
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
CARBARYL
IDENTITY
Synonym
Sevin(R)
Chemical names
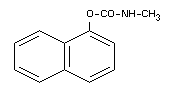
1-naphththyl-N-methylcarbamate; N-methyl-1-naphthyl carbamate;
N-methyl-a-naphthyl urethane.
Formula
 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Some knowledge has been obtained of the changes which the compound
undergoes in plants. Following injection of 14C-carbaryl into the
stems of snapbeans and cotton seedlings, 55 per cent of the activity
injected was present in the beans, and 86 per cent in the cotton, at
28 days. Of the original compound, 5.7 per cent was found in the beans
and 1.7 per cent in the cotton. It is suggested that conversion to
water-soluble metabolite(s), which may have been carbamates, took
place and these were quite stable within the plants (Dorough & Casida,
1964).
A slight rise in free 1-naphthol and a definite rise in conjugated
1-naphthol in the urine were observed during the 48 hours following
oral dosing of carbaryl in rats (Carpenter et al., 1961). Among
workers engaged in the production, handling and shipping of carbaryl,
those most heavily exposed (air concentrations of 0.23 to 31 mg/M3)
excreted large amounts of total 1-naphthol (Best & Murray, 1962).
14C-carbaryl, labelled at the N-methyl, carbonyl and naphthyl-1
positions, and metabolized by rat liver microsomes and insects,
yielded at least 5 metabolites (Dorough et al., 1963). Further work
has shown the existence of 6 metabolites, 5 of which were carbamates.
Three of these were tentatively identified as 1-naphthyl
N-hydroxymethylcarbamate, 4-hydroxy-1-naphthyl-N-methylcarbamate and
5-hydroxy-1-naphthyl-N-methylcarbamate (Dorough & Casida, 1964).
When 14C-carbonyl carbaryl was given orally to a goat in a dose of
1.34 mg/kg, several of these metabolites were found in the milk and
urine. The level of total 14C equivalents of carbaryl reached a peak
of 0.93 ppm in the milk at 8 hours and decreased to below 0.003 ppm at
60 hours. During the 96 hours after dosing, 47 per cent of the
radioactivity was excreted in the urine (Dorough et al., 1963; Dorough
& Casida, 1964).
In rats and guinea-pigs, 7 days after ingestion of labelled carbaryl,
the overall recovery of 14C-methyl, 14C-carbonyl and
14C-naphthyl labels was, respectively, 95, 99 and 91 per cent of the
dose of carbaryl. The only detectable 14C residues in the rat were
from 14C-methyl, representing 2-3 per cent of the dose. Several
metabolites were identified, and two of these, 1-naphthyl glucuronide
and sulfate, were also detected in urine from men exposed to carbaryl
dust (Knaak et al., 1965).
When carbaryl, labelled with 14C at the carbonyl, methylcarbamate or
naphthyl groups, was given to male rats by intraperitoneal injection,
recovery of 14C the expired CO2 after 48 hours was 24.5, 12.3 and
0.2 per cent respectively. At 24 hours, respective urine recoveries
were 62.1, 54.6 and 74.2 per cent and at 48 hours, 2.4, 3.4 and 2.3
per cent. Recoveries from faeces at 48 hours were 2.1., 3.9 and 8.9
per cent, and from carcass, 9.5, 12.7 and 6.7 per cent. After giving
14C-carbonyl carbaryl to male and female rats, no marked sex
differences were noted (Krishna & Casida, 1966).
Technical carbaryl was fed to dairy cows at levels up to 450 ppm for 2
weeks. Samples of milk taken at various times failed to disclose any
measurable residues. Carbaryl, if present, was below the sensitivity
of the method (0.01 ppm) (Gyrisco et al., 1960). In steers fed 50 or
200 ppm daily of technical carbaryl for 27 days, no tissue residues
could be detected (Claborn et al., 1963). Milk from cows 1 hour after
being sprayed to runoff with 0.5 per cent carbaryl solution or after
having received a tablespoon of 50 per cent dust spread down the back,
contained carbaryl residue. Samples taken at 15, 25 and 37 hours
contained no residue (Eheart et al., 1962). Milk taken as early as 5
hours after the last spray from 2 cows sprayed 4 times at 4 day
intervals with 0.5 per cent carbaryl suspension did not contain
carbaryl. Omental fat taken from 2 steers 5 days after the fourth
spraying did not contain carbaryl or 1-naphthol (Roberts et al.,
1960). Carbaryl residue, 1-naphthol and conjugate varied from 0-0.57
ppm in various tissues from steers, sheep, goats and hogs 1 day after
being sprayed to runoff with 1 per cent water suspension of 50 per
cent carbaryl powder. At 7 days no residue could be detected except in
fat and brain of the goat (Claborn et al., 1963).
In laying hens dusted 3 times at 4-day intervals with 4 g of 5 per
cent carbaryl dust per bird and killed 24 hours after the last
dusting, concentrations of less than 0.1 to 2.0 ppm carbaryl residue
and less than 0.1 ppm of 1-naphthol were found in breast and leg
muscle. Liver and gizzard concentrations of both residues were less
than 0.2 ppm. Residue concentrations in eggs remained below 0.2 ppm
throughout the study (Johnson et al., 1963).
A depression of blood and brain cholinesterase activity has been
reported following single large doses of carbaryl. Approximately the
same concentrations are required to produce a 50 per cent inhibition
in the blood of man, rabbit, rat and dog (Mellon Institute, 1958b). No
significant effect was found on dog erythrocyte or plasma
cholinesterase after single intravenous injections of 10 or 15 mg/kg.
In a dog which had received a total of 88.3 mg/kg in 11 doses
intravenously, typical symptoms of cholinesterase inhibition occurred
after 10 and 15 mg/kg, but only a slight reaction was seen after 5
mg/kg (Carpenter at al., 1961). In men exposed to carbaryl air
concentrations of 0.23 to 31 mg/M3 whole blood cholinesterase
activity was occasionally slightly depressed but there were no
clinical signs (Best & Murray, 1962).
Carbaryl is a reversible cholinesterase inhibitor. In fact, the
reversal is so rapid that unless special precautions are taken,
measurements of blood cholinesterase of persons exposed to it are
likely to be inaccurate and always tending to appear normal.
Pyridine-2-aldoxime methiodide, which is a good antidote for some
organophosphorus compounds, is not effective in reversing
cholinesterase inhibition by carbaryl (Mellon Institute, 1958b;
Carpenter at al., 1961). Atropine sulfate was effective in controlling
symptoms in the dog (Carpenter at al., 1961).
Special studies
Some studies have been reported describing the effects of a single
administration of carbaryl on discrete avoidance and food reward
behavioural tests in rats. In one report (Goldberg at al., 1965a) the
dose necessary to suppress avoidance response to 50 per cent
efficiency was slightly lower than the dose required to reduce brain
cholinesterase to 50 per cent of control value. The effects of
carbaryl on behaviour are prevented by atropine pre-treatment, and the
association with Chlorpromazine leads to more than additive effects.
Beta-dimethylaminoethyl-diphenylpropyl-acetate (SKF 525) increases the
behavioural effects of carbaryl without enhancement of cholinesterase
inhibition (Goldberg & Johnson, 1964a; Goldberg & Johnson, 1964b;
Goldberg et al., 1965a; Goldberg et al., 1965b).
Acute toxicity
Animal Route Solvent LD50 References
mg/kg
Mouse i.p. Corn oil 25 Barron et al., 1964
Rat, male oral 10% Tween 80 190 Mellon Institute, 1956a
Rat, male oral 10% Tween 80 310 Mellon Institute, 1958a
in 0.75% NaCl
Rat, male and
female oral 0.25% agar 480-610 Mellon Institute, 195ba
Rat, male oral Peanut oil 850 Gaines, 1960
Rat, female oral Peanut oil 500 Gaines, 1960
Rat, male oral Corn oil 308 Mellon Institute, 1958a
(continued)
Animal Route Solvent LD50 References
mg/kg
Rat, female oral Corn oil 560 Mellon Institute, 1958a
Rat i.v. Propylene glycol 18 Mellon Institute, 1958a
Rat i.v. PEG 400 24 Mellon Institute, 1958a
Rat i.v. Undiluted 93 Mellon Institute, 1958a
Guinea-pig, male oral 0.25% agar 280 Mellon Institute, 1958a
Rabbit, male oral 0.25% agar 707 Mellon Institute, 1958a
Dog oral Powder none died Mellon Institute, 195ba
(250-795 mg/kg)
Chicken. Focal loss of striation and fatty infiltration of muscle
was observed at 3 g/kg subcutaneously. Transient leg weakness for 1-2
days occurred after 2 g/kg and a nephrotoxic action was observed after
2 g/kg or more. No demyelination was seen (Carpenter et al., 1961).
Man. A 19-month-old child swallowed an unknown amount of carbaryl.
Typical early signs of cholinesterase inhibition, i.e. constricted
pupils, excessive salivation, and muscle incoordination, occurred. A
single dose of atropine of 0.3 mg controlled the symptom and the child
recovered in 12 hours. Urine collected 18 hours after poisoning
contained 3140 µg of 1-naphthol per 100 ml (Best & Murray, 1962).
Short-term studies
Mouse. In one experiment 30 A/Jax and 30 C3H mice at the age of 3
months were started on a treatment of weekly subcutaneous injections
of 0.5 mg of carbaryl. Five months later, the survivors (26 A/Jax and
28 C3H) were sacrificed. In neither group did the survival rate or
tumour incidence differ from untreated mice of the same strains
(Mellon Institute, 1958).
Rat. Groups of 10 rats (5 of each sex) were fed 1500 or 2250 ppm
carbaryl in the diet for 96 days. At 2250 ppm, decreased body-weight
in the females, increased liver weight in the males and increased
kidney weight in the females were observed, while at 1500 ppm there
was increased kidney weight in the females. A minor histopathological
change in the form of diffuse cloudy swelling of the kidney tubules
was noted at the higher concentration (Carpenter et al., 1961).
Reproduction studies in 3 generations of CFE rats fed diets containing
carbaryl adjusted to dose levels of 0, 0.0025 or 0.01 mg/kg
body-weight daily, did not show any significant differences between
treatment groups in fertility, gestation, lactation or viability of
pups, mean number of pups per litter, body-weight of pups at weaning
or teratology (Mellon Institute, 1965).
Guinea-pig. Male guinea-pigs were injected with carbaryl and after a
3-week period were given a challenge dose. There was no evidence of
development of sensitization (Carpenter et al., 1961).
Dog. Groups of 3 or 4 dogs were given doses of 0.45, 1.8 and 7.2
mg/kg body-weight/day by capsule, 5 days per week for 1 year. Diffuse
cloudy swelling of the kidney tubules was found in all the dogs at the
7.2 mg/kg level. Focal cloudy swelling was found in the controls. One
female at the 0.45 mg/kg level had transient hind-leg weakness, but no
histological lesion was found at necropsy (Carpenter et al., 1961).
Long-term studies
Mouse. Groups of 48 male and 48 female Cd-1 mice were given 0, 100
and 400 ppm or carbaryl in the diet. After 80 weeks, 12 survivors of
each sex from each group were sacrificed. Survival rate, pathology and
tumour incidence were comparable in all groups (Mellon Institute,
1963)
Rat. In a 2-year experiment, groups of 20 CF-N rats of each sex were
given diets containing 0, 50, 100, 200 and 400 ppm of carbaryl. After
6, 9 and 12 months, 4, 6, or 8 rats of each sex were killed for organ
weight comparison and histopathological examination. The remainder
were sacrificed after 2 years. The highest dose level produced cloudy
swelling of the kidney tubules after 1 year and cloudy swelling of the
central hepatic cords in the males after 2 years. Terminal body-weight
in the males at the high level was reduced. There were no effects at
the lower dose levels (Carpenter et al., 1961).
Comments
While teratogenicity studies using extremely low doses were negative
in the rat, studies in other species have not been reported but would
be very desirable.
Several carbaryl metabolites have been identified in animals but not
yet in plants. Excretion of carbaryl and its metabolites appears to be
rapid in animals. Studies on the identification and toxicological
evaluation of residues occurring in plants would be desirable.
The behavioural studies appear interesting in relation to the mode of
action and as a potential system for detection of toxicity. However,
no data are available concerning experiments with continuous exposure
to the compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 400 ppm in the diet, equivalent to 60 mg/kg/day
Rat: 200 ppm in the diet, equivalent to 10 mg/kg/day
Dog: 1.8 mg/kg/day
Estimate of acceptable daily intake for man
0-0.02 mg/kg per day. (This value is based on experiments carried out
with carbaryl, and thus does not take account of chemical alterations
of the pesticide brought about by the plants to which it has been
applied.)
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Carbaryl has been approved for use on a wide variety of food crops,
including cereals, fruits and vegetables in a number of countries. It
is being used in increasing amounts and is partly replacing more
persistent and toxic compounds. In some countries it is used against
external parasites of cattle.
It is suggested for the control of at least 10 insects which attack
cane fruit (berries), 20 which attack tree fruits, 25 which attack
vegetables, as well as for use on other commodities and livestock. The
usual rate of application for berries and vegetables is 1 or 2 lb per
acre but for tree fruits as high as 10 lb per acre is suggested.
(b) Post-harvest treatments
Carbaryl has been used experimentally on stored cereals but is not so
used on a commercial scale.
National tolerances
Country Product Parts per million
Austria General 10
Canada Various 5, 10 and 25
United States of Sorghum (grain) 10
America Rice 5
Various fruits,
cabbage, lettuce,
carrots 10
Fruits (cane) 12
Garden beet,
parsnips,
radishes 5
Residues resulting from supervised trials
Many data have been obtained from supervised trials on a large number
of food crops produced under varying cultural conditions, rates and
times of application, pre-harvest intervals, etc. This information is
too extensive to reproduce in this monograph. Table 1 therefore
contains estimates of residues for groups of commodities as contained
in these reports. (A file summarizing such data for ca. 85 crops is
held at the FAO headquarters in Rome.) The residue figures quoted are
those likely to be present as two different pre-harvest intervals
following uses at rates of application that are considered to be
useful.
Residues in food moving in commerce
About two thousand samples of domestic and imported food products were
examined by the United States Food and Drug Administration during
1966. Less than 10 per cent were found to contain residues of carbaryl
and less than 0.1 per cent had residues above the tolerance levels
(information from United States Food and Drug Administration).
TABLE 1. AVERAGE RESIDUES FROM GOOD AGRICULTURAL PRACTICE
Food Resulting residue
Type Pre-harvest ppm
period
(days)
Vegetables
Leaf - spinach 14 10
Lettuce 30 1
Other 2 10
7-10 1
Cereal and 35-50 0
cereal products
wheat, rye, oats
Rice 14 5
30 1
Tree fruits 1 10
14-20 1
Caneberries 7 10
20 1
TABLE 1. AVERAGE RESIDUES FROM GOOD AGRICULTURAL PRACTICE
Food Resulting residue
Type Pre-harvest ppm
period
(days)
Citrus 1 10
20-60 1
Shelled nuts 1 1
Fate of residues
In the earlier studies on residues of carbaryl (Bibliography by
Moorefield, 1966), consideration had been given to the parent compound
carbaryl and its hydrolytic product, 1-naphthol. Residue studies on
many crops showed that the quantity of 1-naphthol was small and
difficult to separate from carbaryl. Thus, it was considered that
1-naphthol need not be measured separately from carbaryl. The United
States of America tolerances for carbaryl are based on the assumption
that the major part of the residue is the intact carbamate.
Since the establishment of the initial tolerances for carbaryl, work
has been done which may have some bearing on the quantative and
qualitative aspects of the residue. Light, including artificial and
sunlight, changes carbaryl. Carbaryl in the formulated state slowly
degraded under the influence of ultra-violet and to give several
unidentified products (Okada et al., 1961). Crosby et al. (1965)
reported that ethanol or hexane solutions of some methylcarbamate
insecticides, including carbaryl, gave a variety of
cholinesterase-inhibiting derivatives when exposed to either
artificial or sunlight. The products were separated but not
identified. Abdel-Waheb (1966) reported that the nature of (1) the
surface, (2) the light, and (3) the compound affected the rate of
conversion of carbaryl and other carbamates in vitro in the solid
state.
Since neither the qualitative nor quantative aspects of
photo-decomposition of carbamates have been established, the
significance of these studies in relation to current and future
tolerances for carbaryl must await further study.
(a) In animals
Whitehurst et al. (1963) using the colorimetric method of analysis
sensitive to carbaryl, 1-naphthol and 1-naphthol conjugates concluded
that, when cows were fed on a diet containing carbaryl, no residues,
to the limit of method, were found in the milk. This was confirmation
of the feeding study by Gyrisco et al. (1960), who fed dairy cows
carbaryl at levels of up to 450 ppm. In another study, carbaryl was
dusted and sprayed on cows. No detectable residues were found in milk
when carbaryl was dusted on to animals, but after 48 hours some
carbaryl residues were found in the milk when the spray application
was used (Buttram, 1964; Camp et al., 1963).
In recent work done by Dorough (1966), ring-labelled carbaryl was fed
to a lactating cow. A total of approximately 1 ppm, based upon a
radioactivity measurement calculated as carbaryl, was found in the
skim milk six to 12 hours after administration of 3.05 mg/kg
body-weight. Approximately one half of this residue was chloroform
extractable, and one half was water extractable with a small
unextractable fraction. At the end of 60 hours all the radioactivity
(calculated to be 0.01 ppm as equivalents of carbaryl) was
unextractable from the milk. In the six-hour samples about 30 per cent
of the radioactivity in the milk was characterized as
5,6-dihydro-5,6-dihydroxy 1-naphthol N-methylcarbamate, and
approximately 20 per cent was an unknown metabolite. Neither of these
materials, corresponding to 50 per cent of the total milk residue (0.5
ppm), responded to the standard colorimetric method commonly used for
carbaryl.
Cattle, sheep, goats, and hogs were sprayed four times in two weeks
with a 1.0 per cent suspension of carbaryl and Hereford steers were
fed 200 ppm of carbaryl in the diet for a 27-day period. These animals
were slaughtered at one and seven days after spraying or at the end of
the feeding interval. Residues of carbaryl, 1-naphthol and conjugates
of 1-naphthol were not detected in the body tissues of any of the
cattle, sheep, or hogs seven days after spraying. At this time
interval some small residues were noted in the fat and brain of a
goat. No residues were detected in tissues of cattle fed carbaryl for
27 days (Claborn, 1963). Krishna & Casida (1966) have reported that
less than 10 per cent of the administered acute dose of
carbonyl-C14-carbaryl was recovered after 48 hours as tissue
residues, primarily in those tissues known to be involved in body
contaminant eliminations - (e.g. liver, spleen, kidney). After seven
days Knaak (1966) found no residues resulting from carbonyl-carbaryl.
Laying hens were dusted three times at four-day intervals using 4 gm
of five per cent carbaryl per bird. One day after treatment no
residues were found in each type of edible tissue except skin. Seven
days after treatment this residue was reduced to low levels. Eggs were
found to be free of residue, throughout the study (Johnson,
Critchfield & Arthur, 1963).
(b) In plants
A limited amount of metabolism studies on carbamates in and on plants
has been reported. Studies by Dorough et al. (1963, 1964) found that
radioactive carbaryl yielded water soluble persistent metabolites
which were different than those found in the milk of a goat.
Carbonyl-C14-carbaryl, injected and administered to the surface of
bean plants yielded compounds that largely remain within the plant.
While these compounds have not been characterized, they are stable and
after 12 hours are not extractable by the usual organic solvents.
Abdel-Wahab observed that approximately 50 per cent of the surface
administered dose of C14 radioactivity was recovered after 72 hours.
When bean plants were injected into the stem with radioactive
carbaryl, approximately 75 per cent of the radioactivity persisted for
at least six days as aqueous and unextractable materials. Thus,
reactions within the plant presumably produce carbamates in some
stable form. These non-hydrolytic metabolites in many instances may
not respond to the usual method of analysis for carbaryl because (1)
the metabolites yield a phenolic material different than 1-naphthol
and do not show the same colour reaction, and (2) the major fraction
of some of the metabolites show solubility properties different than
the parent compounds. Preliminary studies on bio-assay of certain
metabolites have indicated a reduced biological activity when compared
with the parent compounds.
(c) In storage and processing
Raw unwashed tomatoes, harvested from field treated applications, were
stored at 55° F for approximately two weeks with samples taken at
varying intervals. Data showed that no loss of carbaryl was realized
under these storage conditions. The harvested tomatoes were subjected
to home and commercial canning operations, and analysis of samples
indicated that 50 per cent or more of the residue was removed by
water-washing and nearly complete removal of all residue was realized
by peeling and canning operations (Farrow et al. 1966).
Methods of residue analysis
The methods available for carbaryl are based on a colorimetric
determination following suitable extraction and clean-up. There are
approximately 20 variations of the basic method for different types
of food products, most of which are not published and no one method
has been tested for all food products.
For most fruits and vegetables the methods of (1) AOAC or that of (2)
Benson & Finocchiaro (1965) are suggested. These methods have a
sensitivity of about 0.1 ppm.
The above methods do not give very adequate clean-up for citrus,
olives, shelled nuts, many cereals and cereal products. Of the many
variations of the method, that developed for wheat and wheat fractions
has the most comprehensive clean-up. It is believed that it would be
useful for all products not amenable to the AOAC method.
RECOMMENDATIONS FOR TOLERANCES
The low acceptable daily intake necessitated a very close evaluation
of residue data to determine tolerances which would permit useful
applications to crops without possibly providing residues which might
exceed the acceptable daily intake.
Some data indicate that there will be losses under some conditions of
storage and processing. However, not enough data are available to make
a calculation at present, therefore no factor for such losses are
included in the calculations. The basic factor used in recommending
temporary tolerances which yield values within the ADI is that of
choosing a long pre-harvest interval.
The recommended tolerance figures are indicated in the following
table.
TEMPORARY TOLERANCE RECOMMENDATIONS FOR CARBARYL
Crop Temporary tolerance
in ppm
Vegetables 1.0
Cereal and cereal products 1.0
Tree fruits 1.0
Caneberries 1.2
Citrus 10.0
Shelled nuts 1.0
Further work or information
In the above calculations no considerations were given to possible
losses during storage, shipping and processing. It is recommended that
such data be developed and made available to the working party.
It is also recommended that further work be done to establish more
definitely the character of the terminal residue in treated plants.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Baron, R. L., Casterline, J. L. & Fitzhugh, O. G. (1964) Toxicol.
appl. Pharmacol., 6, 402
Best, E. M., jr & Murray, B. L. (1962) J. occup. Med., 4, 507
Carpenter, C. P., Weil, C. S., Palm, P. E., Woodside, M. W., Nair,
J. H. & Smyth, H. F., jr (1961) J. Agr. Food Chem., 9, 30
Claborn, H. V., Roberts, R. H., Mann, H. D., Bowman, M. C., Ivey,
M. C., Weidenbach, C. P. & Radeleff, R. D. (1963) J. Agr. Food
Chem., 11, 74
Dorough, H. W., Leeling, N. C. & Casida, J. E. (1963) Science,
140, 170
Dorough, H. W. & Casida, J. E. (1964) J. Agr. Food Chem., 12, 294
Eheart, J. F., Turner, E. C. & Dickinson, J. (1962) J. Econ. Ent.,
55, 504
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Goldberg, M. E. & Johnson, H. E. (1964a) J. Pharm. Pharmacol., 16,
60
Goldberg, M. E. & Johnson, H. E. (1964b) J. Pharmacol. exp. Ther.,
145, 367
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965a)
Psychopharmacologia, 7, 72
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965b) Biochem.
Pharmacol., 13, 1483
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, E. W., Fox,
F. H., Holland, R. F. & Trimberger, G. W. (1960) J. Agr. Food Chem.,
8, 409
Johnson, D. P., Critchfield, F. E. & Arthur, B. W. (1963) J. Agr.
Food Chem., 11, 77
Knaak, J. B., Tallant, M. J., Bartley, W. J. & Sullivan, L. J. (1965)
J. Agr. Food Chem., 13, 537
Krishna, J. G. & Casida, J. E. (1966), J. Agr. Food Chem., 14, 98
Mellon Institute of Industrial Research (1958a) Unpublished Report
Mellon Institute of Industrial Research (1958b) Unpublished Report
Mellon Institute of Industrial Research (1963) Unpublished Report
Mellon Institute of Industrial Research (1965) Unpublished Report
Roberts, R. H., Jackson, J. B., Westlake, W. E., Ackerman, A. J. &
Claborn, H. V. (1960) J. Econ. Ent., 53, 326
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abdel-Wahab, A. M., Kuhr, R. J., and Casida, J. E. (1966) The fate of
C14 C=O labeled aryl methylcarbamate insecticide chemicals in and on
bean plants. J. Agr. Food Chem. 14:290.
AOAC (1965) Methods of Analysis, (10th edition) paras. 24. 188-24.192
Benson, W. R. and Finocchiaro, J. M. (1965) Rapid Procedure for
Carbaryl Residues: Modification of Official Colorimetric Method. J.
Assoc. Offic. Agric. Chem. 48, 676-679
Buttram, J. Ress. (1964) Thesis, Auburn University. The metabolism of
carbamate and organophosphate insecticides by cattle and poultry and
associated residues in livestock products.
Camp, H. B., Buttram, J. R., Hays, K. L., and Arthur, B. W. (1963)
Sevin residues in milk from dairy cows following dermal applications.
J. Econ. Entomol. 56:402-404.
Casida, J. E., and Augustinsson, K.-B. (1959) Reaction of Plasma
Albumin with 1-naphthyl N-methylcarbamate and certain other esters.
Biochem. Biophys. Acta. 36:411-26.
Casida, J. E., Augustinsson, K.-B. and Jonsson, G. (1960) Stability,
toxicity and reaction mechanism with esterases of certain carbamate
insecticides. J. Econ. Entomol. 53:205-12.
Claborn, H. V. (1963) Residues in body tissues of livestock sprayed
with Sevin or Sevin in the diet. J. Agr. Food Chem. 11:74-76.
Crosby, D. C., Leitus, E., and Winterlin, W. L. (1965) The
photodecomposition of carbamate insecticides. J. Agr. Food Chem.
13:204.
Dorough, H. W., Leeling, N. C. and Casida, J. E. (1963) Nonhydrolytic
pathway in metabolism of N-methylcarbamate insecticides. Science
140:170-171.
Dorough, H. W. and Casida, J. E, (1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J. Agr. Food Chem. 12 (4):
294-304.
Dorough, H. W. (1966) Carbaryl-C14 metabolism in a lactating cow.
Private communication and in press.
Farrow, R. P., Lamb, F., Cook, R. W., Bergmans, B., Kimball, J. and
Elkins, E. R. (1966) Removal of Sevin residues from tomatoes during
commercial and home preparative procedures. Paper # 36, Pesticides
Subdivision of Agr. and Food Div., Amer. Chem. Soc. 152nd Meeting,
September 12-16, 1966.
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, E. W., Fox, F.
H., Holland, R. F., and Trimberger, G. W. (1960) The effects of
feeding high levels of Sevin on residue, flavor and odor of the milk
of dairy cattle. J. Agr. Food Chem. 8:409-410.
Hodgson, E. and Casida, J. E. (1960) Biological oxidation of
N,N-dialkyl carbamates. Biochem. Biophys. Acta. 42:184-186
Hodgson, E. and Casida, J. E. (1961) Metabolism of N,N-dialkyl
carbamates and related compounds by rat liver. Biochem. Pharmacol.
8:179-191.
Johnson, D. P., Critchfield, F. E., and Arthur, B. W. (1963)
Determination of Sevin insecticide and its metabolites in poultry
tissues and eggs. J. Agr. Food Chem. 11:77-80.
Knaak, J. B., Tallant, M. J., Bartley, W. J., and Sullivan, L. J.
(1965) The metabolism of carbaryl in the rat, guinea pig and man. J.
Agr. Food Chem. 13:537-543.
Krishna, J. G. and Casida, J. E. (1966) Fate in Rats of the
Radiocarbon from ten variously labeled methyl- and
dimethylcarbamate-C14 insecticide chemicals and their hydrolysis
products. J. Agr. Food Chem. 14:98-105.
Leeling, N. C. and Casida, J. E. (1966) Metabolites of carbaryl in
mammals and enzymatic systems for their formation. J. Agr. Food Chem.
14:282-290.
Morefield, H. H. "Agr. Products Tech. Service Report" bibliography on
Sevin insecticides (carbaryl) containing 1463 references has been
compiled by the Union Carbide Corporation, R & D Dept., Agr. Products,
Olefins Division, P.O. Box 8361, So. Charleston, W. Virginia 25303.
Okada, K., Nomura, K., Yamamoto, S., (1961) (no title). Nippon
Nogaikagaku Kaishi 35:739.
Whitehurst, W. E., Bishop, E. T., Critchfield, F. E., Gyrisco, G. G.,
Huddleston, E. W., Arnold, H., and Lisk, D. J. (1963) The metabolism
of Sevin in dairy cows. J. Agr. Food Chem 11:167-169.
Williams, R. T. (1959) Detoxication Mechanisms. John Wiley & Sons,
Inc., N. Y. 796 p.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Some knowledge has been obtained of the changes which the compound
undergoes in plants. Following injection of 14C-carbaryl into the
stems of snapbeans and cotton seedlings, 55 per cent of the activity
injected was present in the beans, and 86 per cent in the cotton, at
28 days. Of the original compound, 5.7 per cent was found in the beans
and 1.7 per cent in the cotton. It is suggested that conversion to
water-soluble metabolite(s), which may have been carbamates, took
place and these were quite stable within the plants (Dorough & Casida,
1964).
A slight rise in free 1-naphthol and a definite rise in conjugated
1-naphthol in the urine were observed during the 48 hours following
oral dosing of carbaryl in rats (Carpenter et al., 1961). Among
workers engaged in the production, handling and shipping of carbaryl,
those most heavily exposed (air concentrations of 0.23 to 31 mg/M3)
excreted large amounts of total 1-naphthol (Best & Murray, 1962).
14C-carbaryl, labelled at the N-methyl, carbonyl and naphthyl-1
positions, and metabolized by rat liver microsomes and insects,
yielded at least 5 metabolites (Dorough et al., 1963). Further work
has shown the existence of 6 metabolites, 5 of which were carbamates.
Three of these were tentatively identified as 1-naphthyl
N-hydroxymethylcarbamate, 4-hydroxy-1-naphthyl-N-methylcarbamate and
5-hydroxy-1-naphthyl-N-methylcarbamate (Dorough & Casida, 1964).
When 14C-carbonyl carbaryl was given orally to a goat in a dose of
1.34 mg/kg, several of these metabolites were found in the milk and
urine. The level of total 14C equivalents of carbaryl reached a peak
of 0.93 ppm in the milk at 8 hours and decreased to below 0.003 ppm at
60 hours. During the 96 hours after dosing, 47 per cent of the
radioactivity was excreted in the urine (Dorough et al., 1963; Dorough
& Casida, 1964).
In rats and guinea-pigs, 7 days after ingestion of labelled carbaryl,
the overall recovery of 14C-methyl, 14C-carbonyl and
14C-naphthyl labels was, respectively, 95, 99 and 91 per cent of the
dose of carbaryl. The only detectable 14C residues in the rat were
from 14C-methyl, representing 2-3 per cent of the dose. Several
metabolites were identified, and two of these, 1-naphthyl glucuronide
and sulfate, were also detected in urine from men exposed to carbaryl
dust (Knaak et al., 1965).
When carbaryl, labelled with 14C at the carbonyl, methylcarbamate or
naphthyl groups, was given to male rats by intraperitoneal injection,
recovery of 14C the expired CO2 after 48 hours was 24.5, 12.3 and
0.2 per cent respectively. At 24 hours, respective urine recoveries
were 62.1, 54.6 and 74.2 per cent and at 48 hours, 2.4, 3.4 and 2.3
per cent. Recoveries from faeces at 48 hours were 2.1., 3.9 and 8.9
per cent, and from carcass, 9.5, 12.7 and 6.7 per cent. After giving
14C-carbonyl carbaryl to male and female rats, no marked sex
differences were noted (Krishna & Casida, 1966).
Technical carbaryl was fed to dairy cows at levels up to 450 ppm for 2
weeks. Samples of milk taken at various times failed to disclose any
measurable residues. Carbaryl, if present, was below the sensitivity
of the method (0.01 ppm) (Gyrisco et al., 1960). In steers fed 50 or
200 ppm daily of technical carbaryl for 27 days, no tissue residues
could be detected (Claborn et al., 1963). Milk from cows 1 hour after
being sprayed to runoff with 0.5 per cent carbaryl solution or after
having received a tablespoon of 50 per cent dust spread down the back,
contained carbaryl residue. Samples taken at 15, 25 and 37 hours
contained no residue (Eheart et al., 1962). Milk taken as early as 5
hours after the last spray from 2 cows sprayed 4 times at 4 day
intervals with 0.5 per cent carbaryl suspension did not contain
carbaryl. Omental fat taken from 2 steers 5 days after the fourth
spraying did not contain carbaryl or 1-naphthol (Roberts et al.,
1960). Carbaryl residue, 1-naphthol and conjugate varied from 0-0.57
ppm in various tissues from steers, sheep, goats and hogs 1 day after
being sprayed to runoff with 1 per cent water suspension of 50 per
cent carbaryl powder. At 7 days no residue could be detected except in
fat and brain of the goat (Claborn et al., 1963).
In laying hens dusted 3 times at 4-day intervals with 4 g of 5 per
cent carbaryl dust per bird and killed 24 hours after the last
dusting, concentrations of less than 0.1 to 2.0 ppm carbaryl residue
and less than 0.1 ppm of 1-naphthol were found in breast and leg
muscle. Liver and gizzard concentrations of both residues were less
than 0.2 ppm. Residue concentrations in eggs remained below 0.2 ppm
throughout the study (Johnson et al., 1963).
A depression of blood and brain cholinesterase activity has been
reported following single large doses of carbaryl. Approximately the
same concentrations are required to produce a 50 per cent inhibition
in the blood of man, rabbit, rat and dog (Mellon Institute, 1958b). No
significant effect was found on dog erythrocyte or plasma
cholinesterase after single intravenous injections of 10 or 15 mg/kg.
In a dog which had received a total of 88.3 mg/kg in 11 doses
intravenously, typical symptoms of cholinesterase inhibition occurred
after 10 and 15 mg/kg, but only a slight reaction was seen after 5
mg/kg (Carpenter at al., 1961). In men exposed to carbaryl air
concentrations of 0.23 to 31 mg/M3 whole blood cholinesterase
activity was occasionally slightly depressed but there were no
clinical signs (Best & Murray, 1962).
Carbaryl is a reversible cholinesterase inhibitor. In fact, the
reversal is so rapid that unless special precautions are taken,
measurements of blood cholinesterase of persons exposed to it are
likely to be inaccurate and always tending to appear normal.
Pyridine-2-aldoxime methiodide, which is a good antidote for some
organophosphorus compounds, is not effective in reversing
cholinesterase inhibition by carbaryl (Mellon Institute, 1958b;
Carpenter at al., 1961). Atropine sulfate was effective in controlling
symptoms in the dog (Carpenter at al., 1961).
Special studies
Some studies have been reported describing the effects of a single
administration of carbaryl on discrete avoidance and food reward
behavioural tests in rats. In one report (Goldberg at al., 1965a) the
dose necessary to suppress avoidance response to 50 per cent
efficiency was slightly lower than the dose required to reduce brain
cholinesterase to 50 per cent of control value. The effects of
carbaryl on behaviour are prevented by atropine pre-treatment, and the
association with Chlorpromazine leads to more than additive effects.
Beta-dimethylaminoethyl-diphenylpropyl-acetate (SKF 525) increases the
behavioural effects of carbaryl without enhancement of cholinesterase
inhibition (Goldberg & Johnson, 1964a; Goldberg & Johnson, 1964b;
Goldberg et al., 1965a; Goldberg et al., 1965b).
Acute toxicity
Animal Route Solvent LD50 References
mg/kg
Mouse i.p. Corn oil 25 Barron et al., 1964
Rat, male oral 10% Tween 80 190 Mellon Institute, 1956a
Rat, male oral 10% Tween 80 310 Mellon Institute, 1958a
in 0.75% NaCl
Rat, male and
female oral 0.25% agar 480-610 Mellon Institute, 195ba
Rat, male oral Peanut oil 850 Gaines, 1960
Rat, female oral Peanut oil 500 Gaines, 1960
Rat, male oral Corn oil 308 Mellon Institute, 1958a
(continued)
Animal Route Solvent LD50 References
mg/kg
Rat, female oral Corn oil 560 Mellon Institute, 1958a
Rat i.v. Propylene glycol 18 Mellon Institute, 1958a
Rat i.v. PEG 400 24 Mellon Institute, 1958a
Rat i.v. Undiluted 93 Mellon Institute, 1958a
Guinea-pig, male oral 0.25% agar 280 Mellon Institute, 1958a
Rabbit, male oral 0.25% agar 707 Mellon Institute, 1958a
Dog oral Powder none died Mellon Institute, 195ba
(250-795 mg/kg)
Chicken. Focal loss of striation and fatty infiltration of muscle
was observed at 3 g/kg subcutaneously. Transient leg weakness for 1-2
days occurred after 2 g/kg and a nephrotoxic action was observed after
2 g/kg or more. No demyelination was seen (Carpenter et al., 1961).
Man. A 19-month-old child swallowed an unknown amount of carbaryl.
Typical early signs of cholinesterase inhibition, i.e. constricted
pupils, excessive salivation, and muscle incoordination, occurred. A
single dose of atropine of 0.3 mg controlled the symptom and the child
recovered in 12 hours. Urine collected 18 hours after poisoning
contained 3140 µg of 1-naphthol per 100 ml (Best & Murray, 1962).
Short-term studies
Mouse. In one experiment 30 A/Jax and 30 C3H mice at the age of 3
months were started on a treatment of weekly subcutaneous injections
of 0.5 mg of carbaryl. Five months later, the survivors (26 A/Jax and
28 C3H) were sacrificed. In neither group did the survival rate or
tumour incidence differ from untreated mice of the same strains
(Mellon Institute, 1958).
Rat. Groups of 10 rats (5 of each sex) were fed 1500 or 2250 ppm
carbaryl in the diet for 96 days. At 2250 ppm, decreased body-weight
in the females, increased liver weight in the males and increased
kidney weight in the females were observed, while at 1500 ppm there
was increased kidney weight in the females. A minor histopathological
change in the form of diffuse cloudy swelling of the kidney tubules
was noted at the higher concentration (Carpenter et al., 1961).
Reproduction studies in 3 generations of CFE rats fed diets containing
carbaryl adjusted to dose levels of 0, 0.0025 or 0.01 mg/kg
body-weight daily, did not show any significant differences between
treatment groups in fertility, gestation, lactation or viability of
pups, mean number of pups per litter, body-weight of pups at weaning
or teratology (Mellon Institute, 1965).
Guinea-pig. Male guinea-pigs were injected with carbaryl and after a
3-week period were given a challenge dose. There was no evidence of
development of sensitization (Carpenter et al., 1961).
Dog. Groups of 3 or 4 dogs were given doses of 0.45, 1.8 and 7.2
mg/kg body-weight/day by capsule, 5 days per week for 1 year. Diffuse
cloudy swelling of the kidney tubules was found in all the dogs at the
7.2 mg/kg level. Focal cloudy swelling was found in the controls. One
female at the 0.45 mg/kg level had transient hind-leg weakness, but no
histological lesion was found at necropsy (Carpenter et al., 1961).
Long-term studies
Mouse. Groups of 48 male and 48 female Cd-1 mice were given 0, 100
and 400 ppm or carbaryl in the diet. After 80 weeks, 12 survivors of
each sex from each group were sacrificed. Survival rate, pathology and
tumour incidence were comparable in all groups (Mellon Institute,
1963)
Rat. In a 2-year experiment, groups of 20 CF-N rats of each sex were
given diets containing 0, 50, 100, 200 and 400 ppm of carbaryl. After
6, 9 and 12 months, 4, 6, or 8 rats of each sex were killed for organ
weight comparison and histopathological examination. The remainder
were sacrificed after 2 years. The highest dose level produced cloudy
swelling of the kidney tubules after 1 year and cloudy swelling of the
central hepatic cords in the males after 2 years. Terminal body-weight
in the males at the high level was reduced. There were no effects at
the lower dose levels (Carpenter et al., 1961).
Comments
While teratogenicity studies using extremely low doses were negative
in the rat, studies in other species have not been reported but would
be very desirable.
Several carbaryl metabolites have been identified in animals but not
yet in plants. Excretion of carbaryl and its metabolites appears to be
rapid in animals. Studies on the identification and toxicological
evaluation of residues occurring in plants would be desirable.
The behavioural studies appear interesting in relation to the mode of
action and as a potential system for detection of toxicity. However,
no data are available concerning experiments with continuous exposure
to the compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 400 ppm in the diet, equivalent to 60 mg/kg/day
Rat: 200 ppm in the diet, equivalent to 10 mg/kg/day
Dog: 1.8 mg/kg/day
Estimate of acceptable daily intake for man
0-0.02 mg/kg per day. (This value is based on experiments carried out
with carbaryl, and thus does not take account of chemical alterations
of the pesticide brought about by the plants to which it has been
applied.)
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Carbaryl has been approved for use on a wide variety of food crops,
including cereals, fruits and vegetables in a number of countries. It
is being used in increasing amounts and is partly replacing more
persistent and toxic compounds. In some countries it is used against
external parasites of cattle.
It is suggested for the control of at least 10 insects which attack
cane fruit (berries), 20 which attack tree fruits, 25 which attack
vegetables, as well as for use on other commodities and livestock. The
usual rate of application for berries and vegetables is 1 or 2 lb per
acre but for tree fruits as high as 10 lb per acre is suggested.
(b) Post-harvest treatments
Carbaryl has been used experimentally on stored cereals but is not so
used on a commercial scale.
National tolerances
Country Product Parts per million
Austria General 10
Canada Various 5, 10 and 25
United States of Sorghum (grain) 10
America Rice 5
Various fruits,
cabbage, lettuce,
carrots 10
Fruits (cane) 12
Garden beet,
parsnips,
radishes 5
Residues resulting from supervised trials
Many data have been obtained from supervised trials on a large number
of food crops produced under varying cultural conditions, rates and
times of application, pre-harvest intervals, etc. This information is
too extensive to reproduce in this monograph. Table 1 therefore
contains estimates of residues for groups of commodities as contained
in these reports. (A file summarizing such data for ca. 85 crops is
held at the FAO headquarters in Rome.) The residue figures quoted are
those likely to be present as two different pre-harvest intervals
following uses at rates of application that are considered to be
useful.
Residues in food moving in commerce
About two thousand samples of domestic and imported food products were
examined by the United States Food and Drug Administration during
1966. Less than 10 per cent were found to contain residues of carbaryl
and less than 0.1 per cent had residues above the tolerance levels
(information from United States Food and Drug Administration).
TABLE 1. AVERAGE RESIDUES FROM GOOD AGRICULTURAL PRACTICE
Food Resulting residue
Type Pre-harvest ppm
period
(days)
Vegetables
Leaf - spinach 14 10
Lettuce 30 1
Other 2 10
7-10 1
Cereal and 35-50 0
cereal products
wheat, rye, oats
Rice 14 5
30 1
Tree fruits 1 10
14-20 1
Caneberries 7 10
20 1
TABLE 1. AVERAGE RESIDUES FROM GOOD AGRICULTURAL PRACTICE
Food Resulting residue
Type Pre-harvest ppm
period
(days)
Citrus 1 10
20-60 1
Shelled nuts 1 1
Fate of residues
In the earlier studies on residues of carbaryl (Bibliography by
Moorefield, 1966), consideration had been given to the parent compound
carbaryl and its hydrolytic product, 1-naphthol. Residue studies on
many crops showed that the quantity of 1-naphthol was small and
difficult to separate from carbaryl. Thus, it was considered that
1-naphthol need not be measured separately from carbaryl. The United
States of America tolerances for carbaryl are based on the assumption
that the major part of the residue is the intact carbamate.
Since the establishment of the initial tolerances for carbaryl, work
has been done which may have some bearing on the quantative and
qualitative aspects of the residue. Light, including artificial and
sunlight, changes carbaryl. Carbaryl in the formulated state slowly
degraded under the influence of ultra-violet and to give several
unidentified products (Okada et al., 1961). Crosby et al. (1965)
reported that ethanol or hexane solutions of some methylcarbamate
insecticides, including carbaryl, gave a variety of
cholinesterase-inhibiting derivatives when exposed to either
artificial or sunlight. The products were separated but not
identified. Abdel-Waheb (1966) reported that the nature of (1) the
surface, (2) the light, and (3) the compound affected the rate of
conversion of carbaryl and other carbamates in vitro in the solid
state.
Since neither the qualitative nor quantative aspects of
photo-decomposition of carbamates have been established, the
significance of these studies in relation to current and future
tolerances for carbaryl must await further study.
(a) In animals
Whitehurst et al. (1963) using the colorimetric method of analysis
sensitive to carbaryl, 1-naphthol and 1-naphthol conjugates concluded
that, when cows were fed on a diet containing carbaryl, no residues,
to the limit of method, were found in the milk. This was confirmation
of the feeding study by Gyrisco et al. (1960), who fed dairy cows
carbaryl at levels of up to 450 ppm. In another study, carbaryl was
dusted and sprayed on cows. No detectable residues were found in milk
when carbaryl was dusted on to animals, but after 48 hours some
carbaryl residues were found in the milk when the spray application
was used (Buttram, 1964; Camp et al., 1963).
In recent work done by Dorough (1966), ring-labelled carbaryl was fed
to a lactating cow. A total of approximately 1 ppm, based upon a
radioactivity measurement calculated as carbaryl, was found in the
skim milk six to 12 hours after administration of 3.05 mg/kg
body-weight. Approximately one half of this residue was chloroform
extractable, and one half was water extractable with a small
unextractable fraction. At the end of 60 hours all the radioactivity
(calculated to be 0.01 ppm as equivalents of carbaryl) was
unextractable from the milk. In the six-hour samples about 30 per cent
of the radioactivity in the milk was characterized as
5,6-dihydro-5,6-dihydroxy 1-naphthol N-methylcarbamate, and
approximately 20 per cent was an unknown metabolite. Neither of these
materials, corresponding to 50 per cent of the total milk residue (0.5
ppm), responded to the standard colorimetric method commonly used for
carbaryl.
Cattle, sheep, goats, and hogs were sprayed four times in two weeks
with a 1.0 per cent suspension of carbaryl and Hereford steers were
fed 200 ppm of carbaryl in the diet for a 27-day period. These animals
were slaughtered at one and seven days after spraying or at the end of
the feeding interval. Residues of carbaryl, 1-naphthol and conjugates
of 1-naphthol were not detected in the body tissues of any of the
cattle, sheep, or hogs seven days after spraying. At this time
interval some small residues were noted in the fat and brain of a
goat. No residues were detected in tissues of cattle fed carbaryl for
27 days (Claborn, 1963). Krishna & Casida (1966) have reported that
less than 10 per cent of the administered acute dose of
carbonyl-C14-carbaryl was recovered after 48 hours as tissue
residues, primarily in those tissues known to be involved in body
contaminant eliminations - (e.g. liver, spleen, kidney). After seven
days Knaak (1966) found no residues resulting from carbonyl-carbaryl.
Laying hens were dusted three times at four-day intervals using 4 gm
of five per cent carbaryl per bird. One day after treatment no
residues were found in each type of edible tissue except skin. Seven
days after treatment this residue was reduced to low levels. Eggs were
found to be free of residue, throughout the study (Johnson,
Critchfield & Arthur, 1963).
(b) In plants
A limited amount of metabolism studies on carbamates in and on plants
has been reported. Studies by Dorough et al. (1963, 1964) found that
radioactive carbaryl yielded water soluble persistent metabolites
which were different than those found in the milk of a goat.
Carbonyl-C14-carbaryl, injected and administered to the surface of
bean plants yielded compounds that largely remain within the plant.
While these compounds have not been characterized, they are stable and
after 12 hours are not extractable by the usual organic solvents.
Abdel-Wahab observed that approximately 50 per cent of the surface
administered dose of C14 radioactivity was recovered after 72 hours.
When bean plants were injected into the stem with radioactive
carbaryl, approximately 75 per cent of the radioactivity persisted for
at least six days as aqueous and unextractable materials. Thus,
reactions within the plant presumably produce carbamates in some
stable form. These non-hydrolytic metabolites in many instances may
not respond to the usual method of analysis for carbaryl because (1)
the metabolites yield a phenolic material different than 1-naphthol
and do not show the same colour reaction, and (2) the major fraction
of some of the metabolites show solubility properties different than
the parent compounds. Preliminary studies on bio-assay of certain
metabolites have indicated a reduced biological activity when compared
with the parent compounds.
(c) In storage and processing
Raw unwashed tomatoes, harvested from field treated applications, were
stored at 55° F for approximately two weeks with samples taken at
varying intervals. Data showed that no loss of carbaryl was realized
under these storage conditions. The harvested tomatoes were subjected
to home and commercial canning operations, and analysis of samples
indicated that 50 per cent or more of the residue was removed by
water-washing and nearly complete removal of all residue was realized
by peeling and canning operations (Farrow et al. 1966).
Methods of residue analysis
The methods available for carbaryl are based on a colorimetric
determination following suitable extraction and clean-up. There are
approximately 20 variations of the basic method for different types
of food products, most of which are not published and no one method
has been tested for all food products.
For most fruits and vegetables the methods of (1) AOAC or that of (2)
Benson & Finocchiaro (1965) are suggested. These methods have a
sensitivity of about 0.1 ppm.
The above methods do not give very adequate clean-up for citrus,
olives, shelled nuts, many cereals and cereal products. Of the many
variations of the method, that developed for wheat and wheat fractions
has the most comprehensive clean-up. It is believed that it would be
useful for all products not amenable to the AOAC method.
RECOMMENDATIONS FOR TOLERANCES
The low acceptable daily intake necessitated a very close evaluation
of residue data to determine tolerances which would permit useful
applications to crops without possibly providing residues which might
exceed the acceptable daily intake.
Some data indicate that there will be losses under some conditions of
storage and processing. However, not enough data are available to make
a calculation at present, therefore no factor for such losses are
included in the calculations. The basic factor used in recommending
temporary tolerances which yield values within the ADI is that of
choosing a long pre-harvest interval.
The recommended tolerance figures are indicated in the following
table.
TEMPORARY TOLERANCE RECOMMENDATIONS FOR CARBARYL
Crop Temporary tolerance
in ppm
Vegetables 1.0
Cereal and cereal products 1.0
Tree fruits 1.0
Caneberries 1.2
Citrus 10.0
Shelled nuts 1.0
Further work or information
In the above calculations no considerations were given to possible
losses during storage, shipping and processing. It is recommended that
such data be developed and made available to the working party.
It is also recommended that further work be done to establish more
definitely the character of the terminal residue in treated plants.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Baron, R. L., Casterline, J. L. & Fitzhugh, O. G. (1964) Toxicol.
appl. Pharmacol., 6, 402
Best, E. M., jr & Murray, B. L. (1962) J. occup. Med., 4, 507
Carpenter, C. P., Weil, C. S., Palm, P. E., Woodside, M. W., Nair,
J. H. & Smyth, H. F., jr (1961) J. Agr. Food Chem., 9, 30
Claborn, H. V., Roberts, R. H., Mann, H. D., Bowman, M. C., Ivey,
M. C., Weidenbach, C. P. & Radeleff, R. D. (1963) J. Agr. Food
Chem., 11, 74
Dorough, H. W., Leeling, N. C. & Casida, J. E. (1963) Science,
140, 170
Dorough, H. W. & Casida, J. E. (1964) J. Agr. Food Chem., 12, 294
Eheart, J. F., Turner, E. C. & Dickinson, J. (1962) J. Econ. Ent.,
55, 504
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Goldberg, M. E. & Johnson, H. E. (1964a) J. Pharm. Pharmacol., 16,
60
Goldberg, M. E. & Johnson, H. E. (1964b) J. Pharmacol. exp. Ther.,
145, 367
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965a)
Psychopharmacologia, 7, 72
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965b) Biochem.
Pharmacol., 13, 1483
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, E. W., Fox,
F. H., Holland, R. F. & Trimberger, G. W. (1960) J. Agr. Food Chem.,
8, 409
Johnson, D. P., Critchfield, F. E. & Arthur, B. W. (1963) J. Agr.
Food Chem., 11, 77
Knaak, J. B., Tallant, M. J., Bartley, W. J. & Sullivan, L. J. (1965)
J. Agr. Food Chem., 13, 537
Krishna, J. G. & Casida, J. E. (1966), J. Agr. Food Chem., 14, 98
Mellon Institute of Industrial Research (1958a) Unpublished Report
Mellon Institute of Industrial Research (1958b) Unpublished Report
Mellon Institute of Industrial Research (1963) Unpublished Report
Mellon Institute of Industrial Research (1965) Unpublished Report
Roberts, R. H., Jackson, J. B., Westlake, W. E., Ackerman, A. J. &
Claborn, H. V. (1960) J. Econ. Ent., 53, 326
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abdel-Wahab, A. M., Kuhr, R. J., and Casida, J. E. (1966) The fate of
C14 C=O labeled aryl methylcarbamate insecticide chemicals in and on
bean plants. J. Agr. Food Chem. 14:290.
AOAC (1965) Methods of Analysis, (10th edition) paras. 24. 188-24.192
Benson, W. R. and Finocchiaro, J. M. (1965) Rapid Procedure for
Carbaryl Residues: Modification of Official Colorimetric Method. J.
Assoc. Offic. Agric. Chem. 48, 676-679
Buttram, J. Ress. (1964) Thesis, Auburn University. The metabolism of
carbamate and organophosphate insecticides by cattle and poultry and
associated residues in livestock products.
Camp, H. B., Buttram, J. R., Hays, K. L., and Arthur, B. W. (1963)
Sevin residues in milk from dairy cows following dermal applications.
J. Econ. Entomol. 56:402-404.
Casida, J. E., and Augustinsson, K.-B. (1959) Reaction of Plasma
Albumin with 1-naphthyl N-methylcarbamate and certain other esters.
Biochem. Biophys. Acta. 36:411-26.
Casida, J. E., Augustinsson, K.-B. and Jonsson, G. (1960) Stability,
toxicity and reaction mechanism with esterases of certain carbamate
insecticides. J. Econ. Entomol. 53:205-12.
Claborn, H. V. (1963) Residues in body tissues of livestock sprayed
with Sevin or Sevin in the diet. J. Agr. Food Chem. 11:74-76.
Crosby, D. C., Leitus, E., and Winterlin, W. L. (1965) The
photodecomposition of carbamate insecticides. J. Agr. Food Chem.
13:204.
Dorough, H. W., Leeling, N. C. and Casida, J. E. (1963) Nonhydrolytic
pathway in metabolism of N-methylcarbamate insecticides. Science
140:170-171.
Dorough, H. W. and Casida, J. E, (1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J. Agr. Food Chem. 12 (4):
294-304.
Dorough, H. W. (1966) Carbaryl-C14 metabolism in a lactating cow.
Private communication and in press.
Farrow, R. P., Lamb, F., Cook, R. W., Bergmans, B., Kimball, J. and
Elkins, E. R. (1966) Removal of Sevin residues from tomatoes during
commercial and home preparative procedures. Paper # 36, Pesticides
Subdivision of Agr. and Food Div., Amer. Chem. Soc. 152nd Meeting,
September 12-16, 1966.
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, E. W., Fox, F.
H., Holland, R. F., and Trimberger, G. W. (1960) The effects of
feeding high levels of Sevin on residue, flavor and odor of the milk
of dairy cattle. J. Agr. Food Chem. 8:409-410.
Hodgson, E. and Casida, J. E. (1960) Biological oxidation of
N,N-dialkyl carbamates. Biochem. Biophys. Acta. 42:184-186
Hodgson, E. and Casida, J. E. (1961) Metabolism of N,N-dialkyl
carbamates and related compounds by rat liver. Biochem. Pharmacol.
8:179-191.
Johnson, D. P., Critchfield, F. E., and Arthur, B. W. (1963)
Determination of Sevin insecticide and its metabolites in poultry
tissues and eggs. J. Agr. Food Chem. 11:77-80.
Knaak, J. B., Tallant, M. J., Bartley, W. J., and Sullivan, L. J.
(1965) The metabolism of carbaryl in the rat, guinea pig and man. J.
Agr. Food Chem. 13:537-543.
Krishna, J. G. and Casida, J. E. (1966) Fate in Rats of the
Radiocarbon from ten variously labeled methyl- and
dimethylcarbamate-C14 insecticide chemicals and their hydrolysis
products. J. Agr. Food Chem. 14:98-105.
Leeling, N. C. and Casida, J. E. (1966) Metabolites of carbaryl in
mammals and enzymatic systems for their formation. J. Agr. Food Chem.
14:282-290.
Morefield, H. H. "Agr. Products Tech. Service Report" bibliography on
Sevin insecticides (carbaryl) containing 1463 references has been
compiled by the Union Carbide Corporation, R & D Dept., Agr. Products,
Olefins Division, P.O. Box 8361, So. Charleston, W. Virginia 25303.
Okada, K., Nomura, K., Yamamoto, S., (1961) (no title). Nippon
Nogaikagaku Kaishi 35:739.
Whitehurst, W. E., Bishop, E. T., Critchfield, F. E., Gyrisco, G. G.,
Huddleston, E. W., Arnold, H., and Lisk, D. J. (1963) The metabolism
of Sevin in dairy cows. J. Agr. Food Chem 11:167-169.
Williams, R. T. (1959) Detoxication Mechanisms. John Wiley & Sons,
Inc., N. Y. 796 p.
 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Some knowledge has been obtained of the changes which the compound
undergoes in plants. Following injection of 14C-carbaryl into the
stems of snapbeans and cotton seedlings, 55 per cent of the activity
injected was present in the beans, and 86 per cent in the cotton, at
28 days. Of the original compound, 5.7 per cent was found in the beans
and 1.7 per cent in the cotton. It is suggested that conversion to
water-soluble metabolite(s), which may have been carbamates, took
place and these were quite stable within the plants (Dorough & Casida,
1964).
A slight rise in free 1-naphthol and a definite rise in conjugated
1-naphthol in the urine were observed during the 48 hours following
oral dosing of carbaryl in rats (Carpenter et al., 1961). Among
workers engaged in the production, handling and shipping of carbaryl,
those most heavily exposed (air concentrations of 0.23 to 31 mg/M3)
excreted large amounts of total 1-naphthol (Best & Murray, 1962).
14C-carbaryl, labelled at the N-methyl, carbonyl and naphthyl-1
positions, and metabolized by rat liver microsomes and insects,
yielded at least 5 metabolites (Dorough et al., 1963). Further work
has shown the existence of 6 metabolites, 5 of which were carbamates.
Three of these were tentatively identified as 1-naphthyl
N-hydroxymethylcarbamate, 4-hydroxy-1-naphthyl-N-methylcarbamate and
5-hydroxy-1-naphthyl-N-methylcarbamate (Dorough & Casida, 1964).
When 14C-carbonyl carbaryl was given orally to a goat in a dose of
1.34 mg/kg, several of these metabolites were found in the milk and
urine. The level of total 14C equivalents of carbaryl reached a peak
of 0.93 ppm in the milk at 8 hours and decreased to below 0.003 ppm at
60 hours. During the 96 hours after dosing, 47 per cent of the
radioactivity was excreted in the urine (Dorough et al., 1963; Dorough
& Casida, 1964).
In rats and guinea-pigs, 7 days after ingestion of labelled carbaryl,
the overall recovery of 14C-methyl, 14C-carbonyl and
14C-naphthyl labels was, respectively, 95, 99 and 91 per cent of the
dose of carbaryl. The only detectable 14C residues in the rat were
from 14C-methyl, representing 2-3 per cent of the dose. Several
metabolites were identified, and two of these, 1-naphthyl glucuronide
and sulfate, were also detected in urine from men exposed to carbaryl
dust (Knaak et al., 1965).
When carbaryl, labelled with 14C at the carbonyl, methylcarbamate or
naphthyl groups, was given to male rats by intraperitoneal injection,
recovery of 14C the expired CO2 after 48 hours was 24.5, 12.3 and
0.2 per cent respectively. At 24 hours, respective urine recoveries
were 62.1, 54.6 and 74.2 per cent and at 48 hours, 2.4, 3.4 and 2.3
per cent. Recoveries from faeces at 48 hours were 2.1., 3.9 and 8.9
per cent, and from carcass, 9.5, 12.7 and 6.7 per cent. After giving
14C-carbonyl carbaryl to male and female rats, no marked sex
differences were noted (Krishna & Casida, 1966).
Technical carbaryl was fed to dairy cows at levels up to 450 ppm for 2
weeks. Samples of milk taken at various times failed to disclose any
measurable residues. Carbaryl, if present, was below the sensitivity
of the method (0.01 ppm) (Gyrisco et al., 1960). In steers fed 50 or
200 ppm daily of technical carbaryl for 27 days, no tissue residues
could be detected (Claborn et al., 1963). Milk from cows 1 hour after
being sprayed to runoff with 0.5 per cent carbaryl solution or after
having received a tablespoon of 50 per cent dust spread down the back,
contained carbaryl residue. Samples taken at 15, 25 and 37 hours
contained no residue (Eheart et al., 1962). Milk taken as early as 5
hours after the last spray from 2 cows sprayed 4 times at 4 day
intervals with 0.5 per cent carbaryl suspension did not contain
carbaryl. Omental fat taken from 2 steers 5 days after the fourth
spraying did not contain carbaryl or 1-naphthol (Roberts et al.,
1960). Carbaryl residue, 1-naphthol and conjugate varied from 0-0.57
ppm in various tissues from steers, sheep, goats and hogs 1 day after
being sprayed to runoff with 1 per cent water suspension of 50 per
cent carbaryl powder. At 7 days no residue could be detected except in
fat and brain of the goat (Claborn et al., 1963).
In laying hens dusted 3 times at 4-day intervals with 4 g of 5 per
cent carbaryl dust per bird and killed 24 hours after the last
dusting, concentrations of less than 0.1 to 2.0 ppm carbaryl residue
and less than 0.1 ppm of 1-naphthol were found in breast and leg
muscle. Liver and gizzard concentrations of both residues were less
than 0.2 ppm. Residue concentrations in eggs remained below 0.2 ppm
throughout the study (Johnson et al., 1963).
A depression of blood and brain cholinesterase activity has been
reported following single large doses of carbaryl. Approximately the
same concentrations are required to produce a 50 per cent inhibition
in the blood of man, rabbit, rat and dog (Mellon Institute, 1958b). No
significant effect was found on dog erythrocyte or plasma
cholinesterase after single intravenous injections of 10 or 15 mg/kg.
In a dog which had received a total of 88.3 mg/kg in 11 doses
intravenously, typical symptoms of cholinesterase inhibition occurred
after 10 and 15 mg/kg, but only a slight reaction was seen after 5
mg/kg (Carpenter at al., 1961). In men exposed to carbaryl air
concentrations of 0.23 to 31 mg/M3 whole blood cholinesterase
activity was occasionally slightly depressed but there were no
clinical signs (Best & Murray, 1962).
Carbaryl is a reversible cholinesterase inhibitor. In fact, the
reversal is so rapid that unless special precautions are taken,
measurements of blood cholinesterase of persons exposed to it are
likely to be inaccurate and always tending to appear normal.
Pyridine-2-aldoxime methiodide, which is a good antidote for some
organophosphorus compounds, is not effective in reversing
cholinesterase inhibition by carbaryl (Mellon Institute, 1958b;
Carpenter at al., 1961). Atropine sulfate was effective in controlling
symptoms in the dog (Carpenter at al., 1961).
Special studies
Some studies have been reported describing the effects of a single
administration of carbaryl on discrete avoidance and food reward
behavioural tests in rats. In one report (Goldberg at al., 1965a) the
dose necessary to suppress avoidance response to 50 per cent
efficiency was slightly lower than the dose required to reduce brain
cholinesterase to 50 per cent of control value. The effects of
carbaryl on behaviour are prevented by atropine pre-treatment, and the
association with Chlorpromazine leads to more than additive effects.
Beta-dimethylaminoethyl-diphenylpropyl-acetate (SKF 525) increases the
behavioural effects of carbaryl without enhancement of cholinesterase
inhibition (Goldberg & Johnson, 1964a; Goldberg & Johnson, 1964b;
Goldberg et al., 1965a; Goldberg et al., 1965b).
Acute toxicity
Animal Route Solvent LD50 References
mg/kg
Mouse i.p. Corn oil 25 Barron et al., 1964
Rat, male oral 10% Tween 80 190 Mellon Institute, 1956a
Rat, male oral 10% Tween 80 310 Mellon Institute, 1958a
in 0.75% NaCl
Rat, male and
female oral 0.25% agar 480-610 Mellon Institute, 195ba
Rat, male oral Peanut oil 850 Gaines, 1960
Rat, female oral Peanut oil 500 Gaines, 1960
Rat, male oral Corn oil 308 Mellon Institute, 1958a
(continued)
Animal Route Solvent LD50 References
mg/kg
Rat, female oral Corn oil 560 Mellon Institute, 1958a
Rat i.v. Propylene glycol 18 Mellon Institute, 1958a
Rat i.v. PEG 400 24 Mellon Institute, 1958a
Rat i.v. Undiluted 93 Mellon Institute, 1958a
Guinea-pig, male oral 0.25% agar 280 Mellon Institute, 1958a
Rabbit, male oral 0.25% agar 707 Mellon Institute, 1958a
Dog oral Powder none died Mellon Institute, 195ba
(250-795 mg/kg)
Chicken. Focal loss of striation and fatty infiltration of muscle
was observed at 3 g/kg subcutaneously. Transient leg weakness for 1-2
days occurred after 2 g/kg and a nephrotoxic action was observed after
2 g/kg or more. No demyelination was seen (Carpenter et al., 1961).
Man. A 19-month-old child swallowed an unknown amount of carbaryl.
Typical early signs of cholinesterase inhibition, i.e. constricted
pupils, excessive salivation, and muscle incoordination, occurred. A
single dose of atropine of 0.3 mg controlled the symptom and the child
recovered in 12 hours. Urine collected 18 hours after poisoning
contained 3140 µg of 1-naphthol per 100 ml (Best & Murray, 1962).
Short-term studies
Mouse. In one experiment 30 A/Jax and 30 C3H mice at the age of 3
months were started on a treatment of weekly subcutaneous injections
of 0.5 mg of carbaryl. Five months later, the survivors (26 A/Jax and
28 C3H) were sacrificed. In neither group did the survival rate or
tumour incidence differ from untreated mice of the same strains
(Mellon Institute, 1958).
Rat. Groups of 10 rats (5 of each sex) were fed 1500 or 2250 ppm
carbaryl in the diet for 96 days. At 2250 ppm, decreased body-weight
in the females, increased liver weight in the males and increased
kidney weight in the females were observed, while at 1500 ppm there
was increased kidney weight in the females. A minor histopathological
change in the form of diffuse cloudy swelling of the kidney tubules
was noted at the higher concentration (Carpenter et al., 1961).
Reproduction studies in 3 generations of CFE rats fed diets containing
carbaryl adjusted to dose levels of 0, 0.0025 or 0.01 mg/kg
body-weight daily, did not show any significant differences between
treatment groups in fertility, gestation, lactation or viability of
pups, mean number of pups per litter, body-weight of pups at weaning
or teratology (Mellon Institute, 1965).
Guinea-pig. Male guinea-pigs were injected with carbaryl and after a
3-week period were given a challenge dose. There was no evidence of
development of sensitization (Carpenter et al., 1961).
Dog. Groups of 3 or 4 dogs were given doses of 0.45, 1.8 and 7.2
mg/kg body-weight/day by capsule, 5 days per week for 1 year. Diffuse
cloudy swelling of the kidney tubules was found in all the dogs at the
7.2 mg/kg level. Focal cloudy swelling was found in the controls. One
female at the 0.45 mg/kg level had transient hind-leg weakness, but no
histological lesion was found at necropsy (Carpenter et al., 1961).
Long-term studies
Mouse. Groups of 48 male and 48 female Cd-1 mice were given 0, 100
and 400 ppm or carbaryl in the diet. After 80 weeks, 12 survivors of
each sex from each group were sacrificed. Survival rate, pathology and
tumour incidence were comparable in all groups (Mellon Institute,
1963)
Rat. In a 2-year experiment, groups of 20 CF-N rats of each sex were
given diets containing 0, 50, 100, 200 and 400 ppm of carbaryl. After
6, 9 and 12 months, 4, 6, or 8 rats of each sex were killed for organ
weight comparison and histopathological examination. The remainder
were sacrificed after 2 years. The highest dose level produced cloudy
swelling of the kidney tubules after 1 year and cloudy swelling of the
central hepatic cords in the males after 2 years. Terminal body-weight
in the males at the high level was reduced. There were no effects at
the lower dose levels (Carpenter et al., 1961).
Comments
While teratogenicity studies using extremely low doses were negative
in the rat, studies in other species have not been reported but would
be very desirable.
Several carbaryl metabolites have been identified in animals but not
yet in plants. Excretion of carbaryl and its metabolites appears to be
rapid in animals. Studies on the identification and toxicological
evaluation of residues occurring in plants would be desirable.
The behavioural studies appear interesting in relation to the mode of
action and as a potential system for detection of toxicity. However,
no data are available concerning experiments with continuous exposure
to the compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 400 ppm in the diet, equivalent to 60 mg/kg/day
Rat: 200 ppm in the diet, equivalent to 10 mg/kg/day
Dog: 1.8 mg/kg/day
Estimate of acceptable daily intake for man
0-0.02 mg/kg per day. (This value is based on experiments carried out
with carbaryl, and thus does not take account of chemical alterations
of the pesticide brought about by the plants to which it has been
applied.)
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Carbaryl has been approved for use on a wide variety of food crops,
including cereals, fruits and vegetables in a number of countries. It
is being used in increasing amounts and is partly replacing more
persistent and toxic compounds. In some countries it is used against
external parasites of cattle.
It is suggested for the control of at least 10 insects which attack
cane fruit (berries), 20 which attack tree fruits, 25 which attack
vegetables, as well as for use on other commodities and livestock. The
usual rate of application for berries and vegetables is 1 or 2 lb per
acre but for tree fruits as high as 10 lb per acre is suggested.
(b) Post-harvest treatments
Carbaryl has been used experimentally on stored cereals but is not so
used on a commercial scale.
National tolerances
Country Product Parts per million
Austria General 10
Canada Various 5, 10 and 25
United States of Sorghum (grain) 10
America Rice 5
Various fruits,
cabbage, lettuce,
carrots 10
Fruits (cane) 12
Garden beet,
parsnips,
radishes 5
Residues resulting from supervised trials
Many data have been obtained from supervised trials on a large number
of food crops produced under varying cultural conditions, rates and
times of application, pre-harvest intervals, etc. This information is
too extensive to reproduce in this monograph. Table 1 therefore
contains estimates of residues for groups of commodities as contained
in these reports. (A file summarizing such data for ca. 85 crops is
held at the FAO headquarters in Rome.) The residue figures quoted are
those likely to be present as two different pre-harvest intervals
following uses at rates of application that are considered to be
useful.
Residues in food moving in commerce
About two thousand samples of domestic and imported food products were
examined by the United States Food and Drug Administration during
1966. Less than 10 per cent were found to contain residues of carbaryl
and less than 0.1 per cent had residues above the tolerance levels
(information from United States Food and Drug Administration).
TABLE 1. AVERAGE RESIDUES FROM GOOD AGRICULTURAL PRACTICE
Food Resulting residue
Type Pre-harvest ppm
period
(days)
Vegetables
Leaf - spinach 14 10
Lettuce 30 1
Other 2 10
7-10 1
Cereal and 35-50 0
cereal products
wheat, rye, oats
Rice 14 5
30 1
Tree fruits 1 10
14-20 1
Caneberries 7 10
20 1
TABLE 1. AVERAGE RESIDUES FROM GOOD AGRICULTURAL PRACTICE
Food Resulting residue
Type Pre-harvest ppm
period
(days)
Citrus 1 10
20-60 1
Shelled nuts 1 1
Fate of residues
In the earlier studies on residues of carbaryl (Bibliography by
Moorefield, 1966), consideration had been given to the parent compound
carbaryl and its hydrolytic product, 1-naphthol. Residue studies on
many crops showed that the quantity of 1-naphthol was small and
difficult to separate from carbaryl. Thus, it was considered that
1-naphthol need not be measured separately from carbaryl. The United
States of America tolerances for carbaryl are based on the assumption
that the major part of the residue is the intact carbamate.
Since the establishment of the initial tolerances for carbaryl, work
has been done which may have some bearing on the quantative and
qualitative aspects of the residue. Light, including artificial and
sunlight, changes carbaryl. Carbaryl in the formulated state slowly
degraded under the influence of ultra-violet and to give several
unidentified products (Okada et al., 1961). Crosby et al. (1965)
reported that ethanol or hexane solutions of some methylcarbamate
insecticides, including carbaryl, gave a variety of
cholinesterase-inhibiting derivatives when exposed to either
artificial or sunlight. The products were separated but not
identified. Abdel-Waheb (1966) reported that the nature of (1) the
surface, (2) the light, and (3) the compound affected the rate of
conversion of carbaryl and other carbamates in vitro in the solid
state.
Since neither the qualitative nor quantative aspects of
photo-decomposition of carbamates have been established, the
significance of these studies in relation to current and future
tolerances for carbaryl must await further study.
(a) In animals
Whitehurst et al. (1963) using the colorimetric method of analysis
sensitive to carbaryl, 1-naphthol and 1-naphthol conjugates concluded
that, when cows were fed on a diet containing carbaryl, no residues,
to the limit of method, were found in the milk. This was confirmation
of the feeding study by Gyrisco et al. (1960), who fed dairy cows
carbaryl at levels of up to 450 ppm. In another study, carbaryl was
dusted and sprayed on cows. No detectable residues were found in milk
when carbaryl was dusted on to animals, but after 48 hours some
carbaryl residues were found in the milk when the spray application
was used (Buttram, 1964; Camp et al., 1963).
In recent work done by Dorough (1966), ring-labelled carbaryl was fed
to a lactating cow. A total of approximately 1 ppm, based upon a
radioactivity measurement calculated as carbaryl, was found in the
skim milk six to 12 hours after administration of 3.05 mg/kg
body-weight. Approximately one half of this residue was chloroform
extractable, and one half was water extractable with a small
unextractable fraction. At the end of 60 hours all the radioactivity
(calculated to be 0.01 ppm as equivalents of carbaryl) was
unextractable from the milk. In the six-hour samples about 30 per cent
of the radioactivity in the milk was characterized as
5,6-dihydro-5,6-dihydroxy 1-naphthol N-methylcarbamate, and
approximately 20 per cent was an unknown metabolite. Neither of these
materials, corresponding to 50 per cent of the total milk residue (0.5
ppm), responded to the standard colorimetric method commonly used for
carbaryl.
Cattle, sheep, goats, and hogs were sprayed four times in two weeks
with a 1.0 per cent suspension of carbaryl and Hereford steers were
fed 200 ppm of carbaryl in the diet for a 27-day period. These animals
were slaughtered at one and seven days after spraying or at the end of
the feeding interval. Residues of carbaryl, 1-naphthol and conjugates
of 1-naphthol were not detected in the body tissues of any of the
cattle, sheep, or hogs seven days after spraying. At this time
interval some small residues were noted in the fat and brain of a
goat. No residues were detected in tissues of cattle fed carbaryl for
27 days (Claborn, 1963). Krishna & Casida (1966) have reported that
less than 10 per cent of the administered acute dose of
carbonyl-C14-carbaryl was recovered after 48 hours as tissue
residues, primarily in those tissues known to be involved in body
contaminant eliminations - (e.g. liver, spleen, kidney). After seven
days Knaak (1966) found no residues resulting from carbonyl-carbaryl.
Laying hens were dusted three times at four-day intervals using 4 gm
of five per cent carbaryl per bird. One day after treatment no
residues were found in each type of edible tissue except skin. Seven
days after treatment this residue was reduced to low levels. Eggs were
found to be free of residue, throughout the study (Johnson,
Critchfield & Arthur, 1963).
(b) In plants
A limited amount of metabolism studies on carbamates in and on plants
has been reported. Studies by Dorough et al. (1963, 1964) found that
radioactive carbaryl yielded water soluble persistent metabolites
which were different than those found in the milk of a goat.
Carbonyl-C14-carbaryl, injected and administered to the surface of
bean plants yielded compounds that largely remain within the plant.
While these compounds have not been characterized, they are stable and
after 12 hours are not extractable by the usual organic solvents.
Abdel-Wahab observed that approximately 50 per cent of the surface
administered dose of C14 radioactivity was recovered after 72 hours.
When bean plants were injected into the stem with radioactive
carbaryl, approximately 75 per cent of the radioactivity persisted for
at least six days as aqueous and unextractable materials. Thus,
reactions within the plant presumably produce carbamates in some
stable form. These non-hydrolytic metabolites in many instances may
not respond to the usual method of analysis for carbaryl because (1)
the metabolites yield a phenolic material different than 1-naphthol
and do not show the same colour reaction, and (2) the major fraction
of some of the metabolites show solubility properties different than
the parent compounds. Preliminary studies on bio-assay of certain
metabolites have indicated a reduced biological activity when compared
with the parent compounds.
(c) In storage and processing
Raw unwashed tomatoes, harvested from field treated applications, were
stored at 55° F for approximately two weeks with samples taken at
varying intervals. Data showed that no loss of carbaryl was realized
under these storage conditions. The harvested tomatoes were subjected
to home and commercial canning operations, and analysis of samples
indicated that 50 per cent or more of the residue was removed by
water-washing and nearly complete removal of all residue was realized
by peeling and canning operations (Farrow et al. 1966).
Methods of residue analysis
The methods available for carbaryl are based on a colorimetric
determination following suitable extraction and clean-up. There are
approximately 20 variations of the basic method for different types
of food products, most of which are not published and no one method
has been tested for all food products.
For most fruits and vegetables the methods of (1) AOAC or that of (2)
Benson & Finocchiaro (1965) are suggested. These methods have a
sensitivity of about 0.1 ppm.
The above methods do not give very adequate clean-up for citrus,
olives, shelled nuts, many cereals and cereal products. Of the many
variations of the method, that developed for wheat and wheat fractions
has the most comprehensive clean-up. It is believed that it would be
useful for all products not amenable to the AOAC method.
RECOMMENDATIONS FOR TOLERANCES
The low acceptable daily intake necessitated a very close evaluation
of residue data to determine tolerances which would permit useful
applications to crops without possibly providing residues which might
exceed the acceptable daily intake.
Some data indicate that there will be losses under some conditions of
storage and processing. However, not enough data are available to make
a calculation at present, therefore no factor for such losses are
included in the calculations. The basic factor used in recommending
temporary tolerances which yield values within the ADI is that of
choosing a long pre-harvest interval.
The recommended tolerance figures are indicated in the following
table.
TEMPORARY TOLERANCE RECOMMENDATIONS FOR CARBARYL
Crop Temporary tolerance
in ppm
Vegetables 1.0
Cereal and cereal products 1.0
Tree fruits 1.0
Caneberries 1.2
Citrus 10.0
Shelled nuts 1.0
Further work or information
In the above calculations no considerations were given to possible
losses during storage, shipping and processing. It is recommended that
such data be developed and made available to the working party.
It is also recommended that further work be done to establish more
definitely the character of the terminal residue in treated plants.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Baron, R. L., Casterline, J. L. & Fitzhugh, O. G. (1964) Toxicol.
appl. Pharmacol., 6, 402
Best, E. M., jr & Murray, B. L. (1962) J. occup. Med., 4, 507
Carpenter, C. P., Weil, C. S., Palm, P. E., Woodside, M. W., Nair,
J. H. & Smyth, H. F., jr (1961) J. Agr. Food Chem., 9, 30
Claborn, H. V., Roberts, R. H., Mann, H. D., Bowman, M. C., Ivey,
M. C., Weidenbach, C. P. & Radeleff, R. D. (1963) J. Agr. Food
Chem., 11, 74
Dorough, H. W., Leeling, N. C. & Casida, J. E. (1963) Science,
140, 170
Dorough, H. W. & Casida, J. E. (1964) J. Agr. Food Chem., 12, 294
Eheart, J. F., Turner, E. C. & Dickinson, J. (1962) J. Econ. Ent.,
55, 504
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Goldberg, M. E. & Johnson, H. E. (1964a) J. Pharm. Pharmacol., 16,
60
Goldberg, M. E. & Johnson, H. E. (1964b) J. Pharmacol. exp. Ther.,
145, 367
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965a)
Psychopharmacologia, 7, 72
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965b) Biochem.
Pharmacol., 13, 1483
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, E. W., Fox,
F. H., Holland, R. F. & Trimberger, G. W. (1960) J. Agr. Food Chem.,
8, 409
Johnson, D. P., Critchfield, F. E. & Arthur, B. W. (1963) J. Agr.
Food Chem., 11, 77
Knaak, J. B., Tallant, M. J., Bartley, W. J. & Sullivan, L. J. (1965)
J. Agr. Food Chem., 13, 537
Krishna, J. G. & Casida, J. E. (1966), J. Agr. Food Chem., 14, 98
Mellon Institute of Industrial Research (1958a) Unpublished Report
Mellon Institute of Industrial Research (1958b) Unpublished Report
Mellon Institute of Industrial Research (1963) Unpublished Report
Mellon Institute of Industrial Research (1965) Unpublished Report
Roberts, R. H., Jackson, J. B., Westlake, W. E., Ackerman, A. J. &
Claborn, H. V. (1960) J. Econ. Ent., 53, 326
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abdel-Wahab, A. M., Kuhr, R. J., and Casida, J. E. (1966) The fate of
C14 C=O labeled aryl methylcarbamate insecticide chemicals in and on
bean plants. J. Agr. Food Chem. 14:290.
AOAC (1965) Methods of Analysis, (10th edition) paras. 24. 188-24.192
Benson, W. R. and Finocchiaro, J. M. (1965) Rapid Procedure for
Carbaryl Residues: Modification of Official Colorimetric Method. J.
Assoc. Offic. Agric. Chem. 48, 676-679
Buttram, J. Ress. (1964) Thesis, Auburn University. The metabolism of
carbamate and organophosphate insecticides by cattle and poultry and
associated residues in livestock products.
Camp, H. B., Buttram, J. R., Hays, K. L., and Arthur, B. W. (1963)
Sevin residues in milk from dairy cows following dermal applications.
J. Econ. Entomol. 56:402-404.
Casida, J. E., and Augustinsson, K.-B. (1959) Reaction of Plasma
Albumin with 1-naphthyl N-methylcarbamate and certain other esters.
Biochem. Biophys. Acta. 36:411-26.
Casida, J. E., Augustinsson, K.-B. and Jonsson, G. (1960) Stability,
toxicity and reaction mechanism with esterases of certain carbamate
insecticides. J. Econ. Entomol. 53:205-12.
Claborn, H. V. (1963) Residues in body tissues of livestock sprayed
with Sevin or Sevin in the diet. J. Agr. Food Chem. 11:74-76.
Crosby, D. C., Leitus, E., and Winterlin, W. L. (1965) The
photodecomposition of carbamate insecticides. J. Agr. Food Chem.
13:204.
Dorough, H. W., Leeling, N. C. and Casida, J. E. (1963) Nonhydrolytic
pathway in metabolism of N-methylcarbamate insecticides. Science
140:170-171.
Dorough, H. W. and Casida, J. E, (1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J. Agr. Food Chem. 12 (4):
294-304.
Dorough, H. W. (1966) Carbaryl-C14 metabolism in a lactating cow.
Private communication and in press.
Farrow, R. P., Lamb, F., Cook, R. W., Bergmans, B., Kimball, J. and
Elkins, E. R. (1966) Removal of Sevin residues from tomatoes during
commercial and home preparative procedures. Paper # 36, Pesticides
Subdivision of Agr. and Food Div., Amer. Chem. Soc. 152nd Meeting,
September 12-16, 1966.
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, E. W., Fox, F.
H., Holland, R. F., and Trimberger, G. W. (1960) The effects of
feeding high levels of Sevin on residue, flavor and odor of the milk
of dairy cattle. J. Agr. Food Chem. 8:409-410.
Hodgson, E. and Casida, J. E. (1960) Biological oxidation of
N,N-dialkyl carbamates. Biochem. Biophys. Acta. 42:184-186
Hodgson, E. and Casida, J. E. (1961) Metabolism of N,N-dialkyl
carbamates and related compounds by rat liver. Biochem. Pharmacol.
8:179-191.
Johnson, D. P., Critchfield, F. E., and Arthur, B. W. (1963)
Determination of Sevin insecticide and its metabolites in poultry
tissues and eggs. J. Agr. Food Chem. 11:77-80.
Knaak, J. B., Tallant, M. J., Bartley, W. J., and Sullivan, L. J.
(1965) The metabolism of carbaryl in the rat, guinea pig and man. J.
Agr. Food Chem. 13:537-543.
Krishna, J. G. and Casida, J. E. (1966) Fate in Rats of the
Radiocarbon from ten variously labeled methyl- and
dimethylcarbamate-C14 insecticide chemicals and their hydrolysis
products. J. Agr. Food Chem. 14:98-105.
Leeling, N. C. and Casida, J. E. (1966) Metabolites of carbaryl in
mammals and enzymatic systems for their formation. J. Agr. Food Chem.
14:282-290.
Morefield, H. H. "Agr. Products Tech. Service Report" bibliography on
Sevin insecticides (carbaryl) containing 1463 references has been
compiled by the Union Carbide Corporation, R & D Dept., Agr. Products,
Olefins Division, P.O. Box 8361, So. Charleston, W. Virginia 25303.
Okada, K., Nomura, K., Yamamoto, S., (1961) (no title). Nippon
Nogaikagaku Kaishi 35:739.
Whitehurst, W. E., Bishop, E. T., Critchfield, F. E., Gyrisco, G. G.,
Huddleston, E. W., Arnold, H., and Lisk, D. J. (1963) The metabolism
of Sevin in dairy cows. J. Agr. Food Chem 11:167-169.
Williams, R. T. (1959) Detoxication Mechanisms. John Wiley & Sons,
Inc., N. Y. 796 p.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Some knowledge has been obtained of the changes which the compound
undergoes in plants. Following injection of 14C-carbaryl into the
stems of snapbeans and cotton seedlings, 55 per cent of the activity
injected was present in the beans, and 86 per cent in the cotton, at
28 days. Of the original compound, 5.7 per cent was found in the beans
and 1.7 per cent in the cotton. It is suggested that conversion to
water-soluble metabolite(s), which may have been carbamates, took
place and these were quite stable within the plants (Dorough & Casida,
1964).
A slight rise in free 1-naphthol and a definite rise in conjugated
1-naphthol in the urine were observed during the 48 hours following
oral dosing of carbaryl in rats (Carpenter et al., 1961). Among
workers engaged in the production, handling and shipping of carbaryl,
those most heavily exposed (air concentrations of 0.23 to 31 mg/M3)
excreted large amounts of total 1-naphthol (Best & Murray, 1962).
14C-carbaryl, labelled at the N-methyl, carbonyl and naphthyl-1
positions, and metabolized by rat liver microsomes and insects,
yielded at least 5 metabolites (Dorough et al., 1963). Further work
has shown the existence of 6 metabolites, 5 of which were carbamates.
Three of these were tentatively identified as 1-naphthyl
N-hydroxymethylcarbamate, 4-hydroxy-1-naphthyl-N-methylcarbamate and
5-hydroxy-1-naphthyl-N-methylcarbamate (Dorough & Casida, 1964).
When 14C-carbonyl carbaryl was given orally to a goat in a dose of
1.34 mg/kg, several of these metabolites were found in the milk and
urine. The level of total 14C equivalents of carbaryl reached a peak
of 0.93 ppm in the milk at 8 hours and decreased to below 0.003 ppm at
60 hours. During the 96 hours after dosing, 47 per cent of the
radioactivity was excreted in the urine (Dorough et al., 1963; Dorough
& Casida, 1964).
In rats and guinea-pigs, 7 days after ingestion of labelled carbaryl,
the overall recovery of 14C-methyl, 14C-carbonyl and
14C-naphthyl labels was, respectively, 95, 99 and 91 per cent of the
dose of carbaryl. The only detectable 14C residues in the rat were
from 14C-methyl, representing 2-3 per cent of the dose. Several
metabolites were identified, and two of these, 1-naphthyl glucuronide
and sulfate, were also detected in urine from men exposed to carbaryl
dust (Knaak et al., 1965).
When carbaryl, labelled with 14C at the carbonyl, methylcarbamate or
naphthyl groups, was given to male rats by intraperitoneal injection,
recovery of 14C the expired CO2 after 48 hours was 24.5, 12.3 and
0.2 per cent respectively. At 24 hours, respective urine recoveries
were 62.1, 54.6 and 74.2 per cent and at 48 hours, 2.4, 3.4 and 2.3
per cent. Recoveries from faeces at 48 hours were 2.1., 3.9 and 8.9
per cent, and from carcass, 9.5, 12.7 and 6.7 per cent. After giving
14C-carbonyl carbaryl to male and female rats, no marked sex
differences were noted (Krishna & Casida, 1966).
Technical carbaryl was fed to dairy cows at levels up to 450 ppm for 2
weeks. Samples of milk taken at various times failed to disclose any
measurable residues. Carbaryl, if present, was below the sensitivity
of the method (0.01 ppm) (Gyrisco et al., 1960). In steers fed 50 or
200 ppm daily of technical carbaryl for 27 days, no tissue residues
could be detected (Claborn et al., 1963). Milk from cows 1 hour after
being sprayed to runoff with 0.5 per cent carbaryl solution or after
having received a tablespoon of 50 per cent dust spread down the back,
contained carbaryl residue. Samples taken at 15, 25 and 37 hours
contained no residue (Eheart et al., 1962). Milk taken as early as 5
hours after the last spray from 2 cows sprayed 4 times at 4 day
intervals with 0.5 per cent carbaryl suspension did not contain
carbaryl. Omental fat taken from 2 steers 5 days after the fourth
spraying did not contain carbaryl or 1-naphthol (Roberts et al.,
1960). Carbaryl residue, 1-naphthol and conjugate varied from 0-0.57
ppm in various tissues from steers, sheep, goats and hogs 1 day after
being sprayed to runoff with 1 per cent water suspension of 50 per
cent carbaryl powder. At 7 days no residue could be detected except in
fat and brain of the goat (Claborn et al., 1963).
In laying hens dusted 3 times at 4-day intervals with 4 g of 5 per
cent carbaryl dust per bird and killed 24 hours after the last
dusting, concentrations of less than 0.1 to 2.0 ppm carbaryl residue
and less than 0.1 ppm of 1-naphthol were found in breast and leg
muscle. Liver and gizzard concentrations of both residues were less
than 0.2 ppm. Residue concentrations in eggs remained below 0.2 ppm
throughout the study (Johnson et al., 1963).
A depression of blood and brain cholinesterase activity has been
reported following single large doses of carbaryl. Approximately the
same concentrations are required to produce a 50 per cent inhibition
in the blood of man, rabbit, rat and dog (Mellon Institute, 1958b). No
significant effect was found on dog erythrocyte or plasma
cholinesterase after single intravenous injections of 10 or 15 mg/kg.
In a dog which had received a total of 88.3 mg/kg in 11 doses
intravenously, typical symptoms of cholinesterase inhibition occurred
after 10 and 15 mg/kg, but only a slight reaction was seen after 5
mg/kg (Carpenter at al., 1961). In men exposed to carbaryl air
concentrations of 0.23 to 31 mg/M3 whole blood cholinesterase
activity was occasionally slightly depressed but there were no
clinical signs (Best & Murray, 1962).
Carbaryl is a reversible cholinesterase inhibitor. In fact, the
reversal is so rapid that unless special precautions are taken,
measurements of blood cholinesterase of persons exposed to it are
likely to be inaccurate and always tending to appear normal.
Pyridine-2-aldoxime methiodide, which is a good antidote for some
organophosphorus compounds, is not effective in reversing
cholinesterase inhibition by carbaryl (Mellon Institute, 1958b;
Carpenter at al., 1961). Atropine sulfate was effective in controlling
symptoms in the dog (Carpenter at al., 1961).
Special studies
Some studies have been reported describing the effects of a single
administration of carbaryl on discrete avoidance and food reward
behavioural tests in rats. In one report (Goldberg at al., 1965a) the
dose necessary to suppress avoidance response to 50 per cent
efficiency was slightly lower than the dose required to reduce brain
cholinesterase to 50 per cent of control value. The effects of
carbaryl on behaviour are prevented by atropine pre-treatment, and the
association with Chlorpromazine leads to more than additive effects.
Beta-dimethylaminoethyl-diphenylpropyl-acetate (SKF 525) increases the
behavioural effects of carbaryl without enhancement of cholinesterase
inhibition (Goldberg & Johnson, 1964a; Goldberg & Johnson, 1964b;
Goldberg et al., 1965a; Goldberg et al., 1965b).
Acute toxicity
Animal Route Solvent LD50 References
mg/kg
Mouse i.p. Corn oil 25 Barron et al., 1964
Rat, male oral 10% Tween 80 190 Mellon Institute, 1956a
Rat, male oral 10% Tween 80 310 Mellon Institute, 1958a
in 0.75% NaCl
Rat, male and
female oral 0.25% agar 480-610 Mellon Institute, 195ba
Rat, male oral Peanut oil 850 Gaines, 1960
Rat, female oral Peanut oil 500 Gaines, 1960
Rat, male oral Corn oil 308 Mellon Institute, 1958a
(continued)
Animal Route Solvent LD50 References
mg/kg
Rat, female oral Corn oil 560 Mellon Institute, 1958a
Rat i.v. Propylene glycol 18 Mellon Institute, 1958a
Rat i.v. PEG 400 24 Mellon Institute, 1958a
Rat i.v. Undiluted 93 Mellon Institute, 1958a
Guinea-pig, male oral 0.25% agar 280 Mellon Institute, 1958a
Rabbit, male oral 0.25% agar 707 Mellon Institute, 1958a
Dog oral Powder none died Mellon Institute, 195ba
(250-795 mg/kg)
Chicken. Focal loss of striation and fatty infiltration of muscle
was observed at 3 g/kg subcutaneously. Transient leg weakness for 1-2
days occurred after 2 g/kg and a nephrotoxic action was observed after
2 g/kg or more. No demyelination was seen (Carpenter et al., 1961).
Man. A 19-month-old child swallowed an unknown amount of carbaryl.
Typical early signs of cholinesterase inhibition, i.e. constricted
pupils, excessive salivation, and muscle incoordination, occurred. A
single dose of atropine of 0.3 mg controlled the symptom and the child
recovered in 12 hours. Urine collected 18 hours after poisoning
contained 3140 µg of 1-naphthol per 100 ml (Best & Murray, 1962).
Short-term studies
Mouse. In one experiment 30 A/Jax and 30 C3H mice at the age of 3
months were started on a treatment of weekly subcutaneous injections
of 0.5 mg of carbaryl. Five months later, the survivors (26 A/Jax and
28 C3H) were sacrificed. In neither group did the survival rate or
tumour incidence differ from untreated mice of the same strains
(Mellon Institute, 1958).
Rat. Groups of 10 rats (5 of each sex) were fed 1500 or 2250 ppm
carbaryl in the diet for 96 days. At 2250 ppm, decreased body-weight
in the females, increased liver weight in the males and increased
kidney weight in the females were observed, while at 1500 ppm there
was increased kidney weight in the females. A minor histopathological
change in the form of diffuse cloudy swelling of the kidney tubules
was noted at the higher concentration (Carpenter et al., 1961).
Reproduction studies in 3 generations of CFE rats fed diets containing
carbaryl adjusted to dose levels of 0, 0.0025 or 0.01 mg/kg
body-weight daily, did not show any significant differences between
treatment groups in fertility, gestation, lactation or viability of
pups, mean number of pups per litter, body-weight of pups at weaning
or teratology (Mellon Institute, 1965).
Guinea-pig. Male guinea-pigs were injected with carbaryl and after a
3-week period were given a challenge dose. There was no evidence of
development of sensitization (Carpenter et al., 1961).
Dog. Groups of 3 or 4 dogs were given doses of 0.45, 1.8 and 7.2
mg/kg body-weight/day by capsule, 5 days per week for 1 year. Diffuse
cloudy swelling of the kidney tubules was found in all the dogs at the
7.2 mg/kg level. Focal cloudy swelling was found in the controls. One
female at the 0.45 mg/kg level had transient hind-leg weakness, but no
histological lesion was found at necropsy (Carpenter et al., 1961).
Long-term studies
Mouse. Groups of 48 male and 48 female Cd-1 mice were given 0, 100
and 400 ppm or carbaryl in the diet. After 80 weeks, 12 survivors of
each sex from each group were sacrificed. Survival rate, pathology and
tumour incidence were comparable in all groups (Mellon Institute,
1963)
Rat. In a 2-year experiment, groups of 20 CF-N rats of each sex were
given diets containing 0, 50, 100, 200 and 400 ppm of carbaryl. After
6, 9 and 12 months, 4, 6, or 8 rats of each sex were killed for organ
weight comparison and histopathological examination. The remainder
were sacrificed after 2 years. The highest dose level produced cloudy
swelling of the kidney tubules after 1 year and cloudy swelling of the
central hepatic cords in the males after 2 years. Terminal body-weight
in the males at the high level was reduced. There were no effects at
the lower dose levels (Carpenter et al., 1961).
Comments
While teratogenicity studies using extremely low doses were negative
in the rat, studies in other species have not been reported but would
be very desirable.
Several carbaryl metabolites have been identified in animals but not
yet in plants. Excretion of carbaryl and its metabolites appears to be
rapid in animals. Studies on the identification and toxicological
evaluation of residues occurring in plants would be desirable.
The behavioural studies appear interesting in relation to the mode of
action and as a potential system for detection of toxicity. However,
no data are available concerning experiments with continuous exposure
to the compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 400 ppm in the diet, equivalent to 60 mg/kg/day
Rat: 200 ppm in the diet, equivalent to 10 mg/kg/day
Dog: 1.8 mg/kg/day
Estimate of acceptable daily intake for man
0-0.02 mg/kg per day. (This value is based on experiments carried out
with carbaryl, and thus does not take account of chemical alterations
of the pesticide brought about by the plants to which it has been
applied.)
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Carbaryl has been approved for use on a wide variety of food crops,
including cereals, fruits and vegetables in a number of countries. It
is being used in increasing amounts and is partly replacing more
persistent and toxic compounds. In some countries it is used against
external parasites of cattle.
It is suggested for the control of at least 10 insects which attack
cane fruit (berries), 20 which attack tree fruits, 25 which attack
vegetables, as well as for use on other commodities and livestock. The
usual rate of application for berries and vegetables is 1 or 2 lb per
acre but for tree fruits as high as 10 lb per acre is suggested.
(b) Post-harvest treatments
Carbaryl has been used experimentally on stored cereals but is not so
used on a commercial scale.
National tolerances
Country Product Parts per million
Austria General 10
Canada Various 5, 10 and 25
United States of Sorghum (grain) 10
America Rice 5
Various fruits,
cabbage, lettuce,
carrots 10
Fruits (cane) 12
Garden beet,
parsnips,
radishes 5
Residues resulting from supervised trials
Many data have been obtained from supervised trials on a large number
of food crops produced under varying cultural conditions, rates and
times of application, pre-harvest intervals, etc. This information is
too extensive to reproduce in this monograph. Table 1 therefore
contains estimates of residues for groups of commodities as contained
in these reports. (A file summarizing such data for ca. 85 crops is
held at the FAO headquarters in Rome.) The residue figures quoted are
those likely to be present as two different pre-harvest intervals
following uses at rates of application that are considered to be
useful.
Residues in food moving in commerce
About two thousand samples of domestic and imported food products were
examined by the United States Food and Drug Administration during
1966. Less than 10 per cent were found to contain residues of carbaryl
and less than 0.1 per cent had residues above the tolerance levels
(information from United States Food and Drug Administration).
TABLE 1. AVERAGE RESIDUES FROM GOOD AGRICULTURAL PRACTICE
Food Resulting residue
Type Pre-harvest ppm
period
(days)
Vegetables
Leaf - spinach 14 10
Lettuce 30 1
Other 2 10
7-10 1
Cereal and 35-50 0
cereal products
wheat, rye, oats
Rice 14 5
30 1
Tree fruits 1 10
14-20 1
Caneberries 7 10
20 1
TABLE 1. AVERAGE RESIDUES FROM GOOD AGRICULTURAL PRACTICE
Food Resulting residue
Type Pre-harvest ppm
period
(days)
Citrus 1 10
20-60 1
Shelled nuts 1 1
Fate of residues
In the earlier studies on residues of carbaryl (Bibliography by
Moorefield, 1966), consideration had been given to the parent compound
carbaryl and its hydrolytic product, 1-naphthol. Residue studies on
many crops showed that the quantity of 1-naphthol was small and
difficult to separate from carbaryl. Thus, it was considered that
1-naphthol need not be measured separately from carbaryl. The United
States of America tolerances for carbaryl are based on the assumption
that the major part of the residue is the intact carbamate.
Since the establishment of the initial tolerances for carbaryl, work
has been done which may have some bearing on the quantative and
qualitative aspects of the residue. Light, including artificial and
sunlight, changes carbaryl. Carbaryl in the formulated state slowly
degraded under the influence of ultra-violet and to give several
unidentified products (Okada et al., 1961). Crosby et al. (1965)
reported that ethanol or hexane solutions of some methylcarbamate
insecticides, including carbaryl, gave a variety of
cholinesterase-inhibiting derivatives when exposed to either
artificial or sunlight. The products were separated but not
identified. Abdel-Waheb (1966) reported that the nature of (1) the
surface, (2) the light, and (3) the compound affected the rate of
conversion of carbaryl and other carbamates in vitro in the solid
state.
Since neither the qualitative nor quantative aspects of
photo-decomposition of carbamates have been established, the
significance of these studies in relation to current and future
tolerances for carbaryl must await further study.
(a) In animals
Whitehurst et al. (1963) using the colorimetric method of analysis
sensitive to carbaryl, 1-naphthol and 1-naphthol conjugates concluded
that, when cows were fed on a diet containing carbaryl, no residues,
to the limit of method, were found in the milk. This was confirmation
of the feeding study by Gyrisco et al. (1960), who fed dairy cows
carbaryl at levels of up to 450 ppm. In another study, carbaryl was
dusted and sprayed on cows. No detectable residues were found in milk
when carbaryl was dusted on to animals, but after 48 hours some
carbaryl residues were found in the milk when the spray application
was used (Buttram, 1964; Camp et al., 1963).
In recent work done by Dorough (1966), ring-labelled carbaryl was fed
to a lactating cow. A total of approximately 1 ppm, based upon a
radioactivity measurement calculated as carbaryl, was found in the
skim milk six to 12 hours after administration of 3.05 mg/kg
body-weight. Approximately one half of this residue was chloroform
extractable, and one half was water extractable with a small
unextractable fraction. At the end of 60 hours all the radioactivity
(calculated to be 0.01 ppm as equivalents of carbaryl) was
unextractable from the milk. In the six-hour samples about 30 per cent
of the radioactivity in the milk was characterized as
5,6-dihydro-5,6-dihydroxy 1-naphthol N-methylcarbamate, and
approximately 20 per cent was an unknown metabolite. Neither of these
materials, corresponding to 50 per cent of the total milk residue (0.5
ppm), responded to the standard colorimetric method commonly used for
carbaryl.
Cattle, sheep, goats, and hogs were sprayed four times in two weeks
with a 1.0 per cent suspension of carbaryl and Hereford steers were
fed 200 ppm of carbaryl in the diet for a 27-day period. These animals
were slaughtered at one and seven days after spraying or at the end of
the feeding interval. Residues of carbaryl, 1-naphthol and conjugates
of 1-naphthol were not detected in the body tissues of any of the
cattle, sheep, or hogs seven days after spraying. At this time
interval some small residues were noted in the fat and brain of a
goat. No residues were detected in tissues of cattle fed carbaryl for
27 days (Claborn, 1963). Krishna & Casida (1966) have reported that
less than 10 per cent of the administered acute dose of
carbonyl-C14-carbaryl was recovered after 48 hours as tissue
residues, primarily in those tissues known to be involved in body
contaminant eliminations - (e.g. liver, spleen, kidney). After seven
days Knaak (1966) found no residues resulting from carbonyl-carbaryl.
Laying hens were dusted three times at four-day intervals using 4 gm
of five per cent carbaryl per bird. One day after treatment no
residues were found in each type of edible tissue except skin. Seven
days after treatment this residue was reduced to low levels. Eggs were
found to be free of residue, throughout the study (Johnson,
Critchfield & Arthur, 1963).
(b) In plants
A limited amount of metabolism studies on carbamates in and on plants
has been reported. Studies by Dorough et al. (1963, 1964) found that
radioactive carbaryl yielded water soluble persistent metabolites
which were different than those found in the milk of a goat.
Carbonyl-C14-carbaryl, injected and administered to the surface of
bean plants yielded compounds that largely remain within the plant.
While these compounds have not been characterized, they are stable and
after 12 hours are not extractable by the usual organic solvents.
Abdel-Wahab observed that approximately 50 per cent of the surface
administered dose of C14 radioactivity was recovered after 72 hours.
When bean plants were injected into the stem with radioactive
carbaryl, approximately 75 per cent of the radioactivity persisted for
at least six days as aqueous and unextractable materials. Thus,
reactions within the plant presumably produce carbamates in some
stable form. These non-hydrolytic metabolites in many instances may
not respond to the usual method of analysis for carbaryl because (1)
the metabolites yield a phenolic material different than 1-naphthol
and do not show the same colour reaction, and (2) the major fraction
of some of the metabolites show solubility properties different than
the parent compounds. Preliminary studies on bio-assay of certain
metabolites have indicated a reduced biological activity when compared
with the parent compounds.
(c) In storage and processing
Raw unwashed tomatoes, harvested from field treated applications, were
stored at 55° F for approximately two weeks with samples taken at
varying intervals. Data showed that no loss of carbaryl was realized
under these storage conditions. The harvested tomatoes were subjected
to home and commercial canning operations, and analysis of samples
indicated that 50 per cent or more of the residue was removed by
water-washing and nearly complete removal of all residue was realized
by peeling and canning operations (Farrow et al. 1966).
Methods of residue analysis
The methods available for carbaryl are based on a colorimetric
determination following suitable extraction and clean-up. There are
approximately 20 variations of the basic method for different types
of food products, most of which are not published and no one method
has been tested for all food products.
For most fruits and vegetables the methods of (1) AOAC or that of (2)
Benson & Finocchiaro (1965) are suggested. These methods have a
sensitivity of about 0.1 ppm.
The above methods do not give very adequate clean-up for citrus,
olives, shelled nuts, many cereals and cereal products. Of the many
variations of the method, that developed for wheat and wheat fractions
has the most comprehensive clean-up. It is believed that it would be
useful for all products not amenable to the AOAC method.
RECOMMENDATIONS FOR TOLERANCES
The low acceptable daily intake necessitated a very close evaluation
of residue data to determine tolerances which would permit useful
applications to crops without possibly providing residues which might
exceed the acceptable daily intake.
Some data indicate that there will be losses under some conditions of
storage and processing. However, not enough data are available to make
a calculation at present, therefore no factor for such losses are
included in the calculations. The basic factor used in recommending
temporary tolerances which yield values within the ADI is that of
choosing a long pre-harvest interval.
The recommended tolerance figures are indicated in the following
table.
TEMPORARY TOLERANCE RECOMMENDATIONS FOR CARBARYL
Crop Temporary tolerance
in ppm
Vegetables 1.0
Cereal and cereal products 1.0
Tree fruits 1.0
Caneberries 1.2
Citrus 10.0
Shelled nuts 1.0
Further work or information
In the above calculations no considerations were given to possible
losses during storage, shipping and processing. It is recommended that
such data be developed and made available to the working party.
It is also recommended that further work be done to establish more
definitely the character of the terminal residue in treated plants.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Baron, R. L., Casterline, J. L. & Fitzhugh, O. G. (1964) Toxicol.
appl. Pharmacol., 6, 402
Best, E. M., jr & Murray, B. L. (1962) J. occup. Med., 4, 507
Carpenter, C. P., Weil, C. S., Palm, P. E., Woodside, M. W., Nair,
J. H. & Smyth, H. F., jr (1961) J. Agr. Food Chem., 9, 30
Claborn, H. V., Roberts, R. H., Mann, H. D., Bowman, M. C., Ivey,
M. C., Weidenbach, C. P. & Radeleff, R. D. (1963) J. Agr. Food
Chem., 11, 74
Dorough, H. W., Leeling, N. C. & Casida, J. E. (1963) Science,
140, 170
Dorough, H. W. & Casida, J. E. (1964) J. Agr. Food Chem., 12, 294
Eheart, J. F., Turner, E. C. & Dickinson, J. (1962) J. Econ. Ent.,
55, 504
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Goldberg, M. E. & Johnson, H. E. (1964a) J. Pharm. Pharmacol., 16,
60
Goldberg, M. E. & Johnson, H. E. (1964b) J. Pharmacol. exp. Ther.,
145, 367
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965a)
Psychopharmacologia, 7, 72
Goldberg, M. E., Johnson, H. E. & Knaak, J. B. (1965b) Biochem.
Pharmacol., 13, 1483
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, E. W., Fox,
F. H., Holland, R. F. & Trimberger, G. W. (1960) J. Agr. Food Chem.,
8, 409
Johnson, D. P., Critchfield, F. E. & Arthur, B. W. (1963) J. Agr.
Food Chem., 11, 77
Knaak, J. B., Tallant, M. J., Bartley, W. J. & Sullivan, L. J. (1965)
J. Agr. Food Chem., 13, 537
Krishna, J. G. & Casida, J. E. (1966), J. Agr. Food Chem., 14, 98
Mellon Institute of Industrial Research (1958a) Unpublished Report
Mellon Institute of Industrial Research (1958b) Unpublished Report
Mellon Institute of Industrial Research (1963) Unpublished Report
Mellon Institute of Industrial Research (1965) Unpublished Report
Roberts, R. H., Jackson, J. B., Westlake, W. E., Ackerman, A. J. &
Claborn, H. V. (1960) J. Econ. Ent., 53, 326
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abdel-Wahab, A. M., Kuhr, R. J., and Casida, J. E. (1966) The fate of
C14 C=O labeled aryl methylcarbamate insecticide chemicals in and on
bean plants. J. Agr. Food Chem. 14:290.
AOAC (1965) Methods of Analysis, (10th edition) paras. 24. 188-24.192
Benson, W. R. and Finocchiaro, J. M. (1965) Rapid Procedure for
Carbaryl Residues: Modification of Official Colorimetric Method. J.
Assoc. Offic. Agric. Chem. 48, 676-679
Buttram, J. Ress. (1964) Thesis, Auburn University. The metabolism of
carbamate and organophosphate insecticides by cattle and poultry and
associated residues in livestock products.
Camp, H. B., Buttram, J. R., Hays, K. L., and Arthur, B. W. (1963)
Sevin residues in milk from dairy cows following dermal applications.
J. Econ. Entomol. 56:402-404.
Casida, J. E., and Augustinsson, K.-B. (1959) Reaction of Plasma
Albumin with 1-naphthyl N-methylcarbamate and certain other esters.
Biochem. Biophys. Acta. 36:411-26.
Casida, J. E., Augustinsson, K.-B. and Jonsson, G. (1960) Stability,
toxicity and reaction mechanism with esterases of certain carbamate
insecticides. J. Econ. Entomol. 53:205-12.
Claborn, H. V. (1963) Residues in body tissues of livestock sprayed
with Sevin or Sevin in the diet. J. Agr. Food Chem. 11:74-76.
Crosby, D. C., Leitus, E., and Winterlin, W. L. (1965) The
photodecomposition of carbamate insecticides. J. Agr. Food Chem.
13:204.
Dorough, H. W., Leeling, N. C. and Casida, J. E. (1963) Nonhydrolytic
pathway in metabolism of N-methylcarbamate insecticides. Science
140:170-171.
Dorough, H. W. and Casida, J. E, (1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J. Agr. Food Chem. 12 (4):
294-304.
Dorough, H. W. (1966) Carbaryl-C14 metabolism in a lactating cow.
Private communication and in press.
Farrow, R. P., Lamb, F., Cook, R. W., Bergmans, B., Kimball, J. and
Elkins, E. R. (1966) Removal of Sevin residues from tomatoes during
commercial and home preparative procedures. Paper # 36, Pesticides
Subdivision of Agr. and Food Div., Amer. Chem. Soc. 152nd Meeting,
September 12-16, 1966.
Gyrisco, G. G., Lisk, D. J., Fertig, S. N., Huddleston, E. W., Fox, F.
H., Holland, R. F., and Trimberger, G. W. (1960) The effects of
feeding high levels of Sevin on residue, flavor and odor of the milk
of dairy cattle. J. Agr. Food Chem. 8:409-410.
Hodgson, E. and Casida, J. E. (1960) Biological oxidation of
N,N-dialkyl carbamates. Biochem. Biophys. Acta. 42:184-186
Hodgson, E. and Casida, J. E. (1961) Metabolism of N,N-dialkyl
carbamates and related compounds by rat liver. Biochem. Pharmacol.
8:179-191.
Johnson, D. P., Critchfield, F. E., and Arthur, B. W. (1963)
Determination of Sevin insecticide and its metabolites in poultry
tissues and eggs. J. Agr. Food Chem. 11:77-80.
Knaak, J. B., Tallant, M. J., Bartley, W. J., and Sullivan, L. J.
(1965) The metabolism of carbaryl in the rat, guinea pig and man. J.
Agr. Food Chem. 13:537-543.
Krishna, J. G. and Casida, J. E. (1966) Fate in Rats of the
Radiocarbon from ten variously labeled methyl- and
dimethylcarbamate-C14 insecticide chemicals and their hydrolysis
products. J. Agr. Food Chem. 14:98-105.
Leeling, N. C. and Casida, J. E. (1966) Metabolites of carbaryl in
mammals and enzymatic systems for their formation. J. Agr. Food Chem.
14:282-290.
Morefield, H. H. "Agr. Products Tech. Service Report" bibliography on
Sevin insecticides (carbaryl) containing 1463 references has been
compiled by the Union Carbide Corporation, R & D Dept., Agr. Products,
Olefins Division, P.O. Box 8361, So. Charleston, W. Virginia 25303.
Okada, K., Nomura, K., Yamamoto, S., (1961) (no title). Nippon
Nogaikagaku Kaishi 35:739.
Whitehurst, W. E., Bishop, E. T., Critchfield, F. E., Gyrisco, G. G.,
Huddleston, E. W., Arnold, H., and Lisk, D. J. (1963) The metabolism
of Sevin in dairy cows. J. Agr. Food Chem 11:167-169.
Williams, R. T. (1959) Detoxication Mechanisms. John Wiley & Sons,
Inc., N. Y. 796 p.
