administered dose to
appear in the eggs (Andrawes et al., 1972) and milk (Dorough, 1971)
respectively. The metabolic pathway of carbaryl in intact animals is
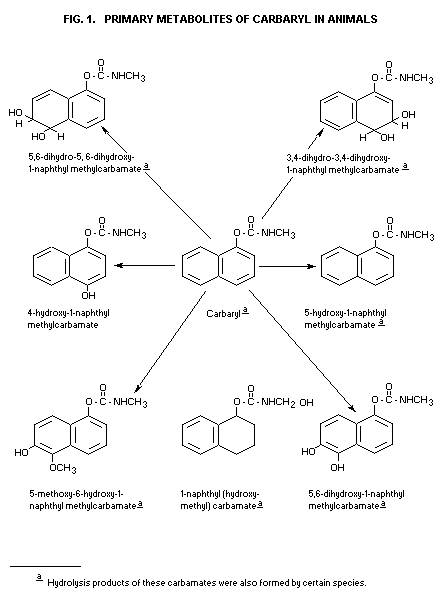
summarized in Fig. 1. Not all metabolites shown are formed by all
species studied. The major portion of urinary metabolites consists of
the water-soluble sulfate and glucuronide conjugates of the primary
products. The metabolites 1-methoxy-5-(methylearbamoyloxy)-2-naphthyl
sulfate and a conjugate of 5-methoxy-1,6-naphthalenediol were only
observed in milk of cows treated with l-naphthyl-14C-carbaryl
(Andrawes, 1973).
TABLE 10. RELATIVE DISTRIBUTION OF METABOLIC PRODUCTS IN THE FOLIAGE (AS PERCENTAGE OF TOTAL RADIO-ACTIVITY IN INTERNAL EXTRACT) IN
DIFFERENT PLANT SPECIES 21 DAYS AFTER APPLICATION OF 1-NAPHTHYL-14C-CARBARYL
Wheat Alfalfa Tomato
Metabolite Ricea Corna Beanb Peanutb Potatob
S,Gh S,Gh S,Ghb I,Ghb S, Gha S,F S,Fb S,Ghb S,F S,F S,Gha I,Gha S,Fb
Free carbaryl 18.5 19.5 71.2 20.7 1.6 7.2 3.7 19.4 2.3 0.8 7.5 1.9 3.7
Combined carbaryl 4.0 1.1 1.6 0.9 13.3 1.7 6.1 7.1 6.4 13.7 5.6 5.1 3.0
I-naphthol 1.8 1.4 0.8 2.5 1.0 1.5 1.8 0.8 14.5 4.2 6.3 3.5 4.2
4-hydroxy 2.0 4.2 18.2 2.6 11.7 2.5 3.6 2.0 9.1 3.3 10.8 7.3 6.4
5-hydroxy 2.9 6.9 6.8 1.5 14.6 2.9 2.5 1.6 5.7 3.1 2.7 1.9 2.4
Hydroxymethyl
(methylol) 4.0 7.7 3.6 1.9 8.5 13.9 7.0 8.5 9.7 11.4 19.9 8.0 15.7
5,6-dihydrodiol 2.1 1.4 1.5 ND 1.4 1.1 ND 4.1 ND ND ND 4.9 ND
Unknowns 1.1 1.9 1.9 ND 2.7 ND 0.3 4.8 2.7 ND 4.0 3.4 2.8
Origin of TLC 6.2 12.5 11.0 8.1 15.0 4.6 14.7 21.6 7.2 19.2 14.2 23.9 7.1
Unhydrolysed 9.7 6.4 3.1 2.0 ND 17.6 18.5 8.8 9.6 11.7 7.1 10.0 13.6
Unextracted 47.9 37.1 34.3 59.8 32.0 40.8 41.1 21.5 32.8 32.6 21.9 30.1 41.2
7-hydroxy ND ND ND ND ND 6.2 1.5 ND ND ND ND NO ND
a Seedlings.
b Mature plants.
Abbreviations: S -surface application; I - injection into stem; Gh - greenhouse; F - field; ND - none detected.
TABLE 11. RELATIVE DISTRIBUTION OF METABOLIC PRODUCTS (AS PERCENTAGE OF TOTAL RADIO-ACTIVITY
IN INTERNAL EXTRACT) IN TOMATO FRUIT AND WHEAT HEAD 21 DAYS APTER APPLICATION OF
1-NAPHTHYL-14C-CARBARYL
Tomato-surfacea Tomato-injectedb Tomatoc Wheat-Steme
Metabolite surface Wheatd
Ripe Green Ripe Green field leaf Chaff Seed
Free carbaryl 6.9 12.8 ND 0.6 4.0 9.1 6.1 4.1
Combined carbaryl 2.5 1.8 1.6 1.5 3.2 3.0 1.5 3.8
1-naphthol 1.2 8.8 0.6 2.4 8.2 1.4 0,9 5.2
4-hydroxy 9.9 7.0 9.0 12.2 0.4 8.9 7,4 7.7
5-hydroxy 6.3 8.1 1.5 3.3 0.3 4.4 4.4 3.1
Methylol 19.5 13.7 32.1 27.8 27.3 2.6 4.7 6.5
5,6-dihydrodiol 4.1 1.2 ND 3.6 ND ND 0.5 ND
Unknowns 0.9 0.7 5.1 4.0 ND ND ND ND
Origin of TLC 11.2 18.3 8.6 9.0 22.7 11.9 11.0 8.0
Unhydrolysed 31.7 18.3 31.9 26.3 22.5 11.1 6.0 10.3
Unextracted 5.8 9.3 9.6 9.3 11.1 47.4 57.0 51.3
7-hydroxy ND ND ND ND ND ND ND ND
a Carbaryl applied to the surface of the fruit in the greenhouse.
b Carbaryl injected into the fruit in the greenhouse.
c Carbaryl applied to the surface of the fruit in the field.
d Carbaryl applied to the leaf blade of mature wheat, the head (chaff plus seeds) was analysed.
e Carbaryl injected into the stem of maturing wheat plants in the greenhouse.
 Potential transfer of residues to meat, milk and eggs
(a) In dairy animals
After oral administration of single does of
1-naphthyl-14C-carbaryl at levels of 0.25 and 3.05 mg/kg,
approximately 0.35% of each dose was detected in the milk (Dorough,
1970). Maximum concentrations were found in samples taken six hours
after dosing which, following the two treatments, were 0.063 and
0.95 ppm, respectively.
In another study, 1-naphthyl-14C-carbaryl was fed to lactating
cows at levels of 0.15, 0.43 and 1.35 mg/kg bw (equivalent to 10, 30
and 100 ppm in the feed) for 14 days (Dorough, 1971). Equilibrium
between intake and elimination was reached within two days of
initiation of the treatment. At each feeding level, approximately 0.2%
of the dose was secreted in the milk. The concentration of total
14C-carbaryl equivalents in the milk was 1/400 of that in the diet.
Most of the 14C-residues (about 90%) were in the aqueous phase. Milk
metabolites and their concentrations after feeding 100 ppm of
l-naphthyl-14C-carbaryl for 14 days are shown in Table 12.
Continuous feeding of l-naphthyl-14C-carbaryl to cows and a
single oral dose of the same material demonstrated that carbaryl
residues do not accumulate in the body tissues (Dorough, 1971).
Furthermore, a good correlation existed between the level of pesticide
fed and that which appeared in the tissues. The distribution of
residues in different tissues and organs of cows receiving carbaryl in
their feed is shown in Table 13, while Table 14 shows the nature of
residues found in various samples.
(b) In poultry and eggs
Following administration of l-naphthyl-14C-carbaryl to hens,
total 14C-residues reached a maximum and dissipated at a much faster
rate in egg white than in egg yolk. In a single dose of 10 mg/kg
(Paulson and Foil, 1969), maximum concentration of 14C-residues in
egg white was 0.12 ppm at one day and dropped to trace amounts on the
second day after treatment. The yolk residues reached a maximum at the
fifth day (0.36 ppm) and had dissipated by the ninth (0.03 ppm). Under
continuous feeding conditions, the total residue in the yolk or white
at each sampling time was dosage related (Andrawes et al., 1972).
Concentration of 14C-carbaryl equivalents (ppm) reached a maximum
(0.10 ppm from 70 ppm in feed; 0.025 ppm from 21 ppm in feed) in the
white after 2-6 days and in the yolk (1.0 ppm from 70 ppm in feed;
0.30 ppm from 21 ppm in feed) after 6-9 days of dosing and remained
level until the end of the treatment period. At plateau levels, the
level of 14C-carbaryl equivalents in the white was one-tenth that in
the yolk; however, the total equivalents were in a ratio of 5:1
between yolk and white. The ratio of the concentration of carbaryl in
whole eggs (white and yolk) to that in the diet was 0.006 at
equilibration. After discontinuation of dosing, residues in the whites
had a half-life of less than one day; for yolk residues the half-life
was approximately 2-3 days. The nature of the metabolites found in
eggs is shown in Table 15.
TABLE 12. CHEMICAL NATURE OF CARBARYL METABOLITES IN COW'S MILK AND THEIR
AVERAGE CONCENTRATIONS AFTER FEEDING WITH 1-NAPHTHYL-14C-
CARBARYL AT LEVEL EQUIVALENT TO 100 ppm IN THE DIET FOR 14 DAYSa
Metabolites ppm in % of
milk total
Carbaryl 17 6
3,4-dihydro-3,4-dihydroxy-l
naphthyl methylearbamate 13 5
5,(3-dihydro-5,6-dihydroxy-l
naphthyl methylcarbamate 94 34
5-hydroxy-l-naphthyl methyl
carbamate 3 1
5,6-dihydro-5,6-dihydroxy-l
naphthol 9 3
l-naphthyl sulfate 72 26
1-methoxy-5-(methylearbamoyloxy)-
2-naphthyl sulfate 63 23
5-methoxy-1,6-naphthalenediol 7 2
a Reference - Dorough, 1971.
TABLE 13. TOTAL CARBARYL-14C EQUIVALENTS IN TISSUES OF COWS FED
CARBARYL-NAPHTHYL-14C FOR 14 DAYS AT RATES OF 10, 30 AND
100 ppm IN THE DIETa
ppm carbaryl-14C equivalents at feeding level of
Tissues
10 ppm 30 ppm 100 ppm
Kidney 0.095 0.531 1.003
Liver 0.033 0.100 0.411
Lung 0.020 0.064 0.207
TABLE 13. (Cont'd.)
ppm carbaryl-14C equivalents at feeding level of
Tissues
10 ppm 30 ppm 100 ppm
Muscle 0.009 0.031 0.104
Heart 0.012 0.038 0.095
Fat 0.000 0.015 0.025
Blood 0.008 0.036 0.141
a Cows were slaughtered 18 hours after the last dose was given.
TABLE 14. RADIO-ACTIVE RESIDUES IN TISSUES OF A COW AFTER BEING FED 100 ppm CARBARYL-14C
IN THE DIET FOR 14 DAYSa
% of total radio-activity in sample
Metabolites
Kidney Liver Lung Muscle Heart Blood
Carbaryl 3.3 9.2 2.1 17.0 3.7 0
5,6-dihydrodihydroxy carbaryl 4.5 3.0 8.8 38.6 31.3 22.0
5,6-dihydrodihydroxy naphthol 1.8 4.1 0 0 4.9 2.0
Naphthyl sulfate 29.3 4.1 27.3 0 4.0 51.8
Water-soluble unknowns 43.2 32.9 47.5 30.6 41.8 7.1
Unextractable unknowns 17.9 46.7 14.3 13.8 14.3 17.1
a cows were slaughtered at 18 hours after the last dose was given.
The distribution of carbaryl residues was determined in hen
tissues after continuous treatment with either 7, 21 or 70 ppm of
l-naphthyl-14C-carbaryl in the diet (Andrawes et al., 1972). Tissue
residues were directly proportional to the concentration of carbaryl
in the diet. The highest residues were found in the blood and tissues
of high blood content (liver, kidney, lung and spleen); body fat,
TABLE 15. METABOLIC PRODUCTS FOUND IN THE EGGS OF HENS FED 1-NAPHTHYL-14C-CARBARYL
FOR 14 DAYS AT A LEVEL EQUIVALENT TO 70 ppm IN THE DIETa
% of the recovered
radio-activity
Chromatographic Identity
fractions Yolk(Y) White(W) Y + W
F1 1-naphthol 17.74 6.25 15.76
F2 Carbaryl 4.59 0.73 3.92
F3 1-naphthyl(hydroxymethyl)-carbamate 4.88 0.15 4.06
S1 - 2.15 3.46 2.38
S2 Unknown A 3.07 8.42 3.99
S3 Unknown B 6.97 46.32 13.70
S4 1-naphthol conjugate 2.73 5.63 3.23
S5 1-naphthyl sulfate 44.05 15.59 39.14
S6 1-naphthol conjugate 5.03 5.68 5.14
S7 Unknown B conjugate 8.79 7.77 8.62
Average total µg of 14C-carbaryl equivalents per eggb 19.7 3.4 19.7
Average ppm of 14C-carbaryl equivalentsb 0.4 0.1 0.4
a Reference -Paulson and Feil, 1969.
b Based on eggs collected after equilibration was established; i.e. between the ninth
and the fourteenth day of dosing.
brain and muscles contained the lowest residues. For example, the
distribution of 14C-carbaryl equivalents one day after treatment for
14 days with 70 ppm in the diet was as follows (in ppm): liver 0.41,
kidney 0.485, thigh 0.03, leg 0.032, breast 0.031, skin 0.043, fat
0.026, gizzard 0.04, heart 0.049, and brain 0.017. The half-life of
total body residues was calculated to be five days.
Comparison of plant and animal metabolites
For the most part, the primary metabolic pathway of carbaryl
metabolism in plants is similar to that found in animals. Recognizable
divergences between plant and animal metabolites are as follows: (1)
conjugation of the primary metabolites in plants yields glycosides
(Casida and Lykken, 1969; Fukuto, 1972; Kuhr, 1968) as compared to
glucuronides, sulfates and pre-mercapturic acids in animals (Dorough,
1970; Fukuto, 1972; Ryan, 1971); (2) the metabolite 7-hydroxy-l-
naphthyl methylearbamate has been detected only in certain plants
(Wiggins et al., 1970) but not in animals; (3) animal metabolites
which are not reported for plants include:
3,4-dihydro-3,4-dihydroxy-1-naphthyl methylearbamate,
3,4-dihydro-3,4-dihydroxy-1-naphthol, 5,6-dihydroxy-l-naphthyl
methylearbamate, 5,6-dihydroxy-1-naphthol,
1-methoxy-5(methylearbamoyloxy)-2-naphthol, and
5-methoxy-1,6-naphthalenediol (Dorough, 1970).
The efficiency and rate of excretion of carbaryl plant
metabolites when fed to rats have been investigated. It was found that
a mixture of radio-labelled water-soluble plant metabolites were
totally eliminated within 96 hours (Dorough and Wiggins, 1969). No
change in the metabolic profile was observed in the excretion
products. In a study conducted on the feeding of plant mare containing
radio-activity designated as unextracted LIC-residues, it was found
that this/these material(s) is/are poorly absorbed by the rat and
is/are excreted primarily through the faeces (Tallent, 1970; Andrawes,
1973).
In storage and processing
No information has been received on the disappearance of residues
during storage and processing of cocoa beans and derived products or
of cereals. However, data on the behaviour of carbaryl residues on
other commodities make it appear probable that: (1) storage would have
little effect on residues; (2) washing, heating, cooking or baking
would likely reduce levels by a substantial amount.
Carbaryl residue degradation and removal during commercial and
home preparative procedures have been determined for green beans
(Elkins et al., 1968), tomatoes (Farrow et al., 1968), spinach (Lamb
et al., 1968), broccoli (Farrow et al., 1969), and spinach and
apricots (Elkins et al., 1972). In general, washing (cold water),
peeling (tomatoes), blanching and cooking were very effective in
removing 50-99% of initial residues. Combinations of these operations
were more effective than single steps. Commercial canning of spinach
and apricots destroyed 44% and 12% of initial residues respectively.
Preprocessing storage of green beans at 45°F and tomatoes at 55°F, and
storage of canned spinach and apricots at ambient temperatures and
100°F had little effect on residues except for canned spinach at 100°F
where a 23% reduction was noted.
Evidence of residues in food in commerce or at consumption
Results of the fifth year (June 1968-April 1969) and sixth year
(June 1969-April 1970) total diet studies of the United States Food
and Drug Administration showed a continuation of the downward trend in
detectable carbaryl residues (Corneliussen, 1970; Corneliussen, 1972).
Carbaryl was detected in three composites in the period 1968-1969. Two
results (in legume vegetables) were below the method sensitivity level
of 0.2 ppm. One fruit composite had 0.3 ppm. Carbaryl was not detected
in any of the diet composites during the 1969-1970 period.
Methods of residue analysis
In spite of vigorous research in recent years to develop a residue
method for carbaryl utilizing gas chromatography, the current method
of choice for regulatory purposes remains the colorimetric procedure
described in the official AOAC method (Official Methods of Analysis
of the AOAC, 11th ed., 1970, p. 493). This method has recently been
extended by the Union Carbide Co. to include determination of the
major carbaryl plant metabolites (total toxic residues). Procedures
have been developed to determine free carbaryl, combined carbaryl, and
the conjugated metabolites 1-naphthol and methylol carbaryl. Of the
known plant metabolites, methylol carbaryl and naphthol are closest in
toxicity to the parent carbaryl. Methylol carbaryl is also either the
major metabolite or a significant metabolite in the plants
investigated. No method yet exists for determining the animal
metabolites such as 5,6-dihydro-5,6-dibydroxy-1-naphthyl methyl
carbamate or water-soluble unknowns (Union Carbide, 1973).
National tolerances
TABLE 16. SUMMARY OF USA CARBARYL TOLERANCES AND LIMITATIONS
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Alfalfa 100 1.6 None
Almonds, shelled 1 8 None
Almond hulls 40 8 None
Apples 10 12 1 day
Apricots 10 8 3 days
Asparagus 10 2 1 day
Bananas 10 1.1 None
Beans 10 2.125 None
Beets, roots 5 2 3 days
Beets, tops 12 2 14 days
Blackberries 12 2 7 days
Blueberries 10 2 None
Boysenberries 12 2 7 days
Broccoli 10 2 3 days
TABLE 16. (cont'd)
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Brussels sprouts 10 2 3 days
Cabbage 10 2 3 days
Cabbage (Chinese) 10 2 14 days
Carrots 10 2 None
Cauliflower 10 2 3 days
Cherries 10 6 1 day
Citrus 10 1.25/100 gal 5 days
Clover 100 1.5 None
Collards 12 2 14 days
Corn forage 100 2 None
Corn kernels 5 3 None
Cotton seed 5 2.5 None
Cotton, forage 100 2.5 None
Cowpeas 5 2 None
Cowpea forage 100 2 None
Cranberries 10 4 1 day
Cucumbers 10 1 None
Dandelion 12 2 14 days
Dewberries 12 2 7 days
Eggplant 10 4 None
Endive (escarole) 10 2 14 days
Filberts, shelled 1 5 None
Grapes 10 3 None
Grapefruit 10 1.25/100 gal 5 days
Grass and hay 100 1.5 None
Horseradish 5 2 3 days
Kale 12 2 14 days
Kohlrabi 10 2 3 days
Lettuce (head) 10 2 3 days
Lettuce (leaf) 10 2 14 days
Loganberries 12 2 7 days
Melons 10 1 None
Mustard greens 12 2 14 days
Nectarines 10 8 3 days
Okra 10 2 None
Olives 10 8 None
Parsley 12 2 14 days
Parsnips 5 2 3 days
Peaches 10 8 1 day
Peanuts, nut and hull 5 1.5 None
Peanut hay 100 1.5 None
Pears 10 12 1 day
Peas and pods 10 2.6 None
Peavine forage 100 2.6 None
Pecans, shelled 1 3 None
Peppers 10 4 None
Plums 10 6 1 day
TABLE 16. (cont'd)
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Potatoes 0.5 interim 2 None
Prunes 10 6 1 day
Pumpkins 10 1 None
Radishes 5 2 3 days
Raspberries 12 2 7 days
Rice 5 2 14 days
Rice straw 100 2 14 days
Rutabagas 5 2 3 days
Salsify roots 5 2 3 days
Salsify tops 10 2 14 days
Sorghum grain 10 2 21 days
Sorghum forage 100 2 None
Soybeans 5 1.5 None
Soybean hay 100 1.5 None
Spinach 12 2 14 days
Squash 10 1 None
Strawberries 10 2 1 day
Sugar beet and tops 100 2 14 days
Swiss chard 12 2 14 days
Tobacco NF 0.6-24 None
Tomatoes 10 4 None
Turnips 5 2 3 days
Turnip tops 12 14 days
Walnuts, nuts 10 5 None
Poultry, meat and fat 5 0.25a 7 days
Poultry eggs 0.5 interim 0.25a 7 days
a Denotes lb ai/100 birds.
Appraisal
Carbaryl is extensively used around the world for control of
insect pests on a wide variety of agricultural crops, ornamentals,
turf, forests, livestock and poultry. Uses are increasing as it is
often selected as a replacement for the persistent organochlorine
insecticides.
Data available on residues in root crop vegetables (except
potatoes) from supervised trials at recommended rates and pre-harvest
intervals indicated that residues should not exceed 2 ppm if good
agricultural practice is followed.
The results of supervised trials on peanuts (groundnuts),
soybeans, sorgbum grain and cowpeas show that residues of up to 2 ppm
could occur on peanuts, up to 1 ppm on soybeans and cowpeas, and up to
24 ppm (rarely) on sorghum grain. On sorghum grain, a tolerance of 10
ppm would be sufficient to provide for the more nearly average residue
of 5 ppm.
Residues on sugar beet roots did not exceed 0.1 ppm at harvest in
supervised trials. Sugar beet tops had residues up to 3.6 ppm in the
same tests.
Field trials on the forage crops, alfalfa, clovers, corn forage,
cowpea foliage, grasses, peanut hay, sorghum forage and soybean
foliage, resulted in maximum residues ranging from 33 to 190 ppm when
treated the same day as harvest. Average residues ranged from 16 to
51 ppm.
The existing temporary tolerance of 3 ppm on cucurbits appears
questionable since data from supervised trials indicate that residues
greater than 5 ppm could occur in these crops with no pre-harvest
limitation. The recommendation was changed from 10 ppm to 3 ppm : in
1969 without giving supportive data.
In accord with the policy of expressing tolerances to one
significant digit (Report of the 1970 Joint FAO/WHO Meeting, 1971,
Wld Hlth Org. techn. Rep. Ser., No. 474, Section 2.13), the figure
for rice (rough) should be changed from 2.5 ppm to 3 ppm. It is
emphasized that this should not be construed as a change in the
tolerance but merely a numerical adjustment.
No information has been received on the disappearance of residues
during storage and processing of cocoa beans and derived products or
of cereals into cereal products. However, data on the behaviour of
carbaryl residues on other commodities make it appear probable that:
(1) storage would have little effect on residues; (2) washing,
heating, cooking or baking would likely reduce levels by a substantial
amount. Since the requirements for data on the disappearance of
residues during storage and processing of cocoa beans and of cereals
into cereal products were initiated in 1968 and no interested party
has responded, it seems reasonable to discontinue these requirements.
Feeding experiments with hens using radio-labelled carbaryl have
shown that residues in whole eggs are dose related, the ratio of
concentration (in eggs) to concentration in diet being 0.006. At a
diet level of 70 ppm of carbaryl, an average level of 0.4 ppm in eggs
(yolk plus white) was reached at equilibrium. After discontinuance of
feedingy residues decreased rapidly, the half-life in yolk being 2-3
days. A tolerance in eggs (shell free) of 0.5 ppm is recommended to
accommodate occasional residues in feed. The regulatory method of
analysis recommended can account for approximately 70% of the residues
(carbaryl, metabolites and their conjugates) in eggs.
Feeding studies with lactating cows using radio-labelled carbaryl
have shown that about 90% of the 14C-residues are found in the
aqueous phase and that at each feeding level approximately 0.2% of the
dose was secreted in the milk. The major metabolites found were
l-naphthyl sulfate (26%), 5,6-dihydro-5,6-dihydroxy-l-naphthyl
methylcarbamate (34%), and l-methoxy-5-(methylearbamoyloxy)-2-naphthyI
sulfate. Unchanged carbaryl was only 6% of the total residue. Since no
method of analysis is available for these compounds, no
recommendations for a tolerance can be made; however, the recommended
tolerance of 100 ppm on forage crops would give assurance that
residues in whole milk would not exceed 0.2 ppm. Since it is very
unlikely that any dairy animal would ever consume as much as 100 ppm
of unchanged carbaryl daily, actual milk residues would be negligibly
small.
Cows fed 100 ppm of 14C-carbaryl in the diet had 1 ppm carbaryl
equivalents in the kidney, 0.4 ppm in the liver, and 0.1 ppm in the
muscle (including heart). In kidney, 43% of the total radio-activity
was water-soluble unknowns, 30% was naphthyl sulfate, 18% was
unextractable, and 3% was carbaryl. In liver, 47% was unextractable,
33% was water-soluble unknowns, and 9% was carbaryl. In muscle, 39%
was 5,6-dihydrodihydroxycarbaryl, 31% was water-soluble unknowns, 14%
was unextractable, and 17% was carbaryl. On the basis of these
results, it would appear that only 13-30% of meat residues are in a
form that can be measured by the present analytical method. It is
therefore recommended that the tolerance for meat of cattle, goat and
sheep be reduced from 1 ppm to 0.2 ppm.
An analytical method suitable for regulatory use on crops, meat,
poultry and eggs has been developed.
RECOMMENDATIONS
Tolerances
The temporary tolerances previously recommended are replaced by
the following tolerances. The values represent the sum of free
carbaryl, combined carbaryl, conjugated naphthol and conjugated
methylol carbaryl expressed as total toxic residues of carbaryl.
Changes from 1970 recommendations are underlined; single
underline - new entry, double underline - re-entry of omitted
commodity or change in value based on new data.
ppm
Animal Feedstuffs (green) (alfalfa, clover, corn
forage, cowpea foliage, grasses, peanut hay,
sorghum forage, soybean foliage, sugar beet tops,
bean and pea vines 100
Apricots, blackberries, boysenberries, nectarines,
peaches, raspberries, asparagus, okra, leafy
vegetables (except brassica), nuts (whole),
olives (fresh), sorghum grain, cherries, plums 10
Blueberries, citrus fruit, cranberries, strawberries 7
Apples, bananas (pulp), grapes, beans, peas
(including pod), brassicas, tomatoes, peppers,
aubergines, pears, poultry skin 5
Cucurbits (including melons), rice (rough) 3
Root crop vegetables (beets, carrots, radishes,
rutabegas, parsnips), peanuts (groundnuts, whole) 2
Cotton seed (whole), sweet corn, (kernels), nuts
(shelled), olives (processed), soybeans (dry
mature seed), cowpeas 1
Poultry (total) (edible portions), eggs (shell free) 0.5
Potatoes, meat of cattle, sheep and goat, sugar beets 0.2
FURTHER WORK OR INFORMATION
Required (before a limit for residues in milk can be recommended)
1. A method suitable for regulatory purposes, for the determination
of total residues of carbaryl in milk.
Desirable
1. Further studies to elucidate the effects of carbaryl on renal
function.
2. Further studies to resolve the differences in observations of
different investigators on reproductive physiology, especially
with regard to nouro-endocrine and behavioural changes.
REFERENCES
Abou-Donia, M.B. and Dieckert, J.W. (1971) Gossypol: subcellular
localization and stimulation of rat liver microsomal oxidases.
Toxicol. Appl. Pharmacol. 18(3): 507-516
Andrawes, N.R. (1973) Metabolism of Sevin insecticide, Summary, 5 June
1973 (Private communication)
Andrawes, N.R. and Chancey, E.L. (1970) Union Carbide Corporation,
Internal Project Report, 2 November 1970
Andrawes, N.R., Chancey, E.L., Crabtree, R.J., Herret, R.A. and
Weiden, M.H.J. (1972) Fate of naphthyl carbaryl in laying chickens. J.
Agr. Food Chem. 20(3): 608-617
AOAC (1970) Official Methods of Analysis of the AOAC, 11th ed., 1970
p.493
Blazques, C.H., Vidyarthi, A.D., Sheehan, T.D., Bennet, M.J. and
McGrew, G.T. (1970) Effect of pinolene (B-pinene polymer) on carbaryl
foliar residues. J. Agr. Food Chem. 18(4): 681-684
Brzheskiy, V.V. (1972) K voprosu ob izuchenii mutagennykh svOxstv
insektitsida iz gruppy karbamatov-Sevina. [Study of the mutagenic
properties of Sevin (carbaryl).] Genetika, 8(6): 151-153
Casida, J.E. and Lykken, L.L. (1969) Ann. Rev. Plant Physiol. 20:
607
Casper, H.H. (1972) Diss. Abstracts, 32(11): 6599 B
Casper, H.H. and Pekas, J.C. (1971) Absorption and excretion of
radiolabeled 1-naphthyl-N-methylearbamate (carbaryl) by the rat. N.
Dak. Acad. Sci. 24(2): 160-166
Chancey, E.L. and Andrawes, N.R. (1971a) Union Carbide Corporation,
Internal Project Report, 27 October 1971
Chancey, E.L. and Andrawes, N.R. (1971b) Union Carbide Corporation,
Internal Project Report, 29 November 1971
Chancey, E.L. and Andrawes, N.R. (1972a) Union Carbide Corporation,
Internal Project Report, 21 July 1972
Chancey, E.L. and Andrawes, N.R. (1972b) Union Carbide Corporation,
Internal Project Report, 23 February 1972
Chancey, E.L., Andrawes, N.R. and Weiden, M.H.J. 1973 (Manuscript in
preparation)
Corneliussen, P.E. (1970) Pesticides Monit. J. 4(3): 89-105
Corneliussen, P.E. Pesticides Monit. J. 5(4): 313-328
Collins, T.F.X., Hansen, W.H. and Keeler, H.W. (1970) Effects of
carbaryl on reproduction of the rat and of the gerbil. Toxicol. Appl.
Pharmacol., 17: 273
Dieringer, C.S., Thomas, J.A. and Stitzel, R.E. (1973) Influence of
carbaryl on androgen metabolism in the prostate gland and liver. The
Pharmacologist, 15: 266
Dorough, H.W. and Wiggins, O.G.J. (1969) Econ. Entomol. 62: 49
Dorough, H.W.J. (1970) Agr. Food Chem. 18: 1015
Dorough, H.W. (1970) Continuous feeding of carbaryl-naphthyl-14C to
lactating cows. Progress Report, 6 January 1970
Dorough, H.W. (1971) Paper presented at the International Symposium on
Pesticide Terminal Residues, Tel Aviv, Israel, 17-19 February 1971
Dorough, H.W., Mehendale, H.M. and Lin, T. (1972) Modification of
carbaryl metabolism in rats with monoamine oxidase inhibitors. J.
Econ. Entomol. 65(4): 958-962
Dougherty, W., Golberg, L. and Coulston, F. (1973) The effect of
carbaryl on reproduction in rhesus monkeys. Unpublished report from
the Institute of Experimental Pathology and Toxicology, Albany Medical
College, United States of America
Elkins, E.R., Lamb, F.C., Farrow, R.P., Cook, R.W., Kawai, M. and
Kimball, J.R.J. (1968) Agr. Food Chem. 16: 962
Elkins, E.R., Farrow, R.P. and Kim, E.S.J. (1972) Agr. Food Chem.
20: 286
FAO/WHO, (1970b) 1969 Evaluation of Some Pesticide Residues in Food.
FAO/PL: 1969/M/17/1; WHO/Food Add./70.38
Farrow, R.P., Lamb, F.C., Cook, R.W., Kimball, J.R. and Elkins, E.R.J.
(1968) Agr. Food Chem. 16: 65
Farrow, R.P., Lamb, F.C., Elkins, E.R., Cook, R.W., Kawai, M. and
Cortes, A.J. (1969) Agr. Food Chem. 17: 75
Fukuto, T.R. (1972) Drug Metab. Revs. 1: 117
Hajkina, B.I. (1970) The mechanism of effect of carbaryl on warm
blooded animals. Gig. prim. toks. pesticid. i, klinika otravl. p. 203
Hassan, A. (1971) Pharmacological effects of carbaryl-I. The effect of
carbaryl on the synthesis and degradation of catecholamines in the
rat. Biochem. Pharmacol. 20(9): 2299-2308
Hoque, M.Z. (1972) Carbaryl - a new chemical mutagen. Curr. Sci.
41(23): 855-856
Homenko, N.P. (1971) Changes in the membrane potentiality of the
motoneurons of the spinal cord under the effect of carbaryl and
chlorfos. Fiziologja, 17(4); 527-528
Jakushko, V.E. (1971) Peculiarities of the glycosis and its regulation
on the cell structure of the cerebrum under the effect of DOT and
carbaryl. Farmakologija i toksikologija, reap. Megived, sb. 6:
187-190
Kagan, Ju. S., Radionev, G.A. and Voroneva, L.Ja. (1970) The effect of
Sevin in animal experiments on the function and structure of liver,
Farmakologija i toksikologija, 33(2): 219-224
Kuhr, R.J.J. (1968) Sci. Food Agric. Suppl. 44
Lamb, F.C., Farrow, R.P., Elkins, E.R., Kimball, J.R. and Cook, R.W.
(1968) J. Agr. Food Chem. 16: 967
Lillie, R.J. (1973) Studies on the reproductive performance and
progeny performance of caged White LeShorns fed malathion and
carbaryl. Poultry Sci. 52(1): 266-272
Locks, R.K. (1972a) Possible in vitro production of N-O-conjugates,
of N-hydroxyearbaryl from carbaryl by tobacco cells in culture. Fed.
Proc. Fed. Am. Soc. Exp. Biol. 31(2): 520
Locks, R.K. (1972b) Thin layer chromatography of l-naphthyl-N
hydroxy-N-methylearbamate and its application in two in vitro
studies involving carbaryl. J. Agr. Food Chem. 20: 1078
Lucier, G.W., McDaniel, O.S., Williams, C. and Klein, R. (1972)
Effects of chlordane and methylmercury on metabolism of carbaryl and
carbofuran in rats. Pest Biochem. Physiol. 2(2): 244-255
Mandzhgaladze, R.N. and Vashakidze, V.I. (1972) Deystviyo
khimicheskikh soyedineniy na potomstvo i razvit iye priznakov pola.
[Action of some chemical compounds on offspring and sexual
characteristics of rats.] Soobsbeh. Akad. Nauk Gruz. SSR, 65(2):
485-488
Moriyama, H., Sugiyama, H. and Shigematsu, H. (1972) A hydroxy
metabolite derived from carbaryl in the silkworm, Bombyx mori. Pest
Biochem. Physiol. 2(1): 1
Natoff, I.L. and Reiff, B. (1973) Effect of oximes on the acute
toxicity of anticholinesterase carbamates. Toxicol. Appl. Pharmacol.
25: 569-575
Paulson, G.D. and Feil, V.J. (1969) Poultry Sci. 48: 1593
Pokas, J.C. (1971) Intestinal metabolism and transport of naphthyl
N-methyl-carbamate in vitro (rat). Amer. J. Physiol. 220(6):
2008-2012
Pekas, J.C. and Paulson, G.D. (1970) Intestinal hydrolysis and
conjugation of a pesticidal carbamate in vitro. Science,
170(3953): 77-78
Rappoport, M.B. (1969) Microscopic and ultrastructural changes of the
thyroid gland under the effect of the insecticide carbaryl. Vrachebnoe
delo, 5: 102-105
Richey, F.A., jr, Bartley, W.J., Fitzpatrick, J.T. and Kurtz, A.P.
(1972) Chemical synthesis of the carbaryl metabolite
trans-5,6-dihydro-5,6-dihydroxy-l-naphthyl methyl-carbamate. J. Agr.
Food Chem. 20(4): 825-828
Ryan, A.J. (1971) CRC Critical Reviews in Toxicology, September 1971,
33
Santolucito, J. A., Whitcomb, E. and Hassan, A. (1972) Pharmacological
effects of carbaryl. Ill. Observations on alteration of smooth
muscle-preparation responses to norepinephrine. Environ. Physiol.
Biochem. 2(3): 142-146
Shah, A.H. and Guthrie, F.E. (1971) In vitro metabolism of
insecticides during midgut penetration. Pest Biochem. Physiol. 1(1):
1-10
Shtenberg, A.I. and Orlova, N.V. (1970) Contribution to the mechanism
of toxic action on Sevin, a pesticide of the carbamate group. Vestn.
Akad. Mod. Nauk SSR, 72-76
Sideroff, S.I. and Santolucito, J.A. (1972) Behavioural and
physiological effects of the cholinesterase inhibitor carbaryl
(I-naphthyl methylearbamate). Physiol. and Behav. 9: 459-462
Stenberg, A.M. (1970) The effect of carbaryl on the status of the
thyroid gland of mice receiving artificial or full value diet with
different content of iodine. Gigiena i sanitarija, 8: 119
Stenberg, A.I. and Otovan, M.B. (1971) Effect of small doses of
carbaryl on the reproductive function of animals in a number of
offsprings. Voprosi pitanija, 30(1): 41-49
Strother, A. (1972) In vitro metabolism of methyl carbamate
insecticides by human and rat liver fractions. Toxicol. Appl.
Pharmacol. 21(1): 112-129
Sullivan, L.J., Eldridge, J.M., Knaak, J.B. and Tallant, M.J. (1972)
5,6-dihydro-5,6-dihydroxy carbaryl glucuronide as a significant
metabolite of carbaryl in a rat. J. Agr. Food Chem. 20: 980-985
Tallant, M.J. (1970) Mellon Institute, Special Report 33-100, 20
October 1970
Thomas, J.A., Schein, L.G. and Dieringer, C.S. (1973) Distribution of
radio-activity in male reproductive organs after the administration
either carbaryl-14C or DDT-3H. The Pharmacologist, 15: 227
Trifonova, T.K., Gladenko, I.N. and Shulyak, V.D. (1970) Deystviye
gamma-izomera GKhTsGi sevina na polovuyu funktsivu. [Effect of
gamma-BHC and Sevin on reproduction] Veterinariya (Moscow), 47(6):
91-93
Union Carbide (1973) Patterns of use for Sevin(R) carbaryl
insecticide. Summary filed with FAO 1 March 1973
Union Carbide (1970) Summary of carbaryl residues. Section V (Private
communication)
Vashakidze, V.I. (1970) The effect of carbaryl on the spermatogenesis
of albino mice. Sbornik trudov Instituta gigiena truda i prof.
zabolevanija, Gruz SSR, 12: 285-288
Weil, C.S., Woodside, M.D., Bernard, J.B., Condra, N.I. and Carpenter,
C.P. (1972a) Studies on rat reproduction and guinea pig teratology of
carbaryl fed in the diet vs stomach tube. Toxicol. Appl. Pharmacol.
22: 318
Weil, C.S., Woodside, M.D., Carpenter, C.P. and Smyth, H.F., jr.
(1972b) Current status of tests of carbaryl for reproductive and
teratogenic effect. Toxicol. Appl. Pharmacol. 21: 390-404
Wiggins, O.G. and Weiden, M.H.J. (1969a) Union Carbide Corporation,
Internal Project Report, 30 June 1969
Wiggins, O.G. and Weiden, M.H.J. (1969b) Union Carbide Corporation,
Internal Project Report, 1 July 1969
Wiggins, O.G., Weiden, M.H.J., Weil, C.S. and Carpenter, C.P. (1970)
Paper presented at the VII International Congress of Plant Protection,
Paris, September 1970
Potential transfer of residues to meat, milk and eggs
(a) In dairy animals
After oral administration of single does of
1-naphthyl-14C-carbaryl at levels of 0.25 and 3.05 mg/kg,
approximately 0.35% of each dose was detected in the milk (Dorough,
1970). Maximum concentrations were found in samples taken six hours
after dosing which, following the two treatments, were 0.063 and
0.95 ppm, respectively.
In another study, 1-naphthyl-14C-carbaryl was fed to lactating
cows at levels of 0.15, 0.43 and 1.35 mg/kg bw (equivalent to 10, 30
and 100 ppm in the feed) for 14 days (Dorough, 1971). Equilibrium
between intake and elimination was reached within two days of
initiation of the treatment. At each feeding level, approximately 0.2%
of the dose was secreted in the milk. The concentration of total
14C-carbaryl equivalents in the milk was 1/400 of that in the diet.
Most of the 14C-residues (about 90%) were in the aqueous phase. Milk
metabolites and their concentrations after feeding 100 ppm of
l-naphthyl-14C-carbaryl for 14 days are shown in Table 12.
Continuous feeding of l-naphthyl-14C-carbaryl to cows and a
single oral dose of the same material demonstrated that carbaryl
residues do not accumulate in the body tissues (Dorough, 1971).
Furthermore, a good correlation existed between the level of pesticide
fed and that which appeared in the tissues. The distribution of
residues in different tissues and organs of cows receiving carbaryl in
their feed is shown in Table 13, while Table 14 shows the nature of
residues found in various samples.
(b) In poultry and eggs
Following administration of l-naphthyl-14C-carbaryl to hens,
total 14C-residues reached a maximum and dissipated at a much faster
rate in egg white than in egg yolk. In a single dose of 10 mg/kg
(Paulson and Foil, 1969), maximum concentration of 14C-residues in
egg white was 0.12 ppm at one day and dropped to trace amounts on the
second day after treatment. The yolk residues reached a maximum at the
fifth day (0.36 ppm) and had dissipated by the ninth (0.03 ppm). Under
continuous feeding conditions, the total residue in the yolk or white
at each sampling time was dosage related (Andrawes et al., 1972).
Concentration of 14C-carbaryl equivalents (ppm) reached a maximum
(0.10 ppm from 70 ppm in feed; 0.025 ppm from 21 ppm in feed) in the
white after 2-6 days and in the yolk (1.0 ppm from 70 ppm in feed;
0.30 ppm from 21 ppm in feed) after 6-9 days of dosing and remained
level until the end of the treatment period. At plateau levels, the
level of 14C-carbaryl equivalents in the white was one-tenth that in
the yolk; however, the total equivalents were in a ratio of 5:1
between yolk and white. The ratio of the concentration of carbaryl in
whole eggs (white and yolk) to that in the diet was 0.006 at
equilibration. After discontinuation of dosing, residues in the whites
had a half-life of less than one day; for yolk residues the half-life
was approximately 2-3 days. The nature of the metabolites found in
eggs is shown in Table 15.
TABLE 12. CHEMICAL NATURE OF CARBARYL METABOLITES IN COW'S MILK AND THEIR
AVERAGE CONCENTRATIONS AFTER FEEDING WITH 1-NAPHTHYL-14C-
CARBARYL AT LEVEL EQUIVALENT TO 100 ppm IN THE DIET FOR 14 DAYSa
Metabolites ppm in % of
milk total
Carbaryl 17 6
3,4-dihydro-3,4-dihydroxy-l
naphthyl methylearbamate 13 5
5,(3-dihydro-5,6-dihydroxy-l
naphthyl methylcarbamate 94 34
5-hydroxy-l-naphthyl methyl
carbamate 3 1
5,6-dihydro-5,6-dihydroxy-l
naphthol 9 3
l-naphthyl sulfate 72 26
1-methoxy-5-(methylearbamoyloxy)-
2-naphthyl sulfate 63 23
5-methoxy-1,6-naphthalenediol 7 2
a Reference - Dorough, 1971.
TABLE 13. TOTAL CARBARYL-14C EQUIVALENTS IN TISSUES OF COWS FED
CARBARYL-NAPHTHYL-14C FOR 14 DAYS AT RATES OF 10, 30 AND
100 ppm IN THE DIETa
ppm carbaryl-14C equivalents at feeding level of
Tissues
10 ppm 30 ppm 100 ppm
Kidney 0.095 0.531 1.003
Liver 0.033 0.100 0.411
Lung 0.020 0.064 0.207
TABLE 13. (Cont'd.)
ppm carbaryl-14C equivalents at feeding level of
Tissues
10 ppm 30 ppm 100 ppm
Muscle 0.009 0.031 0.104
Heart 0.012 0.038 0.095
Fat 0.000 0.015 0.025
Blood 0.008 0.036 0.141
a Cows were slaughtered 18 hours after the last dose was given.
TABLE 14. RADIO-ACTIVE RESIDUES IN TISSUES OF A COW AFTER BEING FED 100 ppm CARBARYL-14C
IN THE DIET FOR 14 DAYSa
% of total radio-activity in sample
Metabolites
Kidney Liver Lung Muscle Heart Blood
Carbaryl 3.3 9.2 2.1 17.0 3.7 0
5,6-dihydrodihydroxy carbaryl 4.5 3.0 8.8 38.6 31.3 22.0
5,6-dihydrodihydroxy naphthol 1.8 4.1 0 0 4.9 2.0
Naphthyl sulfate 29.3 4.1 27.3 0 4.0 51.8
Water-soluble unknowns 43.2 32.9 47.5 30.6 41.8 7.1
Unextractable unknowns 17.9 46.7 14.3 13.8 14.3 17.1
a cows were slaughtered at 18 hours after the last dose was given.
The distribution of carbaryl residues was determined in hen
tissues after continuous treatment with either 7, 21 or 70 ppm of
l-naphthyl-14C-carbaryl in the diet (Andrawes et al., 1972). Tissue
residues were directly proportional to the concentration of carbaryl
in the diet. The highest residues were found in the blood and tissues
of high blood content (liver, kidney, lung and spleen); body fat,
TABLE 15. METABOLIC PRODUCTS FOUND IN THE EGGS OF HENS FED 1-NAPHTHYL-14C-CARBARYL
FOR 14 DAYS AT A LEVEL EQUIVALENT TO 70 ppm IN THE DIETa
% of the recovered
radio-activity
Chromatographic Identity
fractions Yolk(Y) White(W) Y + W
F1 1-naphthol 17.74 6.25 15.76
F2 Carbaryl 4.59 0.73 3.92
F3 1-naphthyl(hydroxymethyl)-carbamate 4.88 0.15 4.06
S1 - 2.15 3.46 2.38
S2 Unknown A 3.07 8.42 3.99
S3 Unknown B 6.97 46.32 13.70
S4 1-naphthol conjugate 2.73 5.63 3.23
S5 1-naphthyl sulfate 44.05 15.59 39.14
S6 1-naphthol conjugate 5.03 5.68 5.14
S7 Unknown B conjugate 8.79 7.77 8.62
Average total µg of 14C-carbaryl equivalents per eggb 19.7 3.4 19.7
Average ppm of 14C-carbaryl equivalentsb 0.4 0.1 0.4
a Reference -Paulson and Feil, 1969.
b Based on eggs collected after equilibration was established; i.e. between the ninth
and the fourteenth day of dosing.
brain and muscles contained the lowest residues. For example, the
distribution of 14C-carbaryl equivalents one day after treatment for
14 days with 70 ppm in the diet was as follows (in ppm): liver 0.41,
kidney 0.485, thigh 0.03, leg 0.032, breast 0.031, skin 0.043, fat
0.026, gizzard 0.04, heart 0.049, and brain 0.017. The half-life of
total body residues was calculated to be five days.
Comparison of plant and animal metabolites
For the most part, the primary metabolic pathway of carbaryl
metabolism in plants is similar to that found in animals. Recognizable
divergences between plant and animal metabolites are as follows: (1)
conjugation of the primary metabolites in plants yields glycosides
(Casida and Lykken, 1969; Fukuto, 1972; Kuhr, 1968) as compared to
glucuronides, sulfates and pre-mercapturic acids in animals (Dorough,
1970; Fukuto, 1972; Ryan, 1971); (2) the metabolite 7-hydroxy-l-
naphthyl methylearbamate has been detected only in certain plants
(Wiggins et al., 1970) but not in animals; (3) animal metabolites
which are not reported for plants include:
3,4-dihydro-3,4-dihydroxy-1-naphthyl methylearbamate,
3,4-dihydro-3,4-dihydroxy-1-naphthol, 5,6-dihydroxy-l-naphthyl
methylearbamate, 5,6-dihydroxy-1-naphthol,
1-methoxy-5(methylearbamoyloxy)-2-naphthol, and
5-methoxy-1,6-naphthalenediol (Dorough, 1970).
The efficiency and rate of excretion of carbaryl plant
metabolites when fed to rats have been investigated. It was found that
a mixture of radio-labelled water-soluble plant metabolites were
totally eliminated within 96 hours (Dorough and Wiggins, 1969). No
change in the metabolic profile was observed in the excretion
products. In a study conducted on the feeding of plant mare containing
radio-activity designated as unextracted LIC-residues, it was found
that this/these material(s) is/are poorly absorbed by the rat and
is/are excreted primarily through the faeces (Tallent, 1970; Andrawes,
1973).
In storage and processing
No information has been received on the disappearance of residues
during storage and processing of cocoa beans and derived products or
of cereals. However, data on the behaviour of carbaryl residues on
other commodities make it appear probable that: (1) storage would have
little effect on residues; (2) washing, heating, cooking or baking
would likely reduce levels by a substantial amount.
Carbaryl residue degradation and removal during commercial and
home preparative procedures have been determined for green beans
(Elkins et al., 1968), tomatoes (Farrow et al., 1968), spinach (Lamb
et al., 1968), broccoli (Farrow et al., 1969), and spinach and
apricots (Elkins et al., 1972). In general, washing (cold water),
peeling (tomatoes), blanching and cooking were very effective in
removing 50-99% of initial residues. Combinations of these operations
were more effective than single steps. Commercial canning of spinach
and apricots destroyed 44% and 12% of initial residues respectively.
Preprocessing storage of green beans at 45°F and tomatoes at 55°F, and
storage of canned spinach and apricots at ambient temperatures and
100°F had little effect on residues except for canned spinach at 100°F
where a 23% reduction was noted.
Evidence of residues in food in commerce or at consumption
Results of the fifth year (June 1968-April 1969) and sixth year
(June 1969-April 1970) total diet studies of the United States Food
and Drug Administration showed a continuation of the downward trend in
detectable carbaryl residues (Corneliussen, 1970; Corneliussen, 1972).
Carbaryl was detected in three composites in the period 1968-1969. Two
results (in legume vegetables) were below the method sensitivity level
of 0.2 ppm. One fruit composite had 0.3 ppm. Carbaryl was not detected
in any of the diet composites during the 1969-1970 period.
Methods of residue analysis
In spite of vigorous research in recent years to develop a residue
method for carbaryl utilizing gas chromatography, the current method
of choice for regulatory purposes remains the colorimetric procedure
described in the official AOAC method (Official Methods of Analysis
of the AOAC, 11th ed., 1970, p. 493). This method has recently been
extended by the Union Carbide Co. to include determination of the
major carbaryl plant metabolites (total toxic residues). Procedures
have been developed to determine free carbaryl, combined carbaryl, and
the conjugated metabolites 1-naphthol and methylol carbaryl. Of the
known plant metabolites, methylol carbaryl and naphthol are closest in
toxicity to the parent carbaryl. Methylol carbaryl is also either the
major metabolite or a significant metabolite in the plants
investigated. No method yet exists for determining the animal
metabolites such as 5,6-dihydro-5,6-dibydroxy-1-naphthyl methyl
carbamate or water-soluble unknowns (Union Carbide, 1973).
National tolerances
TABLE 16. SUMMARY OF USA CARBARYL TOLERANCES AND LIMITATIONS
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Alfalfa 100 1.6 None
Almonds, shelled 1 8 None
Almond hulls 40 8 None
Apples 10 12 1 day
Apricots 10 8 3 days
Asparagus 10 2 1 day
Bananas 10 1.1 None
Beans 10 2.125 None
Beets, roots 5 2 3 days
Beets, tops 12 2 14 days
Blackberries 12 2 7 days
Blueberries 10 2 None
Boysenberries 12 2 7 days
Broccoli 10 2 3 days
TABLE 16. (cont'd)
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Brussels sprouts 10 2 3 days
Cabbage 10 2 3 days
Cabbage (Chinese) 10 2 14 days
Carrots 10 2 None
Cauliflower 10 2 3 days
Cherries 10 6 1 day
Citrus 10 1.25/100 gal 5 days
Clover 100 1.5 None
Collards 12 2 14 days
Corn forage 100 2 None
Corn kernels 5 3 None
Cotton seed 5 2.5 None
Cotton, forage 100 2.5 None
Cowpeas 5 2 None
Cowpea forage 100 2 None
Cranberries 10 4 1 day
Cucumbers 10 1 None
Dandelion 12 2 14 days
Dewberries 12 2 7 days
Eggplant 10 4 None
Endive (escarole) 10 2 14 days
Filberts, shelled 1 5 None
Grapes 10 3 None
Grapefruit 10 1.25/100 gal 5 days
Grass and hay 100 1.5 None
Horseradish 5 2 3 days
Kale 12 2 14 days
Kohlrabi 10 2 3 days
Lettuce (head) 10 2 3 days
Lettuce (leaf) 10 2 14 days
Loganberries 12 2 7 days
Melons 10 1 None
Mustard greens 12 2 14 days
Nectarines 10 8 3 days
Okra 10 2 None
Olives 10 8 None
Parsley 12 2 14 days
Parsnips 5 2 3 days
Peaches 10 8 1 day
Peanuts, nut and hull 5 1.5 None
Peanut hay 100 1.5 None
Pears 10 12 1 day
Peas and pods 10 2.6 None
Peavine forage 100 2.6 None
Pecans, shelled 1 3 None
Peppers 10 4 None
Plums 10 6 1 day
TABLE 16. (cont'd)
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Potatoes 0.5 interim 2 None
Prunes 10 6 1 day
Pumpkins 10 1 None
Radishes 5 2 3 days
Raspberries 12 2 7 days
Rice 5 2 14 days
Rice straw 100 2 14 days
Rutabagas 5 2 3 days
Salsify roots 5 2 3 days
Salsify tops 10 2 14 days
Sorghum grain 10 2 21 days
Sorghum forage 100 2 None
Soybeans 5 1.5 None
Soybean hay 100 1.5 None
Spinach 12 2 14 days
Squash 10 1 None
Strawberries 10 2 1 day
Sugar beet and tops 100 2 14 days
Swiss chard 12 2 14 days
Tobacco NF 0.6-24 None
Tomatoes 10 4 None
Turnips 5 2 3 days
Turnip tops 12 14 days
Walnuts, nuts 10 5 None
Poultry, meat and fat 5 0.25a 7 days
Poultry eggs 0.5 interim 0.25a 7 days
a Denotes lb ai/100 birds.
Appraisal
Carbaryl is extensively used around the world for control of
insect pests on a wide variety of agricultural crops, ornamentals,
turf, forests, livestock and poultry. Uses are increasing as it is
often selected as a replacement for the persistent organochlorine
insecticides.
Data available on residues in root crop vegetables (except
potatoes) from supervised trials at recommended rates and pre-harvest
intervals indicated that residues should not exceed 2 ppm if good
agricultural practice is followed.
The results of supervised trials on peanuts (groundnuts),
soybeans, sorgbum grain and cowpeas show that residues of up to 2 ppm
could occur on peanuts, up to 1 ppm on soybeans and cowpeas, and up to
24 ppm (rarely) on sorghum grain. On sorghum grain, a tolerance of 10
ppm would be sufficient to provide for the more nearly average residue
of 5 ppm.
Residues on sugar beet roots did not exceed 0.1 ppm at harvest in
supervised trials. Sugar beet tops had residues up to 3.6 ppm in the
same tests.
Field trials on the forage crops, alfalfa, clovers, corn forage,
cowpea foliage, grasses, peanut hay, sorghum forage and soybean
foliage, resulted in maximum residues ranging from 33 to 190 ppm when
treated the same day as harvest. Average residues ranged from 16 to
51 ppm.
The existing temporary tolerance of 3 ppm on cucurbits appears
questionable since data from supervised trials indicate that residues
greater than 5 ppm could occur in these crops with no pre-harvest
limitation. The recommendation was changed from 10 ppm to 3 ppm : in
1969 without giving supportive data.
In accord with the policy of expressing tolerances to one
significant digit (Report of the 1970 Joint FAO/WHO Meeting, 1971,
Wld Hlth Org. techn. Rep. Ser., No. 474, Section 2.13), the figure
for rice (rough) should be changed from 2.5 ppm to 3 ppm. It is
emphasized that this should not be construed as a change in the
tolerance but merely a numerical adjustment.
No information has been received on the disappearance of residues
during storage and processing of cocoa beans and derived products or
of cereals into cereal products. However, data on the behaviour of
carbaryl residues on other commodities make it appear probable that:
(1) storage would have little effect on residues; (2) washing,
heating, cooking or baking would likely reduce levels by a substantial
amount. Since the requirements for data on the disappearance of
residues during storage and processing of cocoa beans and of cereals
into cereal products were initiated in 1968 and no interested party
has responded, it seems reasonable to discontinue these requirements.
Feeding experiments with hens using radio-labelled carbaryl have
shown that residues in whole eggs are dose related, the ratio of
concentration (in eggs) to concentration in diet being 0.006. At a
diet level of 70 ppm of carbaryl, an average level of 0.4 ppm in eggs
(yolk plus white) was reached at equilibrium. After discontinuance of
feedingy residues decreased rapidly, the half-life in yolk being 2-3
days. A tolerance in eggs (shell free) of 0.5 ppm is recommended to
accommodate occasional residues in feed. The regulatory method of
analysis recommended can account for approximately 70% of the residues
(carbaryl, metabolites and their conjugates) in eggs.
Feeding studies with lactating cows using radio-labelled carbaryl
have shown that about 90% of the 14C-residues are found in the
aqueous phase and that at each feeding level approximately 0.2% of the
dose was secreted in the milk. The major metabolites found were
l-naphthyl sulfate (26%), 5,6-dihydro-5,6-dihydroxy-l-naphthyl
methylcarbamate (34%), and l-methoxy-5-(methylearbamoyloxy)-2-naphthyI
sulfate. Unchanged carbaryl was only 6% of the total residue. Since no
method of analysis is available for these compounds, no
recommendations for a tolerance can be made; however, the recommended
tolerance of 100 ppm on forage crops would give assurance that
residues in whole milk would not exceed 0.2 ppm. Since it is very
unlikely that any dairy animal would ever consume as much as 100 ppm
of unchanged carbaryl daily, actual milk residues would be negligibly
small.
Cows fed 100 ppm of 14C-carbaryl in the diet had 1 ppm carbaryl
equivalents in the kidney, 0.4 ppm in the liver, and 0.1 ppm in the
muscle (including heart). In kidney, 43% of the total radio-activity
was water-soluble unknowns, 30% was naphthyl sulfate, 18% was
unextractable, and 3% was carbaryl. In liver, 47% was unextractable,
33% was water-soluble unknowns, and 9% was carbaryl. In muscle, 39%
was 5,6-dihydrodihydroxycarbaryl, 31% was water-soluble unknowns, 14%
was unextractable, and 17% was carbaryl. On the basis of these
results, it would appear that only 13-30% of meat residues are in a
form that can be measured by the present analytical method. It is
therefore recommended that the tolerance for meat of cattle, goat and
sheep be reduced from 1 ppm to 0.2 ppm.
An analytical method suitable for regulatory use on crops, meat,
poultry and eggs has been developed.
RECOMMENDATIONS
Tolerances
The temporary tolerances previously recommended are replaced by
the following tolerances. The values represent the sum of free
carbaryl, combined carbaryl, conjugated naphthol and conjugated
methylol carbaryl expressed as total toxic residues of carbaryl.
Changes from 1970 recommendations are underlined; single
underline - new entry, double underline - re-entry of omitted
commodity or change in value based on new data.
ppm
Animal Feedstuffs (green) (alfalfa, clover, corn
forage, cowpea foliage, grasses, peanut hay,
sorghum forage, soybean foliage, sugar beet tops,
bean and pea vines 100
Apricots, blackberries, boysenberries, nectarines,
peaches, raspberries, asparagus, okra, leafy
vegetables (except brassica), nuts (whole),
olives (fresh), sorghum grain, cherries, plums 10
Blueberries, citrus fruit, cranberries, strawberries 7
Apples, bananas (pulp), grapes, beans, peas
(including pod), brassicas, tomatoes, peppers,
aubergines, pears, poultry skin 5
Cucurbits (including melons), rice (rough) 3
Root crop vegetables (beets, carrots, radishes,
rutabegas, parsnips), peanuts (groundnuts, whole) 2
Cotton seed (whole), sweet corn, (kernels), nuts
(shelled), olives (processed), soybeans (dry
mature seed), cowpeas 1
Poultry (total) (edible portions), eggs (shell free) 0.5
Potatoes, meat of cattle, sheep and goat, sugar beets 0.2
FURTHER WORK OR INFORMATION
Required (before a limit for residues in milk can be recommended)
1. A method suitable for regulatory purposes, for the determination
of total residues of carbaryl in milk.
Desirable
1. Further studies to elucidate the effects of carbaryl on renal
function.
2. Further studies to resolve the differences in observations of
different investigators on reproductive physiology, especially
with regard to nouro-endocrine and behavioural changes.
REFERENCES
Abou-Donia, M.B. and Dieckert, J.W. (1971) Gossypol: subcellular
localization and stimulation of rat liver microsomal oxidases.
Toxicol. Appl. Pharmacol. 18(3): 507-516
Andrawes, N.R. (1973) Metabolism of Sevin insecticide, Summary, 5 June
1973 (Private communication)
Andrawes, N.R. and Chancey, E.L. (1970) Union Carbide Corporation,
Internal Project Report, 2 November 1970
Andrawes, N.R., Chancey, E.L., Crabtree, R.J., Herret, R.A. and
Weiden, M.H.J. (1972) Fate of naphthyl carbaryl in laying chickens. J.
Agr. Food Chem. 20(3): 608-617
AOAC (1970) Official Methods of Analysis of the AOAC, 11th ed., 1970
p.493
Blazques, C.H., Vidyarthi, A.D., Sheehan, T.D., Bennet, M.J. and
McGrew, G.T. (1970) Effect of pinolene (B-pinene polymer) on carbaryl
foliar residues. J. Agr. Food Chem. 18(4): 681-684
Brzheskiy, V.V. (1972) K voprosu ob izuchenii mutagennykh svOxstv
insektitsida iz gruppy karbamatov-Sevina. [Study of the mutagenic
properties of Sevin (carbaryl).] Genetika, 8(6): 151-153
Casida, J.E. and Lykken, L.L. (1969) Ann. Rev. Plant Physiol. 20:
607
Casper, H.H. (1972) Diss. Abstracts, 32(11): 6599 B
Casper, H.H. and Pekas, J.C. (1971) Absorption and excretion of
radiolabeled 1-naphthyl-N-methylearbamate (carbaryl) by the rat. N.
Dak. Acad. Sci. 24(2): 160-166
Chancey, E.L. and Andrawes, N.R. (1971a) Union Carbide Corporation,
Internal Project Report, 27 October 1971
Chancey, E.L. and Andrawes, N.R. (1971b) Union Carbide Corporation,
Internal Project Report, 29 November 1971
Chancey, E.L. and Andrawes, N.R. (1972a) Union Carbide Corporation,
Internal Project Report, 21 July 1972
Chancey, E.L. and Andrawes, N.R. (1972b) Union Carbide Corporation,
Internal Project Report, 23 February 1972
Chancey, E.L., Andrawes, N.R. and Weiden, M.H.J. 1973 (Manuscript in
preparation)
Corneliussen, P.E. (1970) Pesticides Monit. J. 4(3): 89-105
Corneliussen, P.E. Pesticides Monit. J. 5(4): 313-328
Collins, T.F.X., Hansen, W.H. and Keeler, H.W. (1970) Effects of
carbaryl on reproduction of the rat and of the gerbil. Toxicol. Appl.
Pharmacol., 17: 273
Dieringer, C.S., Thomas, J.A. and Stitzel, R.E. (1973) Influence of
carbaryl on androgen metabolism in the prostate gland and liver. The
Pharmacologist, 15: 266
Dorough, H.W. and Wiggins, O.G.J. (1969) Econ. Entomol. 62: 49
Dorough, H.W.J. (1970) Agr. Food Chem. 18: 1015
Dorough, H.W. (1970) Continuous feeding of carbaryl-naphthyl-14C to
lactating cows. Progress Report, 6 January 1970
Dorough, H.W. (1971) Paper presented at the International Symposium on
Pesticide Terminal Residues, Tel Aviv, Israel, 17-19 February 1971
Dorough, H.W., Mehendale, H.M. and Lin, T. (1972) Modification of
carbaryl metabolism in rats with monoamine oxidase inhibitors. J.
Econ. Entomol. 65(4): 958-962
Dougherty, W., Golberg, L. and Coulston, F. (1973) The effect of
carbaryl on reproduction in rhesus monkeys. Unpublished report from
the Institute of Experimental Pathology and Toxicology, Albany Medical
College, United States of America
Elkins, E.R., Lamb, F.C., Farrow, R.P., Cook, R.W., Kawai, M. and
Kimball, J.R.J. (1968) Agr. Food Chem. 16: 962
Elkins, E.R., Farrow, R.P. and Kim, E.S.J. (1972) Agr. Food Chem.
20: 286
FAO/WHO, (1970b) 1969 Evaluation of Some Pesticide Residues in Food.
FAO/PL: 1969/M/17/1; WHO/Food Add./70.38
Farrow, R.P., Lamb, F.C., Cook, R.W., Kimball, J.R. and Elkins, E.R.J.
(1968) Agr. Food Chem. 16: 65
Farrow, R.P., Lamb, F.C., Elkins, E.R., Cook, R.W., Kawai, M. and
Cortes, A.J. (1969) Agr. Food Chem. 17: 75
Fukuto, T.R. (1972) Drug Metab. Revs. 1: 117
Hajkina, B.I. (1970) The mechanism of effect of carbaryl on warm
blooded animals. Gig. prim. toks. pesticid. i, klinika otravl. p. 203
Hassan, A. (1971) Pharmacological effects of carbaryl-I. The effect of
carbaryl on the synthesis and degradation of catecholamines in the
rat. Biochem. Pharmacol. 20(9): 2299-2308
Hoque, M.Z. (1972) Carbaryl - a new chemical mutagen. Curr. Sci.
41(23): 855-856
Homenko, N.P. (1971) Changes in the membrane potentiality of the
motoneurons of the spinal cord under the effect of carbaryl and
chlorfos. Fiziologja, 17(4); 527-528
Jakushko, V.E. (1971) Peculiarities of the glycosis and its regulation
on the cell structure of the cerebrum under the effect of DOT and
carbaryl. Farmakologija i toksikologija, reap. Megived, sb. 6:
187-190
Kagan, Ju. S., Radionev, G.A. and Voroneva, L.Ja. (1970) The effect of
Sevin in animal experiments on the function and structure of liver,
Farmakologija i toksikologija, 33(2): 219-224
Kuhr, R.J.J. (1968) Sci. Food Agric. Suppl. 44
Lamb, F.C., Farrow, R.P., Elkins, E.R., Kimball, J.R. and Cook, R.W.
(1968) J. Agr. Food Chem. 16: 967
Lillie, R.J. (1973) Studies on the reproductive performance and
progeny performance of caged White LeShorns fed malathion and
carbaryl. Poultry Sci. 52(1): 266-272
Locks, R.K. (1972a) Possible in vitro production of N-O-conjugates,
of N-hydroxyearbaryl from carbaryl by tobacco cells in culture. Fed.
Proc. Fed. Am. Soc. Exp. Biol. 31(2): 520
Locks, R.K. (1972b) Thin layer chromatography of l-naphthyl-N
hydroxy-N-methylearbamate and its application in two in vitro
studies involving carbaryl. J. Agr. Food Chem. 20: 1078
Lucier, G.W., McDaniel, O.S., Williams, C. and Klein, R. (1972)
Effects of chlordane and methylmercury on metabolism of carbaryl and
carbofuran in rats. Pest Biochem. Physiol. 2(2): 244-255
Mandzhgaladze, R.N. and Vashakidze, V.I. (1972) Deystviyo
khimicheskikh soyedineniy na potomstvo i razvit iye priznakov pola.
[Action of some chemical compounds on offspring and sexual
characteristics of rats.] Soobsbeh. Akad. Nauk Gruz. SSR, 65(2):
485-488
Moriyama, H., Sugiyama, H. and Shigematsu, H. (1972) A hydroxy
metabolite derived from carbaryl in the silkworm, Bombyx mori. Pest
Biochem. Physiol. 2(1): 1
Natoff, I.L. and Reiff, B. (1973) Effect of oximes on the acute
toxicity of anticholinesterase carbamates. Toxicol. Appl. Pharmacol.
25: 569-575
Paulson, G.D. and Feil, V.J. (1969) Poultry Sci. 48: 1593
Pokas, J.C. (1971) Intestinal metabolism and transport of naphthyl
N-methyl-carbamate in vitro (rat). Amer. J. Physiol. 220(6):
2008-2012
Pekas, J.C. and Paulson, G.D. (1970) Intestinal hydrolysis and
conjugation of a pesticidal carbamate in vitro. Science,
170(3953): 77-78
Rappoport, M.B. (1969) Microscopic and ultrastructural changes of the
thyroid gland under the effect of the insecticide carbaryl. Vrachebnoe
delo, 5: 102-105
Richey, F.A., jr, Bartley, W.J., Fitzpatrick, J.T. and Kurtz, A.P.
(1972) Chemical synthesis of the carbaryl metabolite
trans-5,6-dihydro-5,6-dihydroxy-l-naphthyl methyl-carbamate. J. Agr.
Food Chem. 20(4): 825-828
Ryan, A.J. (1971) CRC Critical Reviews in Toxicology, September 1971,
33
Santolucito, J. A., Whitcomb, E. and Hassan, A. (1972) Pharmacological
effects of carbaryl. Ill. Observations on alteration of smooth
muscle-preparation responses to norepinephrine. Environ. Physiol.
Biochem. 2(3): 142-146
Shah, A.H. and Guthrie, F.E. (1971) In vitro metabolism of
insecticides during midgut penetration. Pest Biochem. Physiol. 1(1):
1-10
Shtenberg, A.I. and Orlova, N.V. (1970) Contribution to the mechanism
of toxic action on Sevin, a pesticide of the carbamate group. Vestn.
Akad. Mod. Nauk SSR, 72-76
Sideroff, S.I. and Santolucito, J.A. (1972) Behavioural and
physiological effects of the cholinesterase inhibitor carbaryl
(I-naphthyl methylearbamate). Physiol. and Behav. 9: 459-462
Stenberg, A.M. (1970) The effect of carbaryl on the status of the
thyroid gland of mice receiving artificial or full value diet with
different content of iodine. Gigiena i sanitarija, 8: 119
Stenberg, A.I. and Otovan, M.B. (1971) Effect of small doses of
carbaryl on the reproductive function of animals in a number of
offsprings. Voprosi pitanija, 30(1): 41-49
Strother, A. (1972) In vitro metabolism of methyl carbamate
insecticides by human and rat liver fractions. Toxicol. Appl.
Pharmacol. 21(1): 112-129
Sullivan, L.J., Eldridge, J.M., Knaak, J.B. and Tallant, M.J. (1972)
5,6-dihydro-5,6-dihydroxy carbaryl glucuronide as a significant
metabolite of carbaryl in a rat. J. Agr. Food Chem. 20: 980-985
Tallant, M.J. (1970) Mellon Institute, Special Report 33-100, 20
October 1970
Thomas, J.A., Schein, L.G. and Dieringer, C.S. (1973) Distribution of
radio-activity in male reproductive organs after the administration
either carbaryl-14C or DDT-3H. The Pharmacologist, 15: 227
Trifonova, T.K., Gladenko, I.N. and Shulyak, V.D. (1970) Deystviye
gamma-izomera GKhTsGi sevina na polovuyu funktsivu. [Effect of
gamma-BHC and Sevin on reproduction] Veterinariya (Moscow), 47(6):
91-93
Union Carbide (1973) Patterns of use for Sevin(R) carbaryl
insecticide. Summary filed with FAO 1 March 1973
Union Carbide (1970) Summary of carbaryl residues. Section V (Private
communication)
Vashakidze, V.I. (1970) The effect of carbaryl on the spermatogenesis
of albino mice. Sbornik trudov Instituta gigiena truda i prof.
zabolevanija, Gruz SSR, 12: 285-288
Weil, C.S., Woodside, M.D., Bernard, J.B., Condra, N.I. and Carpenter,
C.P. (1972a) Studies on rat reproduction and guinea pig teratology of
carbaryl fed in the diet vs stomach tube. Toxicol. Appl. Pharmacol.
22: 318
Weil, C.S., Woodside, M.D., Carpenter, C.P. and Smyth, H.F., jr.
(1972b) Current status of tests of carbaryl for reproductive and
teratogenic effect. Toxicol. Appl. Pharmacol. 21: 390-404
Wiggins, O.G. and Weiden, M.H.J. (1969a) Union Carbide Corporation,
Internal Project Report, 30 June 1969
Wiggins, O.G. and Weiden, M.H.J. (1969b) Union Carbide Corporation,
Internal Project Report, 1 July 1969
Wiggins, O.G., Weiden, M.H.J., Weil, C.S. and Carpenter, C.P. (1970)
Paper presented at the VII International Congress of Plant Protection,
Paris, September 1970
 Potential transfer of residues to meat, milk and eggs
(a) In dairy animals
After oral administration of single does of
1-naphthyl-14C-carbaryl at levels of 0.25 and 3.05 mg/kg,
approximately 0.35% of each dose was detected in the milk (Dorough,
1970). Maximum concentrations were found in samples taken six hours
after dosing which, following the two treatments, were 0.063 and
0.95 ppm, respectively.
In another study, 1-naphthyl-14C-carbaryl was fed to lactating
cows at levels of 0.15, 0.43 and 1.35 mg/kg bw (equivalent to 10, 30
and 100 ppm in the feed) for 14 days (Dorough, 1971). Equilibrium
between intake and elimination was reached within two days of
initiation of the treatment. At each feeding level, approximately 0.2%
of the dose was secreted in the milk. The concentration of total
14C-carbaryl equivalents in the milk was 1/400 of that in the diet.
Most of the 14C-residues (about 90%) were in the aqueous phase. Milk
metabolites and their concentrations after feeding 100 ppm of
l-naphthyl-14C-carbaryl for 14 days are shown in Table 12.
Continuous feeding of l-naphthyl-14C-carbaryl to cows and a
single oral dose of the same material demonstrated that carbaryl
residues do not accumulate in the body tissues (Dorough, 1971).
Furthermore, a good correlation existed between the level of pesticide
fed and that which appeared in the tissues. The distribution of
residues in different tissues and organs of cows receiving carbaryl in
their feed is shown in Table 13, while Table 14 shows the nature of
residues found in various samples.
(b) In poultry and eggs
Following administration of l-naphthyl-14C-carbaryl to hens,
total 14C-residues reached a maximum and dissipated at a much faster
rate in egg white than in egg yolk. In a single dose of 10 mg/kg
(Paulson and Foil, 1969), maximum concentration of 14C-residues in
egg white was 0.12 ppm at one day and dropped to trace amounts on the
second day after treatment. The yolk residues reached a maximum at the
fifth day (0.36 ppm) and had dissipated by the ninth (0.03 ppm). Under
continuous feeding conditions, the total residue in the yolk or white
at each sampling time was dosage related (Andrawes et al., 1972).
Concentration of 14C-carbaryl equivalents (ppm) reached a maximum
(0.10 ppm from 70 ppm in feed; 0.025 ppm from 21 ppm in feed) in the
white after 2-6 days and in the yolk (1.0 ppm from 70 ppm in feed;
0.30 ppm from 21 ppm in feed) after 6-9 days of dosing and remained
level until the end of the treatment period. At plateau levels, the
level of 14C-carbaryl equivalents in the white was one-tenth that in
the yolk; however, the total equivalents were in a ratio of 5:1
between yolk and white. The ratio of the concentration of carbaryl in
whole eggs (white and yolk) to that in the diet was 0.006 at
equilibration. After discontinuation of dosing, residues in the whites
had a half-life of less than one day; for yolk residues the half-life
was approximately 2-3 days. The nature of the metabolites found in
eggs is shown in Table 15.
TABLE 12. CHEMICAL NATURE OF CARBARYL METABOLITES IN COW'S MILK AND THEIR
AVERAGE CONCENTRATIONS AFTER FEEDING WITH 1-NAPHTHYL-14C-
CARBARYL AT LEVEL EQUIVALENT TO 100 ppm IN THE DIET FOR 14 DAYSa
Metabolites ppm in % of
milk total
Carbaryl 17 6
3,4-dihydro-3,4-dihydroxy-l
naphthyl methylearbamate 13 5
5,(3-dihydro-5,6-dihydroxy-l
naphthyl methylcarbamate 94 34
5-hydroxy-l-naphthyl methyl
carbamate 3 1
5,6-dihydro-5,6-dihydroxy-l
naphthol 9 3
l-naphthyl sulfate 72 26
1-methoxy-5-(methylearbamoyloxy)-
2-naphthyl sulfate 63 23
5-methoxy-1,6-naphthalenediol 7 2
a Reference - Dorough, 1971.
TABLE 13. TOTAL CARBARYL-14C EQUIVALENTS IN TISSUES OF COWS FED
CARBARYL-NAPHTHYL-14C FOR 14 DAYS AT RATES OF 10, 30 AND
100 ppm IN THE DIETa
ppm carbaryl-14C equivalents at feeding level of
Tissues
10 ppm 30 ppm 100 ppm
Kidney 0.095 0.531 1.003
Liver 0.033 0.100 0.411
Lung 0.020 0.064 0.207
TABLE 13. (Cont'd.)
ppm carbaryl-14C equivalents at feeding level of
Tissues
10 ppm 30 ppm 100 ppm
Muscle 0.009 0.031 0.104
Heart 0.012 0.038 0.095
Fat 0.000 0.015 0.025
Blood 0.008 0.036 0.141
a Cows were slaughtered 18 hours after the last dose was given.
TABLE 14. RADIO-ACTIVE RESIDUES IN TISSUES OF A COW AFTER BEING FED 100 ppm CARBARYL-14C
IN THE DIET FOR 14 DAYSa
% of total radio-activity in sample
Metabolites
Kidney Liver Lung Muscle Heart Blood
Carbaryl 3.3 9.2 2.1 17.0 3.7 0
5,6-dihydrodihydroxy carbaryl 4.5 3.0 8.8 38.6 31.3 22.0
5,6-dihydrodihydroxy naphthol 1.8 4.1 0 0 4.9 2.0
Naphthyl sulfate 29.3 4.1 27.3 0 4.0 51.8
Water-soluble unknowns 43.2 32.9 47.5 30.6 41.8 7.1
Unextractable unknowns 17.9 46.7 14.3 13.8 14.3 17.1
a cows were slaughtered at 18 hours after the last dose was given.
The distribution of carbaryl residues was determined in hen
tissues after continuous treatment with either 7, 21 or 70 ppm of
l-naphthyl-14C-carbaryl in the diet (Andrawes et al., 1972). Tissue
residues were directly proportional to the concentration of carbaryl
in the diet. The highest residues were found in the blood and tissues
of high blood content (liver, kidney, lung and spleen); body fat,
TABLE 15. METABOLIC PRODUCTS FOUND IN THE EGGS OF HENS FED 1-NAPHTHYL-14C-CARBARYL
FOR 14 DAYS AT A LEVEL EQUIVALENT TO 70 ppm IN THE DIETa
% of the recovered
radio-activity
Chromatographic Identity
fractions Yolk(Y) White(W) Y + W
F1 1-naphthol 17.74 6.25 15.76
F2 Carbaryl 4.59 0.73 3.92
F3 1-naphthyl(hydroxymethyl)-carbamate 4.88 0.15 4.06
S1 - 2.15 3.46 2.38
S2 Unknown A 3.07 8.42 3.99
S3 Unknown B 6.97 46.32 13.70
S4 1-naphthol conjugate 2.73 5.63 3.23
S5 1-naphthyl sulfate 44.05 15.59 39.14
S6 1-naphthol conjugate 5.03 5.68 5.14
S7 Unknown B conjugate 8.79 7.77 8.62
Average total µg of 14C-carbaryl equivalents per eggb 19.7 3.4 19.7
Average ppm of 14C-carbaryl equivalentsb 0.4 0.1 0.4
a Reference -Paulson and Feil, 1969.
b Based on eggs collected after equilibration was established; i.e. between the ninth
and the fourteenth day of dosing.
brain and muscles contained the lowest residues. For example, the
distribution of 14C-carbaryl equivalents one day after treatment for
14 days with 70 ppm in the diet was as follows (in ppm): liver 0.41,
kidney 0.485, thigh 0.03, leg 0.032, breast 0.031, skin 0.043, fat
0.026, gizzard 0.04, heart 0.049, and brain 0.017. The half-life of
total body residues was calculated to be five days.
Comparison of plant and animal metabolites
For the most part, the primary metabolic pathway of carbaryl
metabolism in plants is similar to that found in animals. Recognizable
divergences between plant and animal metabolites are as follows: (1)
conjugation of the primary metabolites in plants yields glycosides
(Casida and Lykken, 1969; Fukuto, 1972; Kuhr, 1968) as compared to
glucuronides, sulfates and pre-mercapturic acids in animals (Dorough,
1970; Fukuto, 1972; Ryan, 1971); (2) the metabolite 7-hydroxy-l-
naphthyl methylearbamate has been detected only in certain plants
(Wiggins et al., 1970) but not in animals; (3) animal metabolites
which are not reported for plants include:
3,4-dihydro-3,4-dihydroxy-1-naphthyl methylearbamate,
3,4-dihydro-3,4-dihydroxy-1-naphthol, 5,6-dihydroxy-l-naphthyl
methylearbamate, 5,6-dihydroxy-1-naphthol,
1-methoxy-5(methylearbamoyloxy)-2-naphthol, and
5-methoxy-1,6-naphthalenediol (Dorough, 1970).
The efficiency and rate of excretion of carbaryl plant
metabolites when fed to rats have been investigated. It was found that
a mixture of radio-labelled water-soluble plant metabolites were
totally eliminated within 96 hours (Dorough and Wiggins, 1969). No
change in the metabolic profile was observed in the excretion
products. In a study conducted on the feeding of plant mare containing
radio-activity designated as unextracted LIC-residues, it was found
that this/these material(s) is/are poorly absorbed by the rat and
is/are excreted primarily through the faeces (Tallent, 1970; Andrawes,
1973).
In storage and processing
No information has been received on the disappearance of residues
during storage and processing of cocoa beans and derived products or
of cereals. However, data on the behaviour of carbaryl residues on
other commodities make it appear probable that: (1) storage would have
little effect on residues; (2) washing, heating, cooking or baking
would likely reduce levels by a substantial amount.
Carbaryl residue degradation and removal during commercial and
home preparative procedures have been determined for green beans
(Elkins et al., 1968), tomatoes (Farrow et al., 1968), spinach (Lamb
et al., 1968), broccoli (Farrow et al., 1969), and spinach and
apricots (Elkins et al., 1972). In general, washing (cold water),
peeling (tomatoes), blanching and cooking were very effective in
removing 50-99% of initial residues. Combinations of these operations
were more effective than single steps. Commercial canning of spinach
and apricots destroyed 44% and 12% of initial residues respectively.
Preprocessing storage of green beans at 45°F and tomatoes at 55°F, and
storage of canned spinach and apricots at ambient temperatures and
100°F had little effect on residues except for canned spinach at 100°F
where a 23% reduction was noted.
Evidence of residues in food in commerce or at consumption
Results of the fifth year (June 1968-April 1969) and sixth year
(June 1969-April 1970) total diet studies of the United States Food
and Drug Administration showed a continuation of the downward trend in
detectable carbaryl residues (Corneliussen, 1970; Corneliussen, 1972).
Carbaryl was detected in three composites in the period 1968-1969. Two
results (in legume vegetables) were below the method sensitivity level
of 0.2 ppm. One fruit composite had 0.3 ppm. Carbaryl was not detected
in any of the diet composites during the 1969-1970 period.
Methods of residue analysis
In spite of vigorous research in recent years to develop a residue
method for carbaryl utilizing gas chromatography, the current method
of choice for regulatory purposes remains the colorimetric procedure
described in the official AOAC method (Official Methods of Analysis
of the AOAC, 11th ed., 1970, p. 493). This method has recently been
extended by the Union Carbide Co. to include determination of the
major carbaryl plant metabolites (total toxic residues). Procedures
have been developed to determine free carbaryl, combined carbaryl, and
the conjugated metabolites 1-naphthol and methylol carbaryl. Of the
known plant metabolites, methylol carbaryl and naphthol are closest in
toxicity to the parent carbaryl. Methylol carbaryl is also either the
major metabolite or a significant metabolite in the plants
investigated. No method yet exists for determining the animal
metabolites such as 5,6-dihydro-5,6-dibydroxy-1-naphthyl methyl
carbamate or water-soluble unknowns (Union Carbide, 1973).
National tolerances
TABLE 16. SUMMARY OF USA CARBARYL TOLERANCES AND LIMITATIONS
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Alfalfa 100 1.6 None
Almonds, shelled 1 8 None
Almond hulls 40 8 None
Apples 10 12 1 day
Apricots 10 8 3 days
Asparagus 10 2 1 day
Bananas 10 1.1 None
Beans 10 2.125 None
Beets, roots 5 2 3 days
Beets, tops 12 2 14 days
Blackberries 12 2 7 days
Blueberries 10 2 None
Boysenberries 12 2 7 days
Broccoli 10 2 3 days
TABLE 16. (cont'd)
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Brussels sprouts 10 2 3 days
Cabbage 10 2 3 days
Cabbage (Chinese) 10 2 14 days
Carrots 10 2 None
Cauliflower 10 2 3 days
Cherries 10 6 1 day
Citrus 10 1.25/100 gal 5 days
Clover 100 1.5 None
Collards 12 2 14 days
Corn forage 100 2 None
Corn kernels 5 3 None
Cotton seed 5 2.5 None
Cotton, forage 100 2.5 None
Cowpeas 5 2 None
Cowpea forage 100 2 None
Cranberries 10 4 1 day
Cucumbers 10 1 None
Dandelion 12 2 14 days
Dewberries 12 2 7 days
Eggplant 10 4 None
Endive (escarole) 10 2 14 days
Filberts, shelled 1 5 None
Grapes 10 3 None
Grapefruit 10 1.25/100 gal 5 days
Grass and hay 100 1.5 None
Horseradish 5 2 3 days
Kale 12 2 14 days
Kohlrabi 10 2 3 days
Lettuce (head) 10 2 3 days
Lettuce (leaf) 10 2 14 days
Loganberries 12 2 7 days
Melons 10 1 None
Mustard greens 12 2 14 days
Nectarines 10 8 3 days
Okra 10 2 None
Olives 10 8 None
Parsley 12 2 14 days
Parsnips 5 2 3 days
Peaches 10 8 1 day
Peanuts, nut and hull 5 1.5 None
Peanut hay 100 1.5 None
Pears 10 12 1 day
Peas and pods 10 2.6 None
Peavine forage 100 2.6 None
Pecans, shelled 1 3 None
Peppers 10 4 None
Plums 10 6 1 day
TABLE 16. (cont'd)
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Potatoes 0.5 interim 2 None
Prunes 10 6 1 day
Pumpkins 10 1 None
Radishes 5 2 3 days
Raspberries 12 2 7 days
Rice 5 2 14 days
Rice straw 100 2 14 days
Rutabagas 5 2 3 days
Salsify roots 5 2 3 days
Salsify tops 10 2 14 days
Sorghum grain 10 2 21 days
Sorghum forage 100 2 None
Soybeans 5 1.5 None
Soybean hay 100 1.5 None
Spinach 12 2 14 days
Squash 10 1 None
Strawberries 10 2 1 day
Sugar beet and tops 100 2 14 days
Swiss chard 12 2 14 days
Tobacco NF 0.6-24 None
Tomatoes 10 4 None
Turnips 5 2 3 days
Turnip tops 12 14 days
Walnuts, nuts 10 5 None
Poultry, meat and fat 5 0.25a 7 days
Poultry eggs 0.5 interim 0.25a 7 days
a Denotes lb ai/100 birds.
Appraisal
Carbaryl is extensively used around the world for control of
insect pests on a wide variety of agricultural crops, ornamentals,
turf, forests, livestock and poultry. Uses are increasing as it is
often selected as a replacement for the persistent organochlorine
insecticides.
Data available on residues in root crop vegetables (except
potatoes) from supervised trials at recommended rates and pre-harvest
intervals indicated that residues should not exceed 2 ppm if good
agricultural practice is followed.
The results of supervised trials on peanuts (groundnuts),
soybeans, sorgbum grain and cowpeas show that residues of up to 2 ppm
could occur on peanuts, up to 1 ppm on soybeans and cowpeas, and up to
24 ppm (rarely) on sorghum grain. On sorghum grain, a tolerance of 10
ppm would be sufficient to provide for the more nearly average residue
of 5 ppm.
Residues on sugar beet roots did not exceed 0.1 ppm at harvest in
supervised trials. Sugar beet tops had residues up to 3.6 ppm in the
same tests.
Field trials on the forage crops, alfalfa, clovers, corn forage,
cowpea foliage, grasses, peanut hay, sorghum forage and soybean
foliage, resulted in maximum residues ranging from 33 to 190 ppm when
treated the same day as harvest. Average residues ranged from 16 to
51 ppm.
The existing temporary tolerance of 3 ppm on cucurbits appears
questionable since data from supervised trials indicate that residues
greater than 5 ppm could occur in these crops with no pre-harvest
limitation. The recommendation was changed from 10 ppm to 3 ppm : in
1969 without giving supportive data.
In accord with the policy of expressing tolerances to one
significant digit (Report of the 1970 Joint FAO/WHO Meeting, 1971,
Wld Hlth Org. techn. Rep. Ser., No. 474, Section 2.13), the figure
for rice (rough) should be changed from 2.5 ppm to 3 ppm. It is
emphasized that this should not be construed as a change in the
tolerance but merely a numerical adjustment.
No information has been received on the disappearance of residues
during storage and processing of cocoa beans and derived products or
of cereals into cereal products. However, data on the behaviour of
carbaryl residues on other commodities make it appear probable that:
(1) storage would have little effect on residues; (2) washing,
heating, cooking or baking would likely reduce levels by a substantial
amount. Since the requirements for data on the disappearance of
residues during storage and processing of cocoa beans and of cereals
into cereal products were initiated in 1968 and no interested party
has responded, it seems reasonable to discontinue these requirements.
Feeding experiments with hens using radio-labelled carbaryl have
shown that residues in whole eggs are dose related, the ratio of
concentration (in eggs) to concentration in diet being 0.006. At a
diet level of 70 ppm of carbaryl, an average level of 0.4 ppm in eggs
(yolk plus white) was reached at equilibrium. After discontinuance of
feedingy residues decreased rapidly, the half-life in yolk being 2-3
days. A tolerance in eggs (shell free) of 0.5 ppm is recommended to
accommodate occasional residues in feed. The regulatory method of
analysis recommended can account for approximately 70% of the residues
(carbaryl, metabolites and their conjugates) in eggs.
Feeding studies with lactating cows using radio-labelled carbaryl
have shown that about 90% of the 14C-residues are found in the
aqueous phase and that at each feeding level approximately 0.2% of the
dose was secreted in the milk. The major metabolites found were
l-naphthyl sulfate (26%), 5,6-dihydro-5,6-dihydroxy-l-naphthyl
methylcarbamate (34%), and l-methoxy-5-(methylearbamoyloxy)-2-naphthyI
sulfate. Unchanged carbaryl was only 6% of the total residue. Since no
method of analysis is available for these compounds, no
recommendations for a tolerance can be made; however, the recommended
tolerance of 100 ppm on forage crops would give assurance that
residues in whole milk would not exceed 0.2 ppm. Since it is very
unlikely that any dairy animal would ever consume as much as 100 ppm
of unchanged carbaryl daily, actual milk residues would be negligibly
small.
Cows fed 100 ppm of 14C-carbaryl in the diet had 1 ppm carbaryl
equivalents in the kidney, 0.4 ppm in the liver, and 0.1 ppm in the
muscle (including heart). In kidney, 43% of the total radio-activity
was water-soluble unknowns, 30% was naphthyl sulfate, 18% was
unextractable, and 3% was carbaryl. In liver, 47% was unextractable,
33% was water-soluble unknowns, and 9% was carbaryl. In muscle, 39%
was 5,6-dihydrodihydroxycarbaryl, 31% was water-soluble unknowns, 14%
was unextractable, and 17% was carbaryl. On the basis of these
results, it would appear that only 13-30% of meat residues are in a
form that can be measured by the present analytical method. It is
therefore recommended that the tolerance for meat of cattle, goat and
sheep be reduced from 1 ppm to 0.2 ppm.
An analytical method suitable for regulatory use on crops, meat,
poultry and eggs has been developed.
RECOMMENDATIONS
Tolerances
The temporary tolerances previously recommended are replaced by
the following tolerances. The values represent the sum of free
carbaryl, combined carbaryl, conjugated naphthol and conjugated
methylol carbaryl expressed as total toxic residues of carbaryl.
Changes from 1970 recommendations are underlined; single
underline - new entry, double underline - re-entry of omitted
commodity or change in value based on new data.
ppm
Animal Feedstuffs (green) (alfalfa, clover, corn
forage, cowpea foliage, grasses, peanut hay,
sorghum forage, soybean foliage, sugar beet tops,
bean and pea vines 100
Apricots, blackberries, boysenberries, nectarines,
peaches, raspberries, asparagus, okra, leafy
vegetables (except brassica), nuts (whole),
olives (fresh), sorghum grain, cherries, plums 10
Blueberries, citrus fruit, cranberries, strawberries 7
Apples, bananas (pulp), grapes, beans, peas
(including pod), brassicas, tomatoes, peppers,
aubergines, pears, poultry skin 5
Cucurbits (including melons), rice (rough) 3
Root crop vegetables (beets, carrots, radishes,
rutabegas, parsnips), peanuts (groundnuts, whole) 2
Cotton seed (whole), sweet corn, (kernels), nuts
(shelled), olives (processed), soybeans (dry
mature seed), cowpeas 1
Poultry (total) (edible portions), eggs (shell free) 0.5
Potatoes, meat of cattle, sheep and goat, sugar beets 0.2
FURTHER WORK OR INFORMATION
Required (before a limit for residues in milk can be recommended)
1. A method suitable for regulatory purposes, for the determination
of total residues of carbaryl in milk.
Desirable
1. Further studies to elucidate the effects of carbaryl on renal
function.
2. Further studies to resolve the differences in observations of
different investigators on reproductive physiology, especially
with regard to nouro-endocrine and behavioural changes.
REFERENCES
Abou-Donia, M.B. and Dieckert, J.W. (1971) Gossypol: subcellular
localization and stimulation of rat liver microsomal oxidases.
Toxicol. Appl. Pharmacol. 18(3): 507-516
Andrawes, N.R. (1973) Metabolism of Sevin insecticide, Summary, 5 June
1973 (Private communication)
Andrawes, N.R. and Chancey, E.L. (1970) Union Carbide Corporation,
Internal Project Report, 2 November 1970
Andrawes, N.R., Chancey, E.L., Crabtree, R.J., Herret, R.A. and
Weiden, M.H.J. (1972) Fate of naphthyl carbaryl in laying chickens. J.
Agr. Food Chem. 20(3): 608-617
AOAC (1970) Official Methods of Analysis of the AOAC, 11th ed., 1970
p.493
Blazques, C.H., Vidyarthi, A.D., Sheehan, T.D., Bennet, M.J. and
McGrew, G.T. (1970) Effect of pinolene (B-pinene polymer) on carbaryl
foliar residues. J. Agr. Food Chem. 18(4): 681-684
Brzheskiy, V.V. (1972) K voprosu ob izuchenii mutagennykh svOxstv
insektitsida iz gruppy karbamatov-Sevina. [Study of the mutagenic
properties of Sevin (carbaryl).] Genetika, 8(6): 151-153
Casida, J.E. and Lykken, L.L. (1969) Ann. Rev. Plant Physiol. 20:
607
Casper, H.H. (1972) Diss. Abstracts, 32(11): 6599 B
Casper, H.H. and Pekas, J.C. (1971) Absorption and excretion of
radiolabeled 1-naphthyl-N-methylearbamate (carbaryl) by the rat. N.
Dak. Acad. Sci. 24(2): 160-166
Chancey, E.L. and Andrawes, N.R. (1971a) Union Carbide Corporation,
Internal Project Report, 27 October 1971
Chancey, E.L. and Andrawes, N.R. (1971b) Union Carbide Corporation,
Internal Project Report, 29 November 1971
Chancey, E.L. and Andrawes, N.R. (1972a) Union Carbide Corporation,
Internal Project Report, 21 July 1972
Chancey, E.L. and Andrawes, N.R. (1972b) Union Carbide Corporation,
Internal Project Report, 23 February 1972
Chancey, E.L., Andrawes, N.R. and Weiden, M.H.J. 1973 (Manuscript in
preparation)
Corneliussen, P.E. (1970) Pesticides Monit. J. 4(3): 89-105
Corneliussen, P.E. Pesticides Monit. J. 5(4): 313-328
Collins, T.F.X., Hansen, W.H. and Keeler, H.W. (1970) Effects of
carbaryl on reproduction of the rat and of the gerbil. Toxicol. Appl.
Pharmacol., 17: 273
Dieringer, C.S., Thomas, J.A. and Stitzel, R.E. (1973) Influence of
carbaryl on androgen metabolism in the prostate gland and liver. The
Pharmacologist, 15: 266
Dorough, H.W. and Wiggins, O.G.J. (1969) Econ. Entomol. 62: 49
Dorough, H.W.J. (1970) Agr. Food Chem. 18: 1015
Dorough, H.W. (1970) Continuous feeding of carbaryl-naphthyl-14C to
lactating cows. Progress Report, 6 January 1970
Dorough, H.W. (1971) Paper presented at the International Symposium on
Pesticide Terminal Residues, Tel Aviv, Israel, 17-19 February 1971
Dorough, H.W., Mehendale, H.M. and Lin, T. (1972) Modification of
carbaryl metabolism in rats with monoamine oxidase inhibitors. J.
Econ. Entomol. 65(4): 958-962
Dougherty, W., Golberg, L. and Coulston, F. (1973) The effect of
carbaryl on reproduction in rhesus monkeys. Unpublished report from
the Institute of Experimental Pathology and Toxicology, Albany Medical
College, United States of America
Elkins, E.R., Lamb, F.C., Farrow, R.P., Cook, R.W., Kawai, M. and
Kimball, J.R.J. (1968) Agr. Food Chem. 16: 962
Elkins, E.R., Farrow, R.P. and Kim, E.S.J. (1972) Agr. Food Chem.
20: 286
FAO/WHO, (1970b) 1969 Evaluation of Some Pesticide Residues in Food.
FAO/PL: 1969/M/17/1; WHO/Food Add./70.38
Farrow, R.P., Lamb, F.C., Cook, R.W., Kimball, J.R. and Elkins, E.R.J.
(1968) Agr. Food Chem. 16: 65
Farrow, R.P., Lamb, F.C., Elkins, E.R., Cook, R.W., Kawai, M. and
Cortes, A.J. (1969) Agr. Food Chem. 17: 75
Fukuto, T.R. (1972) Drug Metab. Revs. 1: 117
Hajkina, B.I. (1970) The mechanism of effect of carbaryl on warm
blooded animals. Gig. prim. toks. pesticid. i, klinika otravl. p. 203
Hassan, A. (1971) Pharmacological effects of carbaryl-I. The effect of
carbaryl on the synthesis and degradation of catecholamines in the
rat. Biochem. Pharmacol. 20(9): 2299-2308
Hoque, M.Z. (1972) Carbaryl - a new chemical mutagen. Curr. Sci.
41(23): 855-856
Homenko, N.P. (1971) Changes in the membrane potentiality of the
motoneurons of the spinal cord under the effect of carbaryl and
chlorfos. Fiziologja, 17(4); 527-528
Jakushko, V.E. (1971) Peculiarities of the glycosis and its regulation
on the cell structure of the cerebrum under the effect of DOT and
carbaryl. Farmakologija i toksikologija, reap. Megived, sb. 6:
187-190
Kagan, Ju. S., Radionev, G.A. and Voroneva, L.Ja. (1970) The effect of
Sevin in animal experiments on the function and structure of liver,
Farmakologija i toksikologija, 33(2): 219-224
Kuhr, R.J.J. (1968) Sci. Food Agric. Suppl. 44
Lamb, F.C., Farrow, R.P., Elkins, E.R., Kimball, J.R. and Cook, R.W.
(1968) J. Agr. Food Chem. 16: 967
Lillie, R.J. (1973) Studies on the reproductive performance and
progeny performance of caged White LeShorns fed malathion and
carbaryl. Poultry Sci. 52(1): 266-272
Locks, R.K. (1972a) Possible in vitro production of N-O-conjugates,
of N-hydroxyearbaryl from carbaryl by tobacco cells in culture. Fed.
Proc. Fed. Am. Soc. Exp. Biol. 31(2): 520
Locks, R.K. (1972b) Thin layer chromatography of l-naphthyl-N
hydroxy-N-methylearbamate and its application in two in vitro
studies involving carbaryl. J. Agr. Food Chem. 20: 1078
Lucier, G.W., McDaniel, O.S., Williams, C. and Klein, R. (1972)
Effects of chlordane and methylmercury on metabolism of carbaryl and
carbofuran in rats. Pest Biochem. Physiol. 2(2): 244-255
Mandzhgaladze, R.N. and Vashakidze, V.I. (1972) Deystviyo
khimicheskikh soyedineniy na potomstvo i razvit iye priznakov pola.
[Action of some chemical compounds on offspring and sexual
characteristics of rats.] Soobsbeh. Akad. Nauk Gruz. SSR, 65(2):
485-488
Moriyama, H., Sugiyama, H. and Shigematsu, H. (1972) A hydroxy
metabolite derived from carbaryl in the silkworm, Bombyx mori. Pest
Biochem. Physiol. 2(1): 1
Natoff, I.L. and Reiff, B. (1973) Effect of oximes on the acute
toxicity of anticholinesterase carbamates. Toxicol. Appl. Pharmacol.
25: 569-575
Paulson, G.D. and Feil, V.J. (1969) Poultry Sci. 48: 1593
Pokas, J.C. (1971) Intestinal metabolism and transport of naphthyl
N-methyl-carbamate in vitro (rat). Amer. J. Physiol. 220(6):
2008-2012
Pekas, J.C. and Paulson, G.D. (1970) Intestinal hydrolysis and
conjugation of a pesticidal carbamate in vitro. Science,
170(3953): 77-78
Rappoport, M.B. (1969) Microscopic and ultrastructural changes of the
thyroid gland under the effect of the insecticide carbaryl. Vrachebnoe
delo, 5: 102-105
Richey, F.A., jr, Bartley, W.J., Fitzpatrick, J.T. and Kurtz, A.P.
(1972) Chemical synthesis of the carbaryl metabolite
trans-5,6-dihydro-5,6-dihydroxy-l-naphthyl methyl-carbamate. J. Agr.
Food Chem. 20(4): 825-828
Ryan, A.J. (1971) CRC Critical Reviews in Toxicology, September 1971,
33
Santolucito, J. A., Whitcomb, E. and Hassan, A. (1972) Pharmacological
effects of carbaryl. Ill. Observations on alteration of smooth
muscle-preparation responses to norepinephrine. Environ. Physiol.
Biochem. 2(3): 142-146
Shah, A.H. and Guthrie, F.E. (1971) In vitro metabolism of
insecticides during midgut penetration. Pest Biochem. Physiol. 1(1):
1-10
Shtenberg, A.I. and Orlova, N.V. (1970) Contribution to the mechanism
of toxic action on Sevin, a pesticide of the carbamate group. Vestn.
Akad. Mod. Nauk SSR, 72-76
Sideroff, S.I. and Santolucito, J.A. (1972) Behavioural and
physiological effects of the cholinesterase inhibitor carbaryl
(I-naphthyl methylearbamate). Physiol. and Behav. 9: 459-462
Stenberg, A.M. (1970) The effect of carbaryl on the status of the
thyroid gland of mice receiving artificial or full value diet with
different content of iodine. Gigiena i sanitarija, 8: 119
Stenberg, A.I. and Otovan, M.B. (1971) Effect of small doses of
carbaryl on the reproductive function of animals in a number of
offsprings. Voprosi pitanija, 30(1): 41-49
Strother, A. (1972) In vitro metabolism of methyl carbamate
insecticides by human and rat liver fractions. Toxicol. Appl.
Pharmacol. 21(1): 112-129
Sullivan, L.J., Eldridge, J.M., Knaak, J.B. and Tallant, M.J. (1972)
5,6-dihydro-5,6-dihydroxy carbaryl glucuronide as a significant
metabolite of carbaryl in a rat. J. Agr. Food Chem. 20: 980-985
Tallant, M.J. (1970) Mellon Institute, Special Report 33-100, 20
October 1970
Thomas, J.A., Schein, L.G. and Dieringer, C.S. (1973) Distribution of
radio-activity in male reproductive organs after the administration
either carbaryl-14C or DDT-3H. The Pharmacologist, 15: 227
Trifonova, T.K., Gladenko, I.N. and Shulyak, V.D. (1970) Deystviye
gamma-izomera GKhTsGi sevina na polovuyu funktsivu. [Effect of
gamma-BHC and Sevin on reproduction] Veterinariya (Moscow), 47(6):
91-93
Union Carbide (1973) Patterns of use for Sevin(R) carbaryl
insecticide. Summary filed with FAO 1 March 1973
Union Carbide (1970) Summary of carbaryl residues. Section V (Private
communication)
Vashakidze, V.I. (1970) The effect of carbaryl on the spermatogenesis
of albino mice. Sbornik trudov Instituta gigiena truda i prof.
zabolevanija, Gruz SSR, 12: 285-288
Weil, C.S., Woodside, M.D., Bernard, J.B., Condra, N.I. and Carpenter,
C.P. (1972a) Studies on rat reproduction and guinea pig teratology of
carbaryl fed in the diet vs stomach tube. Toxicol. Appl. Pharmacol.
22: 318
Weil, C.S., Woodside, M.D., Carpenter, C.P. and Smyth, H.F., jr.
(1972b) Current status of tests of carbaryl for reproductive and
teratogenic effect. Toxicol. Appl. Pharmacol. 21: 390-404
Wiggins, O.G. and Weiden, M.H.J. (1969a) Union Carbide Corporation,
Internal Project Report, 30 June 1969
Wiggins, O.G. and Weiden, M.H.J. (1969b) Union Carbide Corporation,
Internal Project Report, 1 July 1969
Wiggins, O.G., Weiden, M.H.J., Weil, C.S. and Carpenter, C.P. (1970)
Paper presented at the VII International Congress of Plant Protection,
Paris, September 1970
Potential transfer of residues to meat, milk and eggs
(a) In dairy animals
After oral administration of single does of
1-naphthyl-14C-carbaryl at levels of 0.25 and 3.05 mg/kg,
approximately 0.35% of each dose was detected in the milk (Dorough,
1970). Maximum concentrations were found in samples taken six hours
after dosing which, following the two treatments, were 0.063 and
0.95 ppm, respectively.
In another study, 1-naphthyl-14C-carbaryl was fed to lactating
cows at levels of 0.15, 0.43 and 1.35 mg/kg bw (equivalent to 10, 30
and 100 ppm in the feed) for 14 days (Dorough, 1971). Equilibrium
between intake and elimination was reached within two days of
initiation of the treatment. At each feeding level, approximately 0.2%
of the dose was secreted in the milk. The concentration of total
14C-carbaryl equivalents in the milk was 1/400 of that in the diet.
Most of the 14C-residues (about 90%) were in the aqueous phase. Milk
metabolites and their concentrations after feeding 100 ppm of
l-naphthyl-14C-carbaryl for 14 days are shown in Table 12.
Continuous feeding of l-naphthyl-14C-carbaryl to cows and a
single oral dose of the same material demonstrated that carbaryl
residues do not accumulate in the body tissues (Dorough, 1971).
Furthermore, a good correlation existed between the level of pesticide
fed and that which appeared in the tissues. The distribution of
residues in different tissues and organs of cows receiving carbaryl in
their feed is shown in Table 13, while Table 14 shows the nature of
residues found in various samples.
(b) In poultry and eggs
Following administration of l-naphthyl-14C-carbaryl to hens,
total 14C-residues reached a maximum and dissipated at a much faster
rate in egg white than in egg yolk. In a single dose of 10 mg/kg
(Paulson and Foil, 1969), maximum concentration of 14C-residues in
egg white was 0.12 ppm at one day and dropped to trace amounts on the
second day after treatment. The yolk residues reached a maximum at the
fifth day (0.36 ppm) and had dissipated by the ninth (0.03 ppm). Under
continuous feeding conditions, the total residue in the yolk or white
at each sampling time was dosage related (Andrawes et al., 1972).
Concentration of 14C-carbaryl equivalents (ppm) reached a maximum
(0.10 ppm from 70 ppm in feed; 0.025 ppm from 21 ppm in feed) in the
white after 2-6 days and in the yolk (1.0 ppm from 70 ppm in feed;
0.30 ppm from 21 ppm in feed) after 6-9 days of dosing and remained
level until the end of the treatment period. At plateau levels, the
level of 14C-carbaryl equivalents in the white was one-tenth that in
the yolk; however, the total equivalents were in a ratio of 5:1
between yolk and white. The ratio of the concentration of carbaryl in
whole eggs (white and yolk) to that in the diet was 0.006 at
equilibration. After discontinuation of dosing, residues in the whites
had a half-life of less than one day; for yolk residues the half-life
was approximately 2-3 days. The nature of the metabolites found in
eggs is shown in Table 15.
TABLE 12. CHEMICAL NATURE OF CARBARYL METABOLITES IN COW'S MILK AND THEIR
AVERAGE CONCENTRATIONS AFTER FEEDING WITH 1-NAPHTHYL-14C-
CARBARYL AT LEVEL EQUIVALENT TO 100 ppm IN THE DIET FOR 14 DAYSa
Metabolites ppm in % of
milk total
Carbaryl 17 6
3,4-dihydro-3,4-dihydroxy-l
naphthyl methylearbamate 13 5
5,(3-dihydro-5,6-dihydroxy-l
naphthyl methylcarbamate 94 34
5-hydroxy-l-naphthyl methyl
carbamate 3 1
5,6-dihydro-5,6-dihydroxy-l
naphthol 9 3
l-naphthyl sulfate 72 26
1-methoxy-5-(methylearbamoyloxy)-
2-naphthyl sulfate 63 23
5-methoxy-1,6-naphthalenediol 7 2
a Reference - Dorough, 1971.
TABLE 13. TOTAL CARBARYL-14C EQUIVALENTS IN TISSUES OF COWS FED
CARBARYL-NAPHTHYL-14C FOR 14 DAYS AT RATES OF 10, 30 AND
100 ppm IN THE DIETa
ppm carbaryl-14C equivalents at feeding level of
Tissues
10 ppm 30 ppm 100 ppm
Kidney 0.095 0.531 1.003
Liver 0.033 0.100 0.411
Lung 0.020 0.064 0.207
TABLE 13. (Cont'd.)
ppm carbaryl-14C equivalents at feeding level of
Tissues
10 ppm 30 ppm 100 ppm
Muscle 0.009 0.031 0.104
Heart 0.012 0.038 0.095
Fat 0.000 0.015 0.025
Blood 0.008 0.036 0.141
a Cows were slaughtered 18 hours after the last dose was given.
TABLE 14. RADIO-ACTIVE RESIDUES IN TISSUES OF A COW AFTER BEING FED 100 ppm CARBARYL-14C
IN THE DIET FOR 14 DAYSa
% of total radio-activity in sample
Metabolites
Kidney Liver Lung Muscle Heart Blood
Carbaryl 3.3 9.2 2.1 17.0 3.7 0
5,6-dihydrodihydroxy carbaryl 4.5 3.0 8.8 38.6 31.3 22.0
5,6-dihydrodihydroxy naphthol 1.8 4.1 0 0 4.9 2.0
Naphthyl sulfate 29.3 4.1 27.3 0 4.0 51.8
Water-soluble unknowns 43.2 32.9 47.5 30.6 41.8 7.1
Unextractable unknowns 17.9 46.7 14.3 13.8 14.3 17.1
a cows were slaughtered at 18 hours after the last dose was given.
The distribution of carbaryl residues was determined in hen
tissues after continuous treatment with either 7, 21 or 70 ppm of
l-naphthyl-14C-carbaryl in the diet (Andrawes et al., 1972). Tissue
residues were directly proportional to the concentration of carbaryl
in the diet. The highest residues were found in the blood and tissues
of high blood content (liver, kidney, lung and spleen); body fat,
TABLE 15. METABOLIC PRODUCTS FOUND IN THE EGGS OF HENS FED 1-NAPHTHYL-14C-CARBARYL
FOR 14 DAYS AT A LEVEL EQUIVALENT TO 70 ppm IN THE DIETa
% of the recovered
radio-activity
Chromatographic Identity
fractions Yolk(Y) White(W) Y + W
F1 1-naphthol 17.74 6.25 15.76
F2 Carbaryl 4.59 0.73 3.92
F3 1-naphthyl(hydroxymethyl)-carbamate 4.88 0.15 4.06
S1 - 2.15 3.46 2.38
S2 Unknown A 3.07 8.42 3.99
S3 Unknown B 6.97 46.32 13.70
S4 1-naphthol conjugate 2.73 5.63 3.23
S5 1-naphthyl sulfate 44.05 15.59 39.14
S6 1-naphthol conjugate 5.03 5.68 5.14
S7 Unknown B conjugate 8.79 7.77 8.62
Average total µg of 14C-carbaryl equivalents per eggb 19.7 3.4 19.7
Average ppm of 14C-carbaryl equivalentsb 0.4 0.1 0.4
a Reference -Paulson and Feil, 1969.
b Based on eggs collected after equilibration was established; i.e. between the ninth
and the fourteenth day of dosing.
brain and muscles contained the lowest residues. For example, the
distribution of 14C-carbaryl equivalents one day after treatment for
14 days with 70 ppm in the diet was as follows (in ppm): liver 0.41,
kidney 0.485, thigh 0.03, leg 0.032, breast 0.031, skin 0.043, fat
0.026, gizzard 0.04, heart 0.049, and brain 0.017. The half-life of
total body residues was calculated to be five days.
Comparison of plant and animal metabolites
For the most part, the primary metabolic pathway of carbaryl
metabolism in plants is similar to that found in animals. Recognizable
divergences between plant and animal metabolites are as follows: (1)
conjugation of the primary metabolites in plants yields glycosides
(Casida and Lykken, 1969; Fukuto, 1972; Kuhr, 1968) as compared to
glucuronides, sulfates and pre-mercapturic acids in animals (Dorough,
1970; Fukuto, 1972; Ryan, 1971); (2) the metabolite 7-hydroxy-l-
naphthyl methylearbamate has been detected only in certain plants
(Wiggins et al., 1970) but not in animals; (3) animal metabolites
which are not reported for plants include:
3,4-dihydro-3,4-dihydroxy-1-naphthyl methylearbamate,
3,4-dihydro-3,4-dihydroxy-1-naphthol, 5,6-dihydroxy-l-naphthyl
methylearbamate, 5,6-dihydroxy-1-naphthol,
1-methoxy-5(methylearbamoyloxy)-2-naphthol, and
5-methoxy-1,6-naphthalenediol (Dorough, 1970).
The efficiency and rate of excretion of carbaryl plant
metabolites when fed to rats have been investigated. It was found that
a mixture of radio-labelled water-soluble plant metabolites were
totally eliminated within 96 hours (Dorough and Wiggins, 1969). No
change in the metabolic profile was observed in the excretion
products. In a study conducted on the feeding of plant mare containing
radio-activity designated as unextracted LIC-residues, it was found
that this/these material(s) is/are poorly absorbed by the rat and
is/are excreted primarily through the faeces (Tallent, 1970; Andrawes,
1973).
In storage and processing
No information has been received on the disappearance of residues
during storage and processing of cocoa beans and derived products or
of cereals. However, data on the behaviour of carbaryl residues on
other commodities make it appear probable that: (1) storage would have
little effect on residues; (2) washing, heating, cooking or baking
would likely reduce levels by a substantial amount.
Carbaryl residue degradation and removal during commercial and
home preparative procedures have been determined for green beans
(Elkins et al., 1968), tomatoes (Farrow et al., 1968), spinach (Lamb
et al., 1968), broccoli (Farrow et al., 1969), and spinach and
apricots (Elkins et al., 1972). In general, washing (cold water),
peeling (tomatoes), blanching and cooking were very effective in
removing 50-99% of initial residues. Combinations of these operations
were more effective than single steps. Commercial canning of spinach
and apricots destroyed 44% and 12% of initial residues respectively.
Preprocessing storage of green beans at 45°F and tomatoes at 55°F, and
storage of canned spinach and apricots at ambient temperatures and
100°F had little effect on residues except for canned spinach at 100°F
where a 23% reduction was noted.
Evidence of residues in food in commerce or at consumption
Results of the fifth year (June 1968-April 1969) and sixth year
(June 1969-April 1970) total diet studies of the United States Food
and Drug Administration showed a continuation of the downward trend in
detectable carbaryl residues (Corneliussen, 1970; Corneliussen, 1972).
Carbaryl was detected in three composites in the period 1968-1969. Two
results (in legume vegetables) were below the method sensitivity level
of 0.2 ppm. One fruit composite had 0.3 ppm. Carbaryl was not detected
in any of the diet composites during the 1969-1970 period.
Methods of residue analysis
In spite of vigorous research in recent years to develop a residue
method for carbaryl utilizing gas chromatography, the current method
of choice for regulatory purposes remains the colorimetric procedure
described in the official AOAC method (Official Methods of Analysis
of the AOAC, 11th ed., 1970, p. 493). This method has recently been
extended by the Union Carbide Co. to include determination of the
major carbaryl plant metabolites (total toxic residues). Procedures
have been developed to determine free carbaryl, combined carbaryl, and
the conjugated metabolites 1-naphthol and methylol carbaryl. Of the
known plant metabolites, methylol carbaryl and naphthol are closest in
toxicity to the parent carbaryl. Methylol carbaryl is also either the
major metabolite or a significant metabolite in the plants
investigated. No method yet exists for determining the animal
metabolites such as 5,6-dihydro-5,6-dibydroxy-1-naphthyl methyl
carbamate or water-soluble unknowns (Union Carbide, 1973).
National tolerances
TABLE 16. SUMMARY OF USA CARBARYL TOLERANCES AND LIMITATIONS
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Alfalfa 100 1.6 None
Almonds, shelled 1 8 None
Almond hulls 40 8 None
Apples 10 12 1 day
Apricots 10 8 3 days
Asparagus 10 2 1 day
Bananas 10 1.1 None
Beans 10 2.125 None
Beets, roots 5 2 3 days
Beets, tops 12 2 14 days
Blackberries 12 2 7 days
Blueberries 10 2 None
Boysenberries 12 2 7 days
Broccoli 10 2 3 days
TABLE 16. (cont'd)
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Brussels sprouts 10 2 3 days
Cabbage 10 2 3 days
Cabbage (Chinese) 10 2 14 days
Carrots 10 2 None
Cauliflower 10 2 3 days
Cherries 10 6 1 day
Citrus 10 1.25/100 gal 5 days
Clover 100 1.5 None
Collards 12 2 14 days
Corn forage 100 2 None
Corn kernels 5 3 None
Cotton seed 5 2.5 None
Cotton, forage 100 2.5 None
Cowpeas 5 2 None
Cowpea forage 100 2 None
Cranberries 10 4 1 day
Cucumbers 10 1 None
Dandelion 12 2 14 days
Dewberries 12 2 7 days
Eggplant 10 4 None
Endive (escarole) 10 2 14 days
Filberts, shelled 1 5 None
Grapes 10 3 None
Grapefruit 10 1.25/100 gal 5 days
Grass and hay 100 1.5 None
Horseradish 5 2 3 days
Kale 12 2 14 days
Kohlrabi 10 2 3 days
Lettuce (head) 10 2 3 days
Lettuce (leaf) 10 2 14 days
Loganberries 12 2 7 days
Melons 10 1 None
Mustard greens 12 2 14 days
Nectarines 10 8 3 days
Okra 10 2 None
Olives 10 8 None
Parsley 12 2 14 days
Parsnips 5 2 3 days
Peaches 10 8 1 day
Peanuts, nut and hull 5 1.5 None
Peanut hay 100 1.5 None
Pears 10 12 1 day
Peas and pods 10 2.6 None
Peavine forage 100 2.6 None
Pecans, shelled 1 3 None
Peppers 10 4 None
Plums 10 6 1 day
TABLE 16. (cont'd)
Tolerance Dosage Pre-harvest
Use ppm lb ai/acre limit, days
Potatoes 0.5 interim 2 None
Prunes 10 6 1 day
Pumpkins 10 1 None
Radishes 5 2 3 days
Raspberries 12 2 7 days
Rice 5 2 14 days
Rice straw 100 2 14 days
Rutabagas 5 2 3 days
Salsify roots 5 2 3 days
Salsify tops 10 2 14 days
Sorghum grain 10 2 21 days
Sorghum forage 100 2 None
Soybeans 5 1.5 None
Soybean hay 100 1.5 None
Spinach 12 2 14 days
Squash 10 1 None
Strawberries 10 2 1 day
Sugar beet and tops 100 2 14 days
Swiss chard 12 2 14 days
Tobacco NF 0.6-24 None
Tomatoes 10 4 None
Turnips 5 2 3 days
Turnip tops 12 14 days
Walnuts, nuts 10 5 None
Poultry, meat and fat 5 0.25a 7 days
Poultry eggs 0.5 interim 0.25a 7 days
a Denotes lb ai/100 birds.
Appraisal
Carbaryl is extensively used around the world for control of
insect pests on a wide variety of agricultural crops, ornamentals,
turf, forests, livestock and poultry. Uses are increasing as it is
often selected as a replacement for the persistent organochlorine
insecticides.
Data available on residues in root crop vegetables (except
potatoes) from supervised trials at recommended rates and pre-harvest
intervals indicated that residues should not exceed 2 ppm if good
agricultural practice is followed.
The results of supervised trials on peanuts (groundnuts),
soybeans, sorgbum grain and cowpeas show that residues of up to 2 ppm
could occur on peanuts, up to 1 ppm on soybeans and cowpeas, and up to
24 ppm (rarely) on sorghum grain. On sorghum grain, a tolerance of 10
ppm would be sufficient to provide for the more nearly average residue
of 5 ppm.
Residues on sugar beet roots did not exceed 0.1 ppm at harvest in
supervised trials. Sugar beet tops had residues up to 3.6 ppm in the
same tests.
Field trials on the forage crops, alfalfa, clovers, corn forage,
cowpea foliage, grasses, peanut hay, sorghum forage and soybean
foliage, resulted in maximum residues ranging from 33 to 190 ppm when
treated the same day as harvest. Average residues ranged from 16 to
51 ppm.
The existing temporary tolerance of 3 ppm on cucurbits appears
questionable since data from supervised trials indicate that residues
greater than 5 ppm could occur in these crops with no pre-harvest
limitation. The recommendation was changed from 10 ppm to 3 ppm : in
1969 without giving supportive data.
In accord with the policy of expressing tolerances to one
significant digit (Report of the 1970 Joint FAO/WHO Meeting, 1971,
Wld Hlth Org. techn. Rep. Ser., No. 474, Section 2.13), the figure
for rice (rough) should be changed from 2.5 ppm to 3 ppm. It is
emphasized that this should not be construed as a change in the
tolerance but merely a numerical adjustment.
No information has been received on the disappearance of residues
during storage and processing of cocoa beans and derived products or
of cereals into cereal products. However, data on the behaviour of
carbaryl residues on other commodities make it appear probable that:
(1) storage would have little effect on residues; (2) washing,
heating, cooking or baking would likely reduce levels by a substantial
amount. Since the requirements for data on the disappearance of
residues during storage and processing of cocoa beans and of cereals
into cereal products were initiated in 1968 and no interested party
has responded, it seems reasonable to discontinue these requirements.
Feeding experiments with hens using radio-labelled carbaryl have
shown that residues in whole eggs are dose related, the ratio of
concentration (in eggs) to concentration in diet being 0.006. At a
diet level of 70 ppm of carbaryl, an average level of 0.4 ppm in eggs
(yolk plus white) was reached at equilibrium. After discontinuance of
feedingy residues decreased rapidly, the half-life in yolk being 2-3
days. A tolerance in eggs (shell free) of 0.5 ppm is recommended to
accommodate occasional residues in feed. The regulatory method of
analysis recommended can account for approximately 70% of the residues
(carbaryl, metabolites and their conjugates) in eggs.
Feeding studies with lactating cows using radio-labelled carbaryl
have shown that about 90% of the 14C-residues are found in the
aqueous phase and that at each feeding level approximately 0.2% of the
dose was secreted in the milk. The major metabolites found were
l-naphthyl sulfate (26%), 5,6-dihydro-5,6-dihydroxy-l-naphthyl
methylcarbamate (34%), and l-methoxy-5-(methylearbamoyloxy)-2-naphthyI
sulfate. Unchanged carbaryl was only 6% of the total residue. Since no
method of analysis is available for these compounds, no
recommendations for a tolerance can be made; however, the recommended
tolerance of 100 ppm on forage crops would give assurance that
residues in whole milk would not exceed 0.2 ppm. Since it is very
unlikely that any dairy animal would ever consume as much as 100 ppm
of unchanged carbaryl daily, actual milk residues would be negligibly
small.
Cows fed 100 ppm of 14C-carbaryl in the diet had 1 ppm carbaryl
equivalents in the kidney, 0.4 ppm in the liver, and 0.1 ppm in the
muscle (including heart). In kidney, 43% of the total radio-activity
was water-soluble unknowns, 30% was naphthyl sulfate, 18% was
unextractable, and 3% was carbaryl. In liver, 47% was unextractable,
33% was water-soluble unknowns, and 9% was carbaryl. In muscle, 39%
was 5,6-dihydrodihydroxycarbaryl, 31% was water-soluble unknowns, 14%
was unextractable, and 17% was carbaryl. On the basis of these
results, it would appear that only 13-30% of meat residues are in a
form that can be measured by the present analytical method. It is
therefore recommended that the tolerance for meat of cattle, goat and
sheep be reduced from 1 ppm to 0.2 ppm.
An analytical method suitable for regulatory use on crops, meat,
poultry and eggs has been developed.
RECOMMENDATIONS
Tolerances
The temporary tolerances previously recommended are replaced by
the following tolerances. The values represent the sum of free
carbaryl, combined carbaryl, conjugated naphthol and conjugated
methylol carbaryl expressed as total toxic residues of carbaryl.
Changes from 1970 recommendations are underlined; single
underline - new entry, double underline - re-entry of omitted
commodity or change in value based on new data.
ppm
Animal Feedstuffs (green) (alfalfa, clover, corn
forage, cowpea foliage, grasses, peanut hay,
sorghum forage, soybean foliage, sugar beet tops,
bean and pea vines 100
Apricots, blackberries, boysenberries, nectarines,
peaches, raspberries, asparagus, okra, leafy
vegetables (except brassica), nuts (whole),
olives (fresh), sorghum grain, cherries, plums 10
Blueberries, citrus fruit, cranberries, strawberries 7
Apples, bananas (pulp), grapes, beans, peas
(including pod), brassicas, tomatoes, peppers,
aubergines, pears, poultry skin 5
Cucurbits (including melons), rice (rough) 3
Root crop vegetables (beets, carrots, radishes,
rutabegas, parsnips), peanuts (groundnuts, whole) 2
Cotton seed (whole), sweet corn, (kernels), nuts
(shelled), olives (processed), soybeans (dry
mature seed), cowpeas 1
Poultry (total) (edible portions), eggs (shell free) 0.5
Potatoes, meat of cattle, sheep and goat, sugar beets 0.2
FURTHER WORK OR INFORMATION
Required (before a limit for residues in milk can be recommended)
1. A method suitable for regulatory purposes, for the determination
of total residues of carbaryl in milk.
Desirable
1. Further studies to elucidate the effects of carbaryl on renal
function.
2. Further studies to resolve the differences in observations of
different investigators on reproductive physiology, especially
with regard to nouro-endocrine and behavioural changes.
REFERENCES
Abou-Donia, M.B. and Dieckert, J.W. (1971) Gossypol: subcellular
localization and stimulation of rat liver microsomal oxidases.
Toxicol. Appl. Pharmacol. 18(3): 507-516
Andrawes, N.R. (1973) Metabolism of Sevin insecticide, Summary, 5 June
1973 (Private communication)
Andrawes, N.R. and Chancey, E.L. (1970) Union Carbide Corporation,
Internal Project Report, 2 November 1970
Andrawes, N.R., Chancey, E.L., Crabtree, R.J., Herret, R.A. and
Weiden, M.H.J. (1972) Fate of naphthyl carbaryl in laying chickens. J.
Agr. Food Chem. 20(3): 608-617
AOAC (1970) Official Methods of Analysis of the AOAC, 11th ed., 1970
p.493
Blazques, C.H., Vidyarthi, A.D., Sheehan, T.D., Bennet, M.J. and
McGrew, G.T. (1970) Effect of pinolene (B-pinene polymer) on carbaryl
foliar residues. J. Agr. Food Chem. 18(4): 681-684
Brzheskiy, V.V. (1972) K voprosu ob izuchenii mutagennykh svOxstv
insektitsida iz gruppy karbamatov-Sevina. [Study of the mutagenic
properties of Sevin (carbaryl).] Genetika, 8(6): 151-153
Casida, J.E. and Lykken, L.L. (1969) Ann. Rev. Plant Physiol. 20:
607
Casper, H.H. (1972) Diss. Abstracts, 32(11): 6599 B
Casper, H.H. and Pekas, J.C. (1971) Absorption and excretion of
radiolabeled 1-naphthyl-N-methylearbamate (carbaryl) by the rat. N.
Dak. Acad. Sci. 24(2): 160-166
Chancey, E.L. and Andrawes, N.R. (1971a) Union Carbide Corporation,
Internal Project Report, 27 October 1971
Chancey, E.L. and Andrawes, N.R. (1971b) Union Carbide Corporation,
Internal Project Report, 29 November 1971
Chancey, E.L. and Andrawes, N.R. (1972a) Union Carbide Corporation,
Internal Project Report, 21 July 1972
Chancey, E.L. and Andrawes, N.R. (1972b) Union Carbide Corporation,
Internal Project Report, 23 February 1972
Chancey, E.L., Andrawes, N.R. and Weiden, M.H.J. 1973 (Manuscript in
preparation)
Corneliussen, P.E. (1970) Pesticides Monit. J. 4(3): 89-105
Corneliussen, P.E. Pesticides Monit. J. 5(4): 313-328
Collins, T.F.X., Hansen, W.H. and Keeler, H.W. (1970) Effects of
carbaryl on reproduction of the rat and of the gerbil. Toxicol. Appl.
Pharmacol., 17: 273
Dieringer, C.S., Thomas, J.A. and Stitzel, R.E. (1973) Influence of
carbaryl on androgen metabolism in the prostate gland and liver. The
Pharmacologist, 15: 266
Dorough, H.W. and Wiggins, O.G.J. (1969) Econ. Entomol. 62: 49
Dorough, H.W.J. (1970) Agr. Food Chem. 18: 1015
Dorough, H.W. (1970) Continuous feeding of carbaryl-naphthyl-14C to
lactating cows. Progress Report, 6 January 1970
Dorough, H.W. (1971) Paper presented at the International Symposium on
Pesticide Terminal Residues, Tel Aviv, Israel, 17-19 February 1971
Dorough, H.W., Mehendale, H.M. and Lin, T. (1972) Modification of
carbaryl metabolism in rats with monoamine oxidase inhibitors. J.
Econ. Entomol. 65(4): 958-962
Dougherty, W., Golberg, L. and Coulston, F. (1973) The effect of
carbaryl on reproduction in rhesus monkeys. Unpublished report from
the Institute of Experimental Pathology and Toxicology, Albany Medical
College, United States of America
Elkins, E.R., Lamb, F.C., Farrow, R.P., Cook, R.W., Kawai, M. and
Kimball, J.R.J. (1968) Agr. Food Chem. 16: 962
Elkins, E.R., Farrow, R.P. and Kim, E.S.J. (1972) Agr. Food Chem.
20: 286
FAO/WHO, (1970b) 1969 Evaluation of Some Pesticide Residues in Food.
FAO/PL: 1969/M/17/1; WHO/Food Add./70.38
Farrow, R.P., Lamb, F.C., Cook, R.W., Kimball, J.R. and Elkins, E.R.J.
(1968) Agr. Food Chem. 16: 65
Farrow, R.P., Lamb, F.C., Elkins, E.R., Cook, R.W., Kawai, M. and
Cortes, A.J. (1969) Agr. Food Chem. 17: 75
Fukuto, T.R. (1972) Drug Metab. Revs. 1: 117
Hajkina, B.I. (1970) The mechanism of effect of carbaryl on warm
blooded animals. Gig. prim. toks. pesticid. i, klinika otravl. p. 203
Hassan, A. (1971) Pharmacological effects of carbaryl-I. The effect of
carbaryl on the synthesis and degradation of catecholamines in the
rat. Biochem. Pharmacol. 20(9): 2299-2308
Hoque, M.Z. (1972) Carbaryl - a new chemical mutagen. Curr. Sci.
41(23): 855-856
Homenko, N.P. (1971) Changes in the membrane potentiality of the
motoneurons of the spinal cord under the effect of carbaryl and
chlorfos. Fiziologja, 17(4); 527-528
Jakushko, V.E. (1971) Peculiarities of the glycosis and its regulation
on the cell structure of the cerebrum under the effect of DOT and
carbaryl. Farmakologija i toksikologija, reap. Megived, sb. 6:
187-190
Kagan, Ju. S., Radionev, G.A. and Voroneva, L.Ja. (1970) The effect of
Sevin in animal experiments on the function and structure of liver,
Farmakologija i toksikologija, 33(2): 219-224
Kuhr, R.J.J. (1968) Sci. Food Agric. Suppl. 44
Lamb, F.C., Farrow, R.P., Elkins, E.R., Kimball, J.R. and Cook, R.W.
(1968) J. Agr. Food Chem. 16: 967
Lillie, R.J. (1973) Studies on the reproductive performance and
progeny performance of caged White LeShorns fed malathion and
carbaryl. Poultry Sci. 52(1): 266-272
Locks, R.K. (1972a) Possible in vitro production of N-O-conjugates,
of N-hydroxyearbaryl from carbaryl by tobacco cells in culture. Fed.
Proc. Fed. Am. Soc. Exp. Biol. 31(2): 520
Locks, R.K. (1972b) Thin layer chromatography of l-naphthyl-N
hydroxy-N-methylearbamate and its application in two in vitro
studies involving carbaryl. J. Agr. Food Chem. 20: 1078
Lucier, G.W., McDaniel, O.S., Williams, C. and Klein, R. (1972)
Effects of chlordane and methylmercury on metabolism of carbaryl and
carbofuran in rats. Pest Biochem. Physiol. 2(2): 244-255
Mandzhgaladze, R.N. and Vashakidze, V.I. (1972) Deystviyo
khimicheskikh soyedineniy na potomstvo i razvit iye priznakov pola.
[Action of some chemical compounds on offspring and sexual
characteristics of rats.] Soobsbeh. Akad. Nauk Gruz. SSR, 65(2):
485-488
Moriyama, H., Sugiyama, H. and Shigematsu, H. (1972) A hydroxy
metabolite derived from carbaryl in the silkworm, Bombyx mori. Pest
Biochem. Physiol. 2(1): 1
Natoff, I.L. and Reiff, B. (1973) Effect of oximes on the acute
toxicity of anticholinesterase carbamates. Toxicol. Appl. Pharmacol.
25: 569-575
Paulson, G.D. and Feil, V.J. (1969) Poultry Sci. 48: 1593
Pokas, J.C. (1971) Intestinal metabolism and transport of naphthyl
N-methyl-carbamate in vitro (rat). Amer. J. Physiol. 220(6):
2008-2012
Pekas, J.C. and Paulson, G.D. (1970) Intestinal hydrolysis and
conjugation of a pesticidal carbamate in vitro. Science,
170(3953): 77-78
Rappoport, M.B. (1969) Microscopic and ultrastructural changes of the
thyroid gland under the effect of the insecticide carbaryl. Vrachebnoe
delo, 5: 102-105
Richey, F.A., jr, Bartley, W.J., Fitzpatrick, J.T. and Kurtz, A.P.
(1972) Chemical synthesis of the carbaryl metabolite
trans-5,6-dihydro-5,6-dihydroxy-l-naphthyl methyl-carbamate. J. Agr.
Food Chem. 20(4): 825-828
Ryan, A.J. (1971) CRC Critical Reviews in Toxicology, September 1971,
33
Santolucito, J. A., Whitcomb, E. and Hassan, A. (1972) Pharmacological
effects of carbaryl. Ill. Observations on alteration of smooth
muscle-preparation responses to norepinephrine. Environ. Physiol.
Biochem. 2(3): 142-146
Shah, A.H. and Guthrie, F.E. (1971) In vitro metabolism of
insecticides during midgut penetration. Pest Biochem. Physiol. 1(1):
1-10
Shtenberg, A.I. and Orlova, N.V. (1970) Contribution to the mechanism
of toxic action on Sevin, a pesticide of the carbamate group. Vestn.
Akad. Mod. Nauk SSR, 72-76
Sideroff, S.I. and Santolucito, J.A. (1972) Behavioural and
physiological effects of the cholinesterase inhibitor carbaryl
(I-naphthyl methylearbamate). Physiol. and Behav. 9: 459-462
Stenberg, A.M. (1970) The effect of carbaryl on the status of the
thyroid gland of mice receiving artificial or full value diet with
different content of iodine. Gigiena i sanitarija, 8: 119
Stenberg, A.I. and Otovan, M.B. (1971) Effect of small doses of
carbaryl on the reproductive function of animals in a number of
offsprings. Voprosi pitanija, 30(1): 41-49
Strother, A. (1972) In vitro metabolism of methyl carbamate
insecticides by human and rat liver fractions. Toxicol. Appl.
Pharmacol. 21(1): 112-129
Sullivan, L.J., Eldridge, J.M., Knaak, J.B. and Tallant, M.J. (1972)
5,6-dihydro-5,6-dihydroxy carbaryl glucuronide as a significant
metabolite of carbaryl in a rat. J. Agr. Food Chem. 20: 980-985
Tallant, M.J. (1970) Mellon Institute, Special Report 33-100, 20
October 1970
Thomas, J.A., Schein, L.G. and Dieringer, C.S. (1973) Distribution of
radio-activity in male reproductive organs after the administration
either carbaryl-14C or DDT-3H. The Pharmacologist, 15: 227
Trifonova, T.K., Gladenko, I.N. and Shulyak, V.D. (1970) Deystviye
gamma-izomera GKhTsGi sevina na polovuyu funktsivu. [Effect of
gamma-BHC and Sevin on reproduction] Veterinariya (Moscow), 47(6):
91-93
Union Carbide (1973) Patterns of use for Sevin(R) carbaryl
insecticide. Summary filed with FAO 1 March 1973
Union Carbide (1970) Summary of carbaryl residues. Section V (Private
communication)
Vashakidze, V.I. (1970) The effect of carbaryl on the spermatogenesis
of albino mice. Sbornik trudov Instituta gigiena truda i prof.
zabolevanija, Gruz SSR, 12: 285-288
Weil, C.S., Woodside, M.D., Bernard, J.B., Condra, N.I. and Carpenter,
C.P. (1972a) Studies on rat reproduction and guinea pig teratology of
carbaryl fed in the diet vs stomach tube. Toxicol. Appl. Pharmacol.
22: 318
Weil, C.S., Woodside, M.D., Carpenter, C.P. and Smyth, H.F., jr.
(1972b) Current status of tests of carbaryl for reproductive and
teratogenic effect. Toxicol. Appl. Pharmacol. 21: 390-404
Wiggins, O.G. and Weiden, M.H.J. (1969a) Union Carbide Corporation,
Internal Project Report, 30 June 1969
Wiggins, O.G. and Weiden, M.H.J. (1969b) Union Carbide Corporation,
Internal Project Report, 1 July 1969
Wiggins, O.G., Weiden, M.H.J., Weil, C.S. and Carpenter, C.P. (1970)
Paper presented at the VII International Congress of Plant Protection,
Paris, September 1970
