
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
CARBARYL
Explanation
Evaluations were made by the Joint Meeting in 1966, 1967 and again in
1968 (FAO/WHO, 1967b, 1968b and 1969b). A review of the new
experimental work which has become available since 1967, when the
acceptable daily intake was last evaluated and since 1968 when only
the residue data ware evaluated, as well as pertinent earlier data,
has been included in this monograph addendum. In the course of this
review it was noted that the Codex Committee on Pesticide Residues at
its fourth session in 1969 had referred back the tolerance
recommendations for further clarification.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Previously reported work indicated that carbaryl was metabolized in
the pig, sheep and rat in a manner similar to that observed in man,
but was metabolized in a different manner in the dog (Knaak and
Sullivan 1967; Knaak at al., 1967). A subsequent report indicated that
the monkey also metabolized carbaryl in a similar manner to man. The
major difference was the extent to which carbaryl was hydrolyzed to
yield 1-naphthol. Little or no hydrolysis occurred in the monkey as
compared to man (Knaak at al., 1968).
Since the last evaluation of carbaryl another organic metabolite of
carbaryl has been found in plants, mammalian urine and the milk of
cows and goats. Its structure has recently been established and it is
now known to be 5,6-dihydro-5, 6-dihydroxy-1-naphthyl
N-methylcarbamate (see Figure 3). The toxicology of this compound is
considered under "Special studies on the toxicity of plant
metabolites" (Baron at al., 1969).
Effect on enzymes and other biochemical parameters
The minimum detectable levels for anticholinesterase activity using
human blood plasma are 0.2 µg for carbaryl, 0.4 µg for the N-hydroxy
derivative, 0.1 µg for the 5 hydroxy derivative and 2.0 µg for the
N-hydroxymethyl derivative (Balba, 1967; Balba and Casida, 1968). The
last of these compounds was later reported to be contaminated with
reduced ring derivatives as impurities which arose in its synthesis
(Balba et al., 1968).
An induction of liver demethylase was observed in rats given carbaryl
orally five times per week for three weeks at a dose of 150 mg/kg
body-weight. In contrast to the usual case in which enzyme stimulation
is observed within one or two days well defined enzyme increases were
not observed until two to three weeks (Serrone, 1966). When the
hepatic microsomal fractions were incubated with carbinoxamine maleate
as substrata, measuring the amount of formaldehyde (HCHO) produced,
the following results were obtained (Coulston, 1966):
TABLE I
Production of formaldehyde on incubation of heptatic
microsomal fractions with carbinoxamine maleate
Group µ moles HCHO/gm/hr
1 week 2 weeks 3 weeks
Control 3.7 4.4 4.2
Carbaryl 4.8 7.2 8.6
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Male and female rats were given carbaryl by 'oral inoculation' at
doses of 50, 100 and 300 mg/kg for three months. In females there was
disturbance of the oestrous cycle, decrease in fertility, interference
with embryo development, and an increase in death rate of the progeny.
In males there was a decrease in sperm motility and deformed
spermatozoa were found (Vashakidze, 1965).
Female rats were given carbaryl in oral doses of 5, 10 or 20 mg/kg for
six months. At the higher doses there was a change in duration of the
phases of the oestrous cycle an increase in of the ovaries and uterus,
an increase in the level of hypophyseal gonadotropins, and disruption
in the reactivity of the vaginal mucosa to administered oestrogens
(Vashakidze, 1967).
Groups of 24 males and 24 female rats were given carbaryl in daily
oral doses of 0, 7, 14 or 70 mg/kg body-weight for 12 months. The
doses were given by intubation of a corn oil suspension of carbaryl.
There was a significant retardation of growth at the two higher levels
but not at the 7 mg/kg level. Blood cholinesterase activity was
significantly reduced during the experiment at the two higher levels
but not at the low dose level. There was a decrease in spermatozoal
motility at 14 and 70 mg/kg at six months. By 12 months this effect
was more pronounced in these groups and was also evident in the 7
mg/kg group. Histological changes occurred in the testes of all three
groups, but were less pronounced at the lowest dose level. In the
females, prolongation of the oestrous cycle occurred at the two higher
levels after six months. This effect became more pronounced in these
two groups by the end of the study but it was not significant at the 7
mg/kg level. An increase in the release of hypophyseal gonadotropic
hormones at all levels was reported. This observation was supported by
histochemical changes detected in the hypophyses. Histological changes
were also observed in adrenals and thyroid (Shtenberg and Rybakova,
1968).
Carbaryl, given orally to male and female rats at 5 or 15 mg/kg per
day over one year, produced a significant change in the enzymic
activity of testes and ovaries, prolonged the oestrous cycle, changed
the functional state of the spermatozoids, and reduced the fertility
of the females. At a dosage level of 2 mg/kg daily there were no
significant effects on the function of the sex glands. A tendency
towards reduction in the esterase levels was evident at this level but
the changes were not significant. Offspring (F1 and F2
generations), receiving 5 and 15 mg/kg, were more significantly
affected than were their parents. Mortality was increased and in
some of the young in these groups and damage to the vertebrae in the
F2 generation was observed (Orlova and Zhalbe, 1968).
Special studies on teratogenicity
Chicken-egg
Injection of carbaryl into the yolk sac of developing hen-eggs has
resulted in non specific malformation at levels of 1 mg/egg (Marliac,
1964), oedema in a limited number of embryos at levels of 1 to 4
mg/kg (Ghadiri and Greenwood, 1966), and foot deformities at levels of
0.01 to 1 mg/egg (Khera, 1965). A recent study demonstrated that
teratogenic manifestations were frequent but not dose related. The
type of malformation was dependent upon the time of injection, with
oedema, haemorrhages and plethora occurring in non-differentiated
tissues up to 15 days incubation, but from thereon being restricted to
mesenchymal tissues (Olefir and Vinogradova, 1968).
Dog
Groups of mated dogs were fed 0 (16 dogs), 3.125 (10 dogs) 6.25 (10
dogs), 12.5 (18 dogs), 25 (nine dogs), or 50 (eight dogs)
mg/kg/body-weight/day in the diet from mating to the termination of
weaning. Conception occurred in 14, 7, 8, 16, 7, and 3 dogs
respectively. Incidence of resorption appears to be high in all test
groups, although the incidence in the control group was not determined
for comparison; litter size was reduced at 25 and 50 mg/kg and foetal
mortality was 100 percent at 50 mg/kg. At parturition an increased
incidence of dystocia was observed in all test groups. Survival of
offspring, to weaning was reduced at 12.5 mg/kg and above. Teratogenic
effects were observed at 6.25 (3/33 pups), 12.5 (14/78 pups), 25 (3/23
pups), and 50 (1/7 pups) mg/kg/body-weight day. At 0, and 3.125 mg/kg,
0/70, and 0/39 pups showed terata. Abnormalities included
abdominal-thoracic fissures, with varying degrees of agenesis and
displacement of abdominal contents, brachygnathia of variable
severity, ecaudate pups, failure of skeletal formation, and
supernumerary phalanges (Smalley at al., 1968).
Four groups of twelve female dogs were fed 0, 2, 5, or 12.5
mg/kg/body-weight/day in the diet from mating to the termination of
weaning. Pregnancy occurred in 9, 7, 9 and 9 females respectively.
Mortality during the study comprised one non-pregnant female at 2
mg/kg, and one from each group at 5, and 12.5 mg/kg during
parturition. Litter size, mean birth-weight and weanling-weights were
comparable between groups, but incidence of stillbirths was increased
at 5, and 12.5 mg/kg, and doubtfully increased at 2 mg/kg. Survival to
weaning was also reduce at 12.5 mg/kg. Defects were observed in 7/57,
and 5/46 pups at 12.5 and 5 mg/kg respectively, These defects
comprised one umbilical hernia, one cleft palate, three fat-like
masses in the heart, and two unilateral microphthalmias in pups from
three litters at 12.5 mg/kg, and one umbilical hernia, one fat-like
mass in the heart, two cases of intussusception of the ileum into the
colon, and one case of extravasation of blood into the myocardium in
pups from three litters at 5 mg/kg (Imming at al., 1969).
Guinea pig
Carbaryl was given by gelatin capsule for 10 consecutive days on days
11 to 20 of gestation at 300 mg/kg body-weight (equivalent to an
LD50 dose each day). Maternal mortality was 38 percent, foetal
mortality 17.5 percent, and offspring with axial skeletal defects,
especially in the cervical vertebrae, were produced (Robens, 1969).
A single dose of 300 mg/kg was given on day 11 or 12 or 13, etc. up
through day 20. Separate groups were used for each treatment day.
Litter size and foetal mortality was similar to that of the control
group. Cervical vertebrae defects occurred in eight foetuses. Two
foetuses from the litter treated on day 13 had no kidneys or genital
organs (Robens, 1969).
Hamster
Carbaryl, when administered during organogenesis, produced no terata
in hamsters at the sublethal levels of 125 and 250 mg/kg body-weight
(Robens, 1969).
Monkey
A study on monkeys was undertaken to determine if carbaryl interfered
with reproduction in the non-human primate. Twenty-seven mature parous
female Rhesus monkeys with a past history of regular menstrual cycles
were chosen for this study. Each female was mated from the eleventh to
the sixteenth day of the cycle. When sperm were found in the vaginal
smear, oral medication of carbaryl was started at 2 mg or 20 mg/kg
body-weight. Pregnancy was determined by a modification of the
Ascheim-Zondak pregnancy test which measures the level of monkey
chorionic gonadotropin in serum. Seven control monkeys were mated;
five of these had positive pregnancy tests and four delivered
normally, the fifth monkey aborted early in pregnancy. Fourteen
carbaryl-treated monkeys were mated; eight had positive pregnancy
tests and three delivered normally, two aborted at 93 and 116 days of
pregnancy and it is suspected that the other three aborted early in
pregnancy. All the positive pregnancy tests in the control and
medicated monkeys occurred by the third mating. Any monkey that failed
to conceive after the fourth mating never conceived. It appears that
carbaryl does not interfere with conception or delivery in the Rhesus
monkey. However, the results seem to indicate that it may induce
abortion in these monkeys (Coulston, 1969).
Rabbit
Three groups of pregnant New Zealand White rabbits were treated with 0
(12 rabbits), 10 (nine rabbits), or 30 (10 rabbits)
mg/kg/body-weight/day from day 9 to day 16 of gestation, day 0 being
the day of insemination. Pregnancy was terminated by Caesarean section
on day 30 of gestation. Incidence of viable litters was 7, 7, and 5 at
0, 10, and 30 mg/kg respectively. Percentage resorptions (37, 19 and
43 percent at 0, 10 and 30 mg/kg respectively) appears to have been
increased at 30 mg/kg. Similarly mean litter size (6.7, 6.6 and 5.4
respectively) appears to be decreased. Group sizes were, however,
insufficiently large for definite confirmation of these apparent
trends. Foetal weight, and 24 hour incubator survival of pups were
comparable in all groups. Skeletal and visceral abnormalities were
within normal limits (Shaffer and Levy, 1968).
Doses up to 200 mg/kg administered orally, daily, for 10 day periods,
to pregnant rabbits, failed to produce terata (Robens, 1969).
Sheep
Three groups of sheep commenced feeding on diets containing 0 (23
sheep), 100 (26 sheep), or 250 (22 sheep) ppm carbaryl, four days
prior to pairing. Thirty sheep were lame at pairing, due to a viral
polyarthritis, and thus all animals were treated with 450,000 units of
benzathine penicillin just after pairing commenced. Incidence of
pregnancy was comparable between groups, and resulted in 25, 22 and 24
offspring (of which 15, 14 and 16 were delivered by Caesarean section
prior to parturition) from the 0, 100 and 250 ppm levels respectively.
Abnormalities were restricted to the 250 ppm group, where one
Caesarean, and one normal parturition lamb were found to show
intraventricular septal defects in the heart (Panciera, 1967).
Special studies on toxicity of plant metabolites
Toxicological studies have been made on authenticated, synthetic
samples of all of the major metabolites of carbaryl (Carpenter, 1969a;
1969b; Weil, 1968; 1969a; 1969b; 1969c; 1969d). The findings are
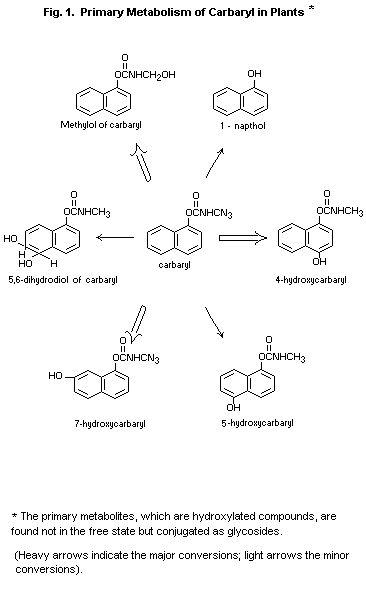
summarized in Table II and Figure 1. The least prevalent metabolite,
5,6-dihydro-5,6-dihydroxycarbaryl, occurs to the extent of about 2
percent of the carbaryl taken into the plant (Wiggins, 1969). Although
this compound has not yet yielded to synthesis, its structure has been
confirmed by spectral measurements and it is produced in vivo as a
result of animal metabolism of carbaryl (Baron at al., 1969). It has
also been noted that in comparative cholinesterase assays with
purified 5,6-dihidro-5,6-dihydroxycarbaryl, isolated from cow urine,
its cholinesterase inhibition capacity was only one fifth that of
carbaryl, indicating that the metabolite truly represents a
detoxication step. Using bovine erythrocyte, cholinesterase the I50
value for carbaryl was 1.0 × 10-6M compared with a 150 value of 5.0 ×
10-6m for 5,6-dihydro-5,6-dihydroxycarbaryl (Baron at al., 1969)
(see also BIOCHEMICAL ASPECTS - Biotransformation).
In one series of experiments, a mixture of the radioactive,
water-soluble, plant metabolites of carbaryl were fed to the rat.
Total elimination of the carbon14 from the body was achieved within
96 hours (Dorough and Wiggins, 1969).
TABLE II
Toxicity of carbaryl plant metabolites
Compound Acute oral LD50 Seven day no effect level Bovine
mg/kg body-weight Rat (mg/kg body-weight) erythrocyte
Carbaryl 430* > 125 < 250 5 × 10-8
4-hydroxycarbaryl 1190 >1000 4.6 × 10-7
5-hydroxycarbaryl 297 >1000 4.6 × 10-8
7-hydroxycarbaryl 4760 >1000 not determined
1-naphthyl-N-hydroxymethyl
carbamate 5360 > 250 < 500 1.4 × 10-5
1-naphthol 2590 > 500 < 1000 > 1 × 10-3
* median of 13 assays over 16 years
Groups of five male and five female rate wore given 1-naphthyl
N-hydroxymethylcarbamate in daily doses of 0, 62.5, 125, 250 or 500
mg/kg body-weight. The compound was added to the diet and fed for
three months. There were no effects of the metabolite on mortality,
appetite, growth, relative liver or kidney-weight or on plasma,
erythrocyte and brain cholinesterase activity at 125 mg/kg. Of these
criteria only relative liver-weight was increased at 250 mg/kg.
Body-weight and plasma cholinesterase were decreased at 500 mg/kg.
Liver degeneration with signs of regeneration was seen in most of the
rats at 250 and 500, but only in one of 10 rats at 125 mg/kg (Weil,
1969d).
Acute toxicity
Animal Route Vehicle LD50
mg/kg
body-weight Reference
Mouse oral sunflower oil 438 Rybakova, 1966
Mouse oral - 650 Coulston, 1966
Mouse i.p. dimethylsulfoxide 29 Balba and Casida, 1968
Rat i.v. propylene glycol 18 Carpenter et al., 1961
Rat i.v. PEG 400 24 Carpenter et al., 1961
Rat i.v. ethyl alcohol 33 Carpenter et al., 1961
Rat i.v. - 42 Wilhelm and Vandekar, 1966
Rat i.p. - 200 Wilhelm and Vandekar, 1966
Rat oral 0.25% agar 510 Carpenter et al., 1961
Rat oral sunflower oil 515 Rybakova, 1966
Rat oral - approx. 600 Coulston, 1966
Guinea-pig oral - 280 Carpenter et al., 1961
Rabbit oral - 710 Carpenter et al., 1961
Rabbit i.p. - 223 Carpenter et al., 1961
Dog oral - < 500 Coulston, 1966
Monkey oral - > 1000 Coulston, 1966
Short-term studies
Dog
In a one-year feeding study in dogs at dose levels of 0.45, 1.8, and
7.2 mg/kg body-weight (equivalent to 25, 100 and 400 ppm in the diet)
no significant adverse effects were reported. However, diffuse cloudy
swelling was observed in the proximal tubular epithelium of the kidney
at the 7.2 mg/kg level (Weil and Palm, 1958).
Monkey
Kidney alterations similar to those found in rats were also observed
in monkeys given doses of 150, 300, and 600 mg/kg orally over a
38-week period (Coulston, 1967).
Rat
When high doses of carbaryl were given to the rat by oral intubation
(75, 150, and 300 mg/kg body-weight) for three months, cytoplasmic
vacuolation was observed in the proximal tubules (Coulston, 1967).
Long-term studies
Rat
Similar kidney changes to those found in the one-year dog study were
observed in the rat in a two-year study in which dietary levels of 50,
100, 200 and 400 ppm were given. The kidney changes (cloudy swelling)
were again observed primarily in the high dose group (Carpenter et
al., 1961).
OBSERVATIONS IN MAN
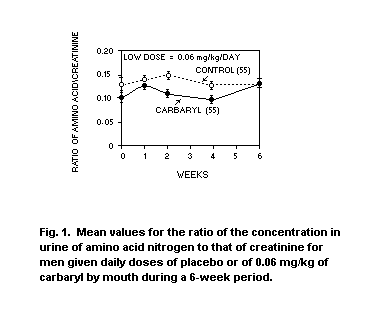
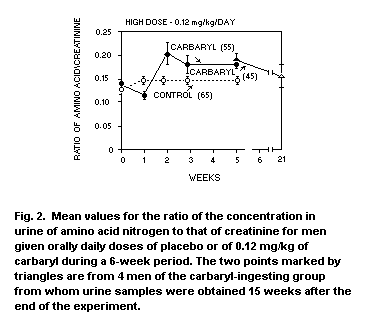
In the 1967 evaluation of carbaryl (FAO/WHO, 1968) mention was made of
a study wherein daily doses of 0.06 and 0 12 mg/kg body-weight/day
were given to groups of five or six men for six weeks (Wills at al.,
1967). At that time both levels were reported as being no effect
levels. However, following more complete evaluation of the data, a
decrease in the ratio of the concentration in urine of amino
acid-nitrogen to that of creatinine was reported for the 0.12 mg/kg
group. In the low dose group this ratio was similar to or below that
of the placebo group throughout the six-week study (see Figures 1 and
2). This increase in the ratio of urinary amino acid-nitrogen to
creatinine was interpreted as a slight decrease in the ability of the
proximal convoluted tubule to reabsorb amino acids. Consequently, the
authors concluded that a daily dose of 0.12 mg/kg of carbaryl may not
be a safe one (Wills et al., 1968).
COMMENT
Additional reports of effects on reproductive function in rats have
appeared since the last evaluation of carbaryl. All the reports
indicate that, under the experimental conditions employed there are
adverse effects from carbaryl given in daily doses of 5 mg/kg
body-weight or higher. In a rat study no significant effects were
reported from a dose-level of 2 mg/kg given orally for one year. In
all these studies carbaryl was administered by daily oral intubation
and never as an added component of the diet. Similar studies, using
carbaryl added to the diet, should be carried out, using the same
parameters for assessing the effects.
 Further investigations of the teratogenic potential of carbaryl have
been carried out using hamsters, guinea pigs, rabbits, sheep, dogs
(two studies) and monkeys. Teratogenic effects were observed in dogs
in the first study at all dosages except the lowest level tested
(3.125 mg/kg). The second dog study was somewhat inconclusive but
cannot be considered to negate the results of the first experiment,
since defects were observed at 5 and 12.5 mg/kg, but not at 2 mg/kg.
Because the dog metabolizes carbaryl differently than man, rat, and
other species the relevance of these findings in the dog to the safety
of carbaryl residues in food for man cannot be considered an important
factor. Carbaryl exerts a possible abortive effect in monkeys but this
work has not yet been completed.
Studies in man, in which daily oral doses of 0.12 mg/kg were given for
six weeks, have indicated an adverse effect upon the reabsorbing
functions of the proximal tubules of the kidneys. A level of 0.06
mg/kg was without effect in man. Cloudy swelling to vacuolation of the
epithelium of the proximal tubule have been observed in rats, dogs,
and monkeys.
Recent studies have identified the major metabolites of carbaryl in
plants. Acute oral LD50 and seven-day no-effect levels in rats have
been determined and demonstrate that they possess lower toxicities
than the parent compound.
The studies on reproductive physiology suggests that the no-effect
level in rats should be reduced. Because of the differing metabolism
of carbaryl in the dog, it was decided not to use the no-effect level
in this species as a basis for estimating the acceptable daily intake.
The 2 mg/kg level in rats appears to have no significant toxicological
effect but a dose level of 0.12 mg/kg may have some effect on
kidney-function in man. For this reason, and because additional
studies are essential on carbaryl, it was decided to given a temporary
acceptable daily intake at a reduced level from that previously
assigned.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.01 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Studies on metabolites in plants
Characterization of both the organo-soluble and water-soluble plant
metabolites of carbaryl has been accomplished (Kuhr and Casida, 1967;
Wiggins, 1969). The pathway is shown graphically in Figure 1. Studies
with carbaryl labelled with carbon14 in the position of the
naphthalene ring now confirm that approximately 10 percent of a
carbaryl spray deposit penetrates into plant tissue, and at a
relatively slow rate (Herrett and Bagley, 1965; Kuhr and Casida,
1967). The surface residue is comprised mainly of carbaryl itself,
even after aging under natural sunlight, indicating that
photo-oxidation has a very minor influence on carbaryl residues
(Wiggins, 1969).
The metabolic pathway for carbaryl in plants is identical whether the
compound in introduced by stem injection or applied to the leaf
surface. Laboratory or field conditions do not affect or alter the
metabolic course. Only after it gains entrance into the plant tissue
does carbaryl undergo biotransformation to its primary metabolites,
which are similar to the ones formed by animals. The major metabolites
in decreasing order of quantitative importance are 1-naphthyl
N-hydroxymethylcarbamate (or the methylol of carbaryl),
7-hydroxycarbaryl and 4-hydroxycarbaryl; the minor ones,
5-hydroxycarbaryl, 1-naphthol and 5,6-dihydro-5,6-dihydroxy-carbaryl.
These hydroxylated metabolites, which are less toxic than carbaryl
Itself, are conjugated by plants to form water-soluble glycosides. The
formation of the primary metabolites appears to be the rate-limiting
step and subsequent conjugation a rapid conversion, since the primary
metabolites are not found free in plant tissue, (Dorough and Wiggins,
1969; Kuhr and Casida, 1967; Wiggins, 1969).
APPRAISAL
Extensive new data show that the metabolic pathways of carbaryl are
the same whether the pesticide is ingested into plants or applied by
surface treatments. Data are available which showed that residues on
fruit and vegetables ware greatly reduced (up to 75 percent) by home
processing or canning.
It was noted that the recommendations made at the 1967 and 1968 Joint
Meetings were designed to accommodate the residues occurring at very
short intervals of time, 0 to 5 days, between last application of
pesticide and enforcement of the tolerance, the compound being one
which may be used close to harvest.
The meeting noted that preliminary studies with radio labelled
compounds fed to dairy cattle have indicated water-soluble as well as
lipid-soluble metabolites in milk and, as the total of such residues
may well exceed the level recommended in 1968 as a tolerance for milk
it was agreed to withdraw the recommendation and to re-consider it in
1972.
It was noted that the data on which the Joint Meeting had made its
previous recommendations were based on work done mainly in the U.S.A.
prior to 1966 and that it did not take into account the data, recently
received from the Netherlands, on supervised trials with raspberries,
peas and apples carried out between 1961 and 1963. New data from the
U.S.A. on crops following registered uses of carbaryl were also
considered.
Further investigations of the teratogenic potential of carbaryl have
been carried out using hamsters, guinea pigs, rabbits, sheep, dogs
(two studies) and monkeys. Teratogenic effects were observed in dogs
in the first study at all dosages except the lowest level tested
(3.125 mg/kg). The second dog study was somewhat inconclusive but
cannot be considered to negate the results of the first experiment,
since defects were observed at 5 and 12.5 mg/kg, but not at 2 mg/kg.
Because the dog metabolizes carbaryl differently than man, rat, and
other species the relevance of these findings in the dog to the safety
of carbaryl residues in food for man cannot be considered an important
factor. Carbaryl exerts a possible abortive effect in monkeys but this
work has not yet been completed.
Studies in man, in which daily oral doses of 0.12 mg/kg were given for
six weeks, have indicated an adverse effect upon the reabsorbing
functions of the proximal tubules of the kidneys. A level of 0.06
mg/kg was without effect in man. Cloudy swelling to vacuolation of the
epithelium of the proximal tubule have been observed in rats, dogs,
and monkeys.
Recent studies have identified the major metabolites of carbaryl in
plants. Acute oral LD50 and seven-day no-effect levels in rats have
been determined and demonstrate that they possess lower toxicities
than the parent compound.
The studies on reproductive physiology suggests that the no-effect
level in rats should be reduced. Because of the differing metabolism
of carbaryl in the dog, it was decided not to use the no-effect level
in this species as a basis for estimating the acceptable daily intake.
The 2 mg/kg level in rats appears to have no significant toxicological
effect but a dose level of 0.12 mg/kg may have some effect on
kidney-function in man. For this reason, and because additional
studies are essential on carbaryl, it was decided to given a temporary
acceptable daily intake at a reduced level from that previously
assigned.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.01 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Studies on metabolites in plants
Characterization of both the organo-soluble and water-soluble plant
metabolites of carbaryl has been accomplished (Kuhr and Casida, 1967;
Wiggins, 1969). The pathway is shown graphically in Figure 1. Studies
with carbaryl labelled with carbon14 in the position of the
naphthalene ring now confirm that approximately 10 percent of a
carbaryl spray deposit penetrates into plant tissue, and at a
relatively slow rate (Herrett and Bagley, 1965; Kuhr and Casida,
1967). The surface residue is comprised mainly of carbaryl itself,
even after aging under natural sunlight, indicating that
photo-oxidation has a very minor influence on carbaryl residues
(Wiggins, 1969).
The metabolic pathway for carbaryl in plants is identical whether the
compound in introduced by stem injection or applied to the leaf
surface. Laboratory or field conditions do not affect or alter the
metabolic course. Only after it gains entrance into the plant tissue
does carbaryl undergo biotransformation to its primary metabolites,
which are similar to the ones formed by animals. The major metabolites
in decreasing order of quantitative importance are 1-naphthyl
N-hydroxymethylcarbamate (or the methylol of carbaryl),
7-hydroxycarbaryl and 4-hydroxycarbaryl; the minor ones,
5-hydroxycarbaryl, 1-naphthol and 5,6-dihydro-5,6-dihydroxy-carbaryl.
These hydroxylated metabolites, which are less toxic than carbaryl
Itself, are conjugated by plants to form water-soluble glycosides. The
formation of the primary metabolites appears to be the rate-limiting
step and subsequent conjugation a rapid conversion, since the primary
metabolites are not found free in plant tissue, (Dorough and Wiggins,
1969; Kuhr and Casida, 1967; Wiggins, 1969).
APPRAISAL
Extensive new data show that the metabolic pathways of carbaryl are
the same whether the pesticide is ingested into plants or applied by
surface treatments. Data are available which showed that residues on
fruit and vegetables ware greatly reduced (up to 75 percent) by home
processing or canning.
It was noted that the recommendations made at the 1967 and 1968 Joint
Meetings were designed to accommodate the residues occurring at very
short intervals of time, 0 to 5 days, between last application of
pesticide and enforcement of the tolerance, the compound being one
which may be used close to harvest.
The meeting noted that preliminary studies with radio labelled
compounds fed to dairy cattle have indicated water-soluble as well as
lipid-soluble metabolites in milk and, as the total of such residues
may well exceed the level recommended in 1968 as a tolerance for milk
it was agreed to withdraw the recommendation and to re-consider it in
1972.
It was noted that the data on which the Joint Meeting had made its
previous recommendations were based on work done mainly in the U.S.A.
prior to 1966 and that it did not take into account the data, recently
received from the Netherlands, on supervised trials with raspberries,
peas and apples carried out between 1961 and 1963. New data from the
U.S.A. on crops following registered uses of carbaryl were also
considered.
 RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
In the light of the above considerations, it was decided to make
recommendations based on practical periods between treatments and
harvesting as follows:
TEMPORARY TOLERANCES
Pre-harvest use Temporary tolerance
Commodity limit (days) (ppm) Comments
Apples 14 5
Bananas (pulp) 3 5
Cane berries 7 10
(raspberries,
blackberries,
boysenberries,)
Citrus 5 7
Grapes 3 5
Rice 14 2.5
Stone fruit 1-3 10
Strawberries/
blueberries 1 7
Asparagus and okra 1 10
Beans and peas (pod) 1-3 5
Brassica 3 5
Corn, sweet (kernels) 1 1
Cotton seed (whole) 0 1
Cucurbits 1-3 3
Leaf vegetables 7-14 10
except brassica
(continued)
Pre-harvest use Temporary tolerance
Commodity limit (days) (ppm) Comments
Nuts (whole) - 10 )
) These figures are
Nuts (shelled) - 1 ) amendments to those
) made in 1968 and are
Olives (fresh) 14 10 ) based on new data.
Olives (processed) 14 1
Potatoes 0 0.2
Tomatoes, peppers, 0-1 5
egg plant
Poultry (use against 7 5 Calculated on the
external parasites) whole of the edible
parts.
Meat of cattle, goats
and sheep 1
Milk Recommendation made in
1968 withdrawn.
These Temporary Tolerances will be subject to review in 1973.
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Clarification of the effect upon reproductive physiology in several
species of animal.
2. Further studies to establish a no-effect level with respect to
kidney dysfunction in animals and/or man.
REFERENCES
Balba, M.H. (1967) Synthesis of possible metabolites of
methylcarbamate insecticide chemicals. Ph.D Thesis, University of
California, Berkeley, Diss. Abstr. B28(7):2761 (1968); Univ.
Microfilms Order No.68-28, 141 pages
Balba, M.H. and Casida, J.E. (1968) Synthesis of possible metabolites
of methylcarbamate insecticide chemicals. Hydroxyaryl and
hydroxyalkylphenyl methylcarbamates J. Agr. Food Chem. 16:561-67
Balba, M.H., Singer, M.S., Slade, M. and Casida, J.E. (1968) Synthesis
of possible metabolites of methylcarbamate insecticide chemicals.
Substituted-aryl N-hydroxy methylcarbamates. J. Agr. Food Chem.
16:821-25
Baron, R.C., Sphon, J.A., Chen, J.T., Lustig, E., Doherty, J.D.,
Hansen, E.A. and Kolbye, S.M. (1969) Confirmatory isolation and
identification of a metabolite of carbaryl in wine and milk. J. Agr.
Food Chem. 17:883-87
Carpenter, C.P., Weil, C.S., Palm, P.E., Woodside, M.H., Nair, J.H.,
and Smyth, H.F. (1961) Mammalian toxicity of
1-naphthyl-N-methylcarbamate (Sevin Insecticide). J. Agr. Food Chem.
9:30-39
Carpenter, C.P. (1969a) Carbaryl; data on plant metabolites.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Carpenter, C.P. (1969b) Carbaryl; data on Plant metabolites.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Coulston, F. (1966) Proceedings. Conference on non-human primate
toxicology. Session 3. Qualitative and quantitative relationships
between toxicity of drugs in man, lower mammals and non-human
primates. F.D.A., Arlie House, Warrenton, Virginia, 12-14 June 1966,
23 pages
Coulston, F. (1967) Unpublished information on the toxicity of
carbaryl to rats and monkeys. Institute of Experimental Pathology and
Toxicology. Albany Medical College, Albany, N.Y.
Coulston, F. (1969) Unpublished information on the effect of carbaryl
upon reproduction in monkeys. Institute of Experimental Pathology and
Toxicology, Albany Medical College, Albany, N.Y.
Dorough, H.W. and Wiggins, O.G. (1969) Nature of the water soluble
metabolites of carbaryl in bean plants and their fate on rats. J.
Econ. Entnmol. 62:49-53
FAO/WHO (1967b) Evaluation of some pesticide residues in food
FAO/PL:CP/15: WHO/Food Add./67.32
FAO/WHO (1968b) 1967 Evaluations of some pesticide residues in food
FAO/PL:1967/M/11/1; WHO/Food Add./68.30
Ghadiri, W. and Greenwood, D.A. (1966) Toxicity and biologic effects
of malathion, Phosdrin, and Sevin in the chick embryo. Toxicol. appl.
Pharmacol. 8:342
Herrett, R.A. and Bagley, W.P. (1965) and metabolism of Sevin in
plants. Unpublished report prepared and submitted by Union Carbide
Corporation
Imming, R.J., Shaffer, B.C. and Woodard, G. (1969) Sevin Safety
evaluation by feeding to female beagles from day one of gestation
through weaning of the offspring. Unpublished report of Woodard
Research Corporation submitted by Union Carbide Corporation.
Khera, K.S. (1966) Toxic and teratogenic effects of insecticides in
duck and chick embryos Toxicol. appl. Pharmacol. 8:345
Knaak, J.B. and Sullivan, L.J. (1967) Carbaryl - C14
(1-naphthyl-N-methyl carbamate) Excretion and metabolism by the dog.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.J. (1967)
The metabolism of carbaryl in man, monkey, pig and sheep. Unpublished
report of the Mellon Institute submitted by Union Carbide Corporation
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.J. (1968)
The Metabolism of carbaryl in man, monkey, pig and sheep. J. Agr.
Fond Chem. 16:465-70
Kuhr, R.J. and Casida, J.E. (1967) Persistent glycosides of metabolism
of methylcarbamate insecticide chemicals formed by hydroxylation in
bean plants. J. Agr. Food Chem. 15:814-24
Marliac, J.P. (1964) Toxicity and teratogenic effects of 12 pesticides
in the chick embryo Fed. Free. 23:105
Olefir, A.I. and Vinogradova, V.K.E. (1968) Embryotoxic effects of the
pesticides Sevin and cyram (Russian). Vroch. Delo. 11:103-06 (Chem.
Abstr. 70:46402f, (1969)
Orlova, N.V. and Zhalbe, E.P. (1968) Maximum permissible mounts of
Sevin in food products (Russian). Vop. Pitan. 27(6): 49-55 (Chem.
Abstr. 70: 46402f., (1969)
Panciera, R.J. (1967) Determination of teratogenic properties of
orally administered 1-naphthyl-N-methylcarbamate in sheep. Unpublished
report from the Department of Veterinary Pathology, Oklahoma State
University, Stillwater submitted by Union Carbide Corporation
Robens, J.P. (1969) Teratologic studies of carbaryl, diazinon, Norea,
and thiram in small laboratory animals. Toxicol. appl. Pharmacol.
15:152-163
Rybakova, M.N. (1966) Toxic effect of Sevin on animals (Russian).
Gig.Sanit, 31(9):42-47
Serrone, D. (1966) Unpublished information on the effect of carbaryl
on liver microsomal enzymes. Institute of Experimental Pathology and
Toxicolgy, Albany Medical College, Albany, N.Y.
Shaffer, B.C. and Levy, A.C. (1968) Evaluation of the teratogenic
potential of rabbits fed the compound from day 9 to day 16 of the
gestation period. Unpublished report of Woodard Research Corporation
submitted by Union Carbide Corporation
Shtenberg, A.I. and Rybakova, M.N. (1968) Effect of carbaryl on the
neuroendocrine system of rats. Food Cosmet. Toxicol. 6:461-467
Smalley, H.E., Curtis, J.M. and Earl, F.L. (1968) Teratogenic action of
carbaryl in beagle dogs. Toxicol. appl. Pharmacol. 13:392-403
Vashakidze, Y.I. (1965) Effect of Sevin Intoxication on sexual
function of experimental animals (Russian). Soobsch. Akad. Nauk. Gruz.
SSR, 39(2):471-474[(Chem. Abstr. 64:2689h (1966)]
Vashakidze, V.I. (1967) Mechanism of action of pesticides (granosan,
Sevin, dinoc) on the reproductive cycle of experimental animals
(Russian). Soobsch, Akad. Nauk. Gruz. SSR, 48(1):219-224[(Chem. Abstr.
68:28750x, (1968)]
Weil, C.S. and Palm, P.E. (1958) Chronic toxicity of Sevin for dogs.
Unpublished report of Mellon Institute submitted by Union Carbide
Corporation
Weil, C.S. (1968) 4-Hydroxy Sevin and 5-Hydroxy Sevin; results of
feeding in the diets of rats for 7 to 9 days. Unpublished report of
the Mellon Institute submitted by Union Carbide Corporation
Weil, C.S. (1969a) 1-Naphthyl N-hydroxymethylcarbamate; results of
feeding in the diets of rats for one week. Unpublished report of the
Mellon Institute submitted by Union Carbide Corporation
Weil, C.S. (1969b) 4-Hydroxy-1-naphthyl N-methylcarbamate,
5-hydroxy-1-naphthyl N-methyl carbamate, and 1-naphthyl
N-hydroxymethyl carbamate; results of feeding in the diets of rats for
one week. Unpublished report of the Mellon Institute submitted by
Union Carbide Corporation
Weil, C.S. (1969c) Alpha naphthol; results of feeding in the diets of
rate for one week Unpublished report of the Mellon Institute
submitted by Union Carbide Corporation
Weil, C.S. (1969d) 1-Naphthyl hydroxymethylcarbamate. Results of
feeding in the diets of rats for three months. Unpublished report of
the Mellon Institute submitted by Union Carbide Corporation
Wiggins, O.G. (1969) Fate of Sevin applied to the surface of bean
leaves under field conditions. Unpublished report prepared and
submitted by Union Carbide Corporation
Wilhelm, E. and Vandekar, M. (1966) Studies in the toxicology of
N-Methylcarbarnates. 1. Comparative toxicity tests and estimation of
persistence of inhibiters in the body. Proc. Int. Congr. Occ. Hlth.,
Vienna, P. 517-20
Wills, J.H., Jameson, E., Stein, A., Serrone, D. and Coulston, F.
(1967) Toxicol. appl. Pharmacol 10:390
Wills, J.H., Jameson, E., Coulston, F. (1968) Clin. Toxicol.,
1(3):265-71
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
In the light of the above considerations, it was decided to make
recommendations based on practical periods between treatments and
harvesting as follows:
TEMPORARY TOLERANCES
Pre-harvest use Temporary tolerance
Commodity limit (days) (ppm) Comments
Apples 14 5
Bananas (pulp) 3 5
Cane berries 7 10
(raspberries,
blackberries,
boysenberries,)
Citrus 5 7
Grapes 3 5
Rice 14 2.5
Stone fruit 1-3 10
Strawberries/
blueberries 1 7
Asparagus and okra 1 10
Beans and peas (pod) 1-3 5
Brassica 3 5
Corn, sweet (kernels) 1 1
Cotton seed (whole) 0 1
Cucurbits 1-3 3
Leaf vegetables 7-14 10
except brassica
(continued)
Pre-harvest use Temporary tolerance
Commodity limit (days) (ppm) Comments
Nuts (whole) - 10 )
) These figures are
Nuts (shelled) - 1 ) amendments to those
) made in 1968 and are
Olives (fresh) 14 10 ) based on new data.
Olives (processed) 14 1
Potatoes 0 0.2
Tomatoes, peppers, 0-1 5
egg plant
Poultry (use against 7 5 Calculated on the
external parasites) whole of the edible
parts.
Meat of cattle, goats
and sheep 1
Milk Recommendation made in
1968 withdrawn.
These Temporary Tolerances will be subject to review in 1973.
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Clarification of the effect upon reproductive physiology in several
species of animal.
2. Further studies to establish a no-effect level with respect to
kidney dysfunction in animals and/or man.
REFERENCES
Balba, M.H. (1967) Synthesis of possible metabolites of
methylcarbamate insecticide chemicals. Ph.D Thesis, University of
California, Berkeley, Diss. Abstr. B28(7):2761 (1968); Univ.
Microfilms Order No.68-28, 141 pages
Balba, M.H. and Casida, J.E. (1968) Synthesis of possible metabolites
of methylcarbamate insecticide chemicals. Hydroxyaryl and
hydroxyalkylphenyl methylcarbamates J. Agr. Food Chem. 16:561-67
Balba, M.H., Singer, M.S., Slade, M. and Casida, J.E. (1968) Synthesis
of possible metabolites of methylcarbamate insecticide chemicals.
Substituted-aryl N-hydroxy methylcarbamates. J. Agr. Food Chem.
16:821-25
Baron, R.C., Sphon, J.A., Chen, J.T., Lustig, E., Doherty, J.D.,
Hansen, E.A. and Kolbye, S.M. (1969) Confirmatory isolation and
identification of a metabolite of carbaryl in wine and milk. J. Agr.
Food Chem. 17:883-87
Carpenter, C.P., Weil, C.S., Palm, P.E., Woodside, M.H., Nair, J.H.,
and Smyth, H.F. (1961) Mammalian toxicity of
1-naphthyl-N-methylcarbamate (Sevin Insecticide). J. Agr. Food Chem.
9:30-39
Carpenter, C.P. (1969a) Carbaryl; data on plant metabolites.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Carpenter, C.P. (1969b) Carbaryl; data on Plant metabolites.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Coulston, F. (1966) Proceedings. Conference on non-human primate
toxicology. Session 3. Qualitative and quantitative relationships
between toxicity of drugs in man, lower mammals and non-human
primates. F.D.A., Arlie House, Warrenton, Virginia, 12-14 June 1966,
23 pages
Coulston, F. (1967) Unpublished information on the toxicity of
carbaryl to rats and monkeys. Institute of Experimental Pathology and
Toxicology. Albany Medical College, Albany, N.Y.
Coulston, F. (1969) Unpublished information on the effect of carbaryl
upon reproduction in monkeys. Institute of Experimental Pathology and
Toxicology, Albany Medical College, Albany, N.Y.
Dorough, H.W. and Wiggins, O.G. (1969) Nature of the water soluble
metabolites of carbaryl in bean plants and their fate on rats. J.
Econ. Entnmol. 62:49-53
FAO/WHO (1967b) Evaluation of some pesticide residues in food
FAO/PL:CP/15: WHO/Food Add./67.32
FAO/WHO (1968b) 1967 Evaluations of some pesticide residues in food
FAO/PL:1967/M/11/1; WHO/Food Add./68.30
Ghadiri, W. and Greenwood, D.A. (1966) Toxicity and biologic effects
of malathion, Phosdrin, and Sevin in the chick embryo. Toxicol. appl.
Pharmacol. 8:342
Herrett, R.A. and Bagley, W.P. (1965) and metabolism of Sevin in
plants. Unpublished report prepared and submitted by Union Carbide
Corporation
Imming, R.J., Shaffer, B.C. and Woodard, G. (1969) Sevin Safety
evaluation by feeding to female beagles from day one of gestation
through weaning of the offspring. Unpublished report of Woodard
Research Corporation submitted by Union Carbide Corporation.
Khera, K.S. (1966) Toxic and teratogenic effects of insecticides in
duck and chick embryos Toxicol. appl. Pharmacol. 8:345
Knaak, J.B. and Sullivan, L.J. (1967) Carbaryl - C14
(1-naphthyl-N-methyl carbamate) Excretion and metabolism by the dog.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.J. (1967)
The metabolism of carbaryl in man, monkey, pig and sheep. Unpublished
report of the Mellon Institute submitted by Union Carbide Corporation
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.J. (1968)
The Metabolism of carbaryl in man, monkey, pig and sheep. J. Agr.
Fond Chem. 16:465-70
Kuhr, R.J. and Casida, J.E. (1967) Persistent glycosides of metabolism
of methylcarbamate insecticide chemicals formed by hydroxylation in
bean plants. J. Agr. Food Chem. 15:814-24
Marliac, J.P. (1964) Toxicity and teratogenic effects of 12 pesticides
in the chick embryo Fed. Free. 23:105
Olefir, A.I. and Vinogradova, V.K.E. (1968) Embryotoxic effects of the
pesticides Sevin and cyram (Russian). Vroch. Delo. 11:103-06 (Chem.
Abstr. 70:46402f, (1969)
Orlova, N.V. and Zhalbe, E.P. (1968) Maximum permissible mounts of
Sevin in food products (Russian). Vop. Pitan. 27(6): 49-55 (Chem.
Abstr. 70: 46402f., (1969)
Panciera, R.J. (1967) Determination of teratogenic properties of
orally administered 1-naphthyl-N-methylcarbamate in sheep. Unpublished
report from the Department of Veterinary Pathology, Oklahoma State
University, Stillwater submitted by Union Carbide Corporation
Robens, J.P. (1969) Teratologic studies of carbaryl, diazinon, Norea,
and thiram in small laboratory animals. Toxicol. appl. Pharmacol.
15:152-163
Rybakova, M.N. (1966) Toxic effect of Sevin on animals (Russian).
Gig.Sanit, 31(9):42-47
Serrone, D. (1966) Unpublished information on the effect of carbaryl
on liver microsomal enzymes. Institute of Experimental Pathology and
Toxicolgy, Albany Medical College, Albany, N.Y.
Shaffer, B.C. and Levy, A.C. (1968) Evaluation of the teratogenic
potential of rabbits fed the compound from day 9 to day 16 of the
gestation period. Unpublished report of Woodard Research Corporation
submitted by Union Carbide Corporation
Shtenberg, A.I. and Rybakova, M.N. (1968) Effect of carbaryl on the
neuroendocrine system of rats. Food Cosmet. Toxicol. 6:461-467
Smalley, H.E., Curtis, J.M. and Earl, F.L. (1968) Teratogenic action of
carbaryl in beagle dogs. Toxicol. appl. Pharmacol. 13:392-403
Vashakidze, Y.I. (1965) Effect of Sevin Intoxication on sexual
function of experimental animals (Russian). Soobsch. Akad. Nauk. Gruz.
SSR, 39(2):471-474[(Chem. Abstr. 64:2689h (1966)]
Vashakidze, V.I. (1967) Mechanism of action of pesticides (granosan,
Sevin, dinoc) on the reproductive cycle of experimental animals
(Russian). Soobsch, Akad. Nauk. Gruz. SSR, 48(1):219-224[(Chem. Abstr.
68:28750x, (1968)]
Weil, C.S. and Palm, P.E. (1958) Chronic toxicity of Sevin for dogs.
Unpublished report of Mellon Institute submitted by Union Carbide
Corporation
Weil, C.S. (1968) 4-Hydroxy Sevin and 5-Hydroxy Sevin; results of
feeding in the diets of rats for 7 to 9 days. Unpublished report of
the Mellon Institute submitted by Union Carbide Corporation
Weil, C.S. (1969a) 1-Naphthyl N-hydroxymethylcarbamate; results of
feeding in the diets of rats for one week. Unpublished report of the
Mellon Institute submitted by Union Carbide Corporation
Weil, C.S. (1969b) 4-Hydroxy-1-naphthyl N-methylcarbamate,
5-hydroxy-1-naphthyl N-methyl carbamate, and 1-naphthyl
N-hydroxymethyl carbamate; results of feeding in the diets of rats for
one week. Unpublished report of the Mellon Institute submitted by
Union Carbide Corporation
Weil, C.S. (1969c) Alpha naphthol; results of feeding in the diets of
rate for one week Unpublished report of the Mellon Institute
submitted by Union Carbide Corporation
Weil, C.S. (1969d) 1-Naphthyl hydroxymethylcarbamate. Results of
feeding in the diets of rats for three months. Unpublished report of
the Mellon Institute submitted by Union Carbide Corporation
Wiggins, O.G. (1969) Fate of Sevin applied to the surface of bean
leaves under field conditions. Unpublished report prepared and
submitted by Union Carbide Corporation
Wilhelm, E. and Vandekar, M. (1966) Studies in the toxicology of
N-Methylcarbarnates. 1. Comparative toxicity tests and estimation of
persistence of inhibiters in the body. Proc. Int. Congr. Occ. Hlth.,
Vienna, P. 517-20
Wills, J.H., Jameson, E., Stein, A., Serrone, D. and Coulston, F.
(1967) Toxicol. appl. Pharmacol 10:390
Wills, J.H., Jameson, E., Coulston, F. (1968) Clin. Toxicol.,
1(3):265-71

 Further investigations of the teratogenic potential of carbaryl have
been carried out using hamsters, guinea pigs, rabbits, sheep, dogs
(two studies) and monkeys. Teratogenic effects were observed in dogs
in the first study at all dosages except the lowest level tested
(3.125 mg/kg). The second dog study was somewhat inconclusive but
cannot be considered to negate the results of the first experiment,
since defects were observed at 5 and 12.5 mg/kg, but not at 2 mg/kg.
Because the dog metabolizes carbaryl differently than man, rat, and
other species the relevance of these findings in the dog to the safety
of carbaryl residues in food for man cannot be considered an important
factor. Carbaryl exerts a possible abortive effect in monkeys but this
work has not yet been completed.
Studies in man, in which daily oral doses of 0.12 mg/kg were given for
six weeks, have indicated an adverse effect upon the reabsorbing
functions of the proximal tubules of the kidneys. A level of 0.06
mg/kg was without effect in man. Cloudy swelling to vacuolation of the
epithelium of the proximal tubule have been observed in rats, dogs,
and monkeys.
Recent studies have identified the major metabolites of carbaryl in
plants. Acute oral LD50 and seven-day no-effect levels in rats have
been determined and demonstrate that they possess lower toxicities
than the parent compound.
The studies on reproductive physiology suggests that the no-effect
level in rats should be reduced. Because of the differing metabolism
of carbaryl in the dog, it was decided not to use the no-effect level
in this species as a basis for estimating the acceptable daily intake.
The 2 mg/kg level in rats appears to have no significant toxicological
effect but a dose level of 0.12 mg/kg may have some effect on
kidney-function in man. For this reason, and because additional
studies are essential on carbaryl, it was decided to given a temporary
acceptable daily intake at a reduced level from that previously
assigned.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.01 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Studies on metabolites in plants
Characterization of both the organo-soluble and water-soluble plant
metabolites of carbaryl has been accomplished (Kuhr and Casida, 1967;
Wiggins, 1969). The pathway is shown graphically in Figure 1. Studies
with carbaryl labelled with carbon14 in the position of the
naphthalene ring now confirm that approximately 10 percent of a
carbaryl spray deposit penetrates into plant tissue, and at a
relatively slow rate (Herrett and Bagley, 1965; Kuhr and Casida,
1967). The surface residue is comprised mainly of carbaryl itself,
even after aging under natural sunlight, indicating that
photo-oxidation has a very minor influence on carbaryl residues
(Wiggins, 1969).
The metabolic pathway for carbaryl in plants is identical whether the
compound in introduced by stem injection or applied to the leaf
surface. Laboratory or field conditions do not affect or alter the
metabolic course. Only after it gains entrance into the plant tissue
does carbaryl undergo biotransformation to its primary metabolites,
which are similar to the ones formed by animals. The major metabolites
in decreasing order of quantitative importance are 1-naphthyl
N-hydroxymethylcarbamate (or the methylol of carbaryl),
7-hydroxycarbaryl and 4-hydroxycarbaryl; the minor ones,
5-hydroxycarbaryl, 1-naphthol and 5,6-dihydro-5,6-dihydroxy-carbaryl.
These hydroxylated metabolites, which are less toxic than carbaryl
Itself, are conjugated by plants to form water-soluble glycosides. The
formation of the primary metabolites appears to be the rate-limiting
step and subsequent conjugation a rapid conversion, since the primary
metabolites are not found free in plant tissue, (Dorough and Wiggins,
1969; Kuhr and Casida, 1967; Wiggins, 1969).
APPRAISAL
Extensive new data show that the metabolic pathways of carbaryl are
the same whether the pesticide is ingested into plants or applied by
surface treatments. Data are available which showed that residues on
fruit and vegetables ware greatly reduced (up to 75 percent) by home
processing or canning.
It was noted that the recommendations made at the 1967 and 1968 Joint
Meetings were designed to accommodate the residues occurring at very
short intervals of time, 0 to 5 days, between last application of
pesticide and enforcement of the tolerance, the compound being one
which may be used close to harvest.
The meeting noted that preliminary studies with radio labelled
compounds fed to dairy cattle have indicated water-soluble as well as
lipid-soluble metabolites in milk and, as the total of such residues
may well exceed the level recommended in 1968 as a tolerance for milk
it was agreed to withdraw the recommendation and to re-consider it in
1972.
It was noted that the data on which the Joint Meeting had made its
previous recommendations were based on work done mainly in the U.S.A.
prior to 1966 and that it did not take into account the data, recently
received from the Netherlands, on supervised trials with raspberries,
peas and apples carried out between 1961 and 1963. New data from the
U.S.A. on crops following registered uses of carbaryl were also
considered.
Further investigations of the teratogenic potential of carbaryl have
been carried out using hamsters, guinea pigs, rabbits, sheep, dogs
(two studies) and monkeys. Teratogenic effects were observed in dogs
in the first study at all dosages except the lowest level tested
(3.125 mg/kg). The second dog study was somewhat inconclusive but
cannot be considered to negate the results of the first experiment,
since defects were observed at 5 and 12.5 mg/kg, but not at 2 mg/kg.
Because the dog metabolizes carbaryl differently than man, rat, and
other species the relevance of these findings in the dog to the safety
of carbaryl residues in food for man cannot be considered an important
factor. Carbaryl exerts a possible abortive effect in monkeys but this
work has not yet been completed.
Studies in man, in which daily oral doses of 0.12 mg/kg were given for
six weeks, have indicated an adverse effect upon the reabsorbing
functions of the proximal tubules of the kidneys. A level of 0.06
mg/kg was without effect in man. Cloudy swelling to vacuolation of the
epithelium of the proximal tubule have been observed in rats, dogs,
and monkeys.
Recent studies have identified the major metabolites of carbaryl in
plants. Acute oral LD50 and seven-day no-effect levels in rats have
been determined and demonstrate that they possess lower toxicities
than the parent compound.
The studies on reproductive physiology suggests that the no-effect
level in rats should be reduced. Because of the differing metabolism
of carbaryl in the dog, it was decided not to use the no-effect level
in this species as a basis for estimating the acceptable daily intake.
The 2 mg/kg level in rats appears to have no significant toxicological
effect but a dose level of 0.12 mg/kg may have some effect on
kidney-function in man. For this reason, and because additional
studies are essential on carbaryl, it was decided to given a temporary
acceptable daily intake at a reduced level from that previously
assigned.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.01 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Studies on metabolites in plants
Characterization of both the organo-soluble and water-soluble plant
metabolites of carbaryl has been accomplished (Kuhr and Casida, 1967;
Wiggins, 1969). The pathway is shown graphically in Figure 1. Studies
with carbaryl labelled with carbon14 in the position of the
naphthalene ring now confirm that approximately 10 percent of a
carbaryl spray deposit penetrates into plant tissue, and at a
relatively slow rate (Herrett and Bagley, 1965; Kuhr and Casida,
1967). The surface residue is comprised mainly of carbaryl itself,
even after aging under natural sunlight, indicating that
photo-oxidation has a very minor influence on carbaryl residues
(Wiggins, 1969).
The metabolic pathway for carbaryl in plants is identical whether the
compound in introduced by stem injection or applied to the leaf
surface. Laboratory or field conditions do not affect or alter the
metabolic course. Only after it gains entrance into the plant tissue
does carbaryl undergo biotransformation to its primary metabolites,
which are similar to the ones formed by animals. The major metabolites
in decreasing order of quantitative importance are 1-naphthyl
N-hydroxymethylcarbamate (or the methylol of carbaryl),
7-hydroxycarbaryl and 4-hydroxycarbaryl; the minor ones,
5-hydroxycarbaryl, 1-naphthol and 5,6-dihydro-5,6-dihydroxy-carbaryl.
These hydroxylated metabolites, which are less toxic than carbaryl
Itself, are conjugated by plants to form water-soluble glycosides. The
formation of the primary metabolites appears to be the rate-limiting
step and subsequent conjugation a rapid conversion, since the primary
metabolites are not found free in plant tissue, (Dorough and Wiggins,
1969; Kuhr and Casida, 1967; Wiggins, 1969).
APPRAISAL
Extensive new data show that the metabolic pathways of carbaryl are
the same whether the pesticide is ingested into plants or applied by
surface treatments. Data are available which showed that residues on
fruit and vegetables ware greatly reduced (up to 75 percent) by home
processing or canning.
It was noted that the recommendations made at the 1967 and 1968 Joint
Meetings were designed to accommodate the residues occurring at very
short intervals of time, 0 to 5 days, between last application of
pesticide and enforcement of the tolerance, the compound being one
which may be used close to harvest.
The meeting noted that preliminary studies with radio labelled
compounds fed to dairy cattle have indicated water-soluble as well as
lipid-soluble metabolites in milk and, as the total of such residues
may well exceed the level recommended in 1968 as a tolerance for milk
it was agreed to withdraw the recommendation and to re-consider it in
1972.
It was noted that the data on which the Joint Meeting had made its
previous recommendations were based on work done mainly in the U.S.A.
prior to 1966 and that it did not take into account the data, recently
received from the Netherlands, on supervised trials with raspberries,
peas and apples carried out between 1961 and 1963. New data from the
U.S.A. on crops following registered uses of carbaryl were also
considered.
 RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
In the light of the above considerations, it was decided to make
recommendations based on practical periods between treatments and
harvesting as follows:
TEMPORARY TOLERANCES
Pre-harvest use Temporary tolerance
Commodity limit (days) (ppm) Comments
Apples 14 5
Bananas (pulp) 3 5
Cane berries 7 10
(raspberries,
blackberries,
boysenberries,)
Citrus 5 7
Grapes 3 5
Rice 14 2.5
Stone fruit 1-3 10
Strawberries/
blueberries 1 7
Asparagus and okra 1 10
Beans and peas (pod) 1-3 5
Brassica 3 5
Corn, sweet (kernels) 1 1
Cotton seed (whole) 0 1
Cucurbits 1-3 3
Leaf vegetables 7-14 10
except brassica
(continued)
Pre-harvest use Temporary tolerance
Commodity limit (days) (ppm) Comments
Nuts (whole) - 10 )
) These figures are
Nuts (shelled) - 1 ) amendments to those
) made in 1968 and are
Olives (fresh) 14 10 ) based on new data.
Olives (processed) 14 1
Potatoes 0 0.2
Tomatoes, peppers, 0-1 5
egg plant
Poultry (use against 7 5 Calculated on the
external parasites) whole of the edible
parts.
Meat of cattle, goats
and sheep 1
Milk Recommendation made in
1968 withdrawn.
These Temporary Tolerances will be subject to review in 1973.
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Clarification of the effect upon reproductive physiology in several
species of animal.
2. Further studies to establish a no-effect level with respect to
kidney dysfunction in animals and/or man.
REFERENCES
Balba, M.H. (1967) Synthesis of possible metabolites of
methylcarbamate insecticide chemicals. Ph.D Thesis, University of
California, Berkeley, Diss. Abstr. B28(7):2761 (1968); Univ.
Microfilms Order No.68-28, 141 pages
Balba, M.H. and Casida, J.E. (1968) Synthesis of possible metabolites
of methylcarbamate insecticide chemicals. Hydroxyaryl and
hydroxyalkylphenyl methylcarbamates J. Agr. Food Chem. 16:561-67
Balba, M.H., Singer, M.S., Slade, M. and Casida, J.E. (1968) Synthesis
of possible metabolites of methylcarbamate insecticide chemicals.
Substituted-aryl N-hydroxy methylcarbamates. J. Agr. Food Chem.
16:821-25
Baron, R.C., Sphon, J.A., Chen, J.T., Lustig, E., Doherty, J.D.,
Hansen, E.A. and Kolbye, S.M. (1969) Confirmatory isolation and
identification of a metabolite of carbaryl in wine and milk. J. Agr.
Food Chem. 17:883-87
Carpenter, C.P., Weil, C.S., Palm, P.E., Woodside, M.H., Nair, J.H.,
and Smyth, H.F. (1961) Mammalian toxicity of
1-naphthyl-N-methylcarbamate (Sevin Insecticide). J. Agr. Food Chem.
9:30-39
Carpenter, C.P. (1969a) Carbaryl; data on plant metabolites.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Carpenter, C.P. (1969b) Carbaryl; data on Plant metabolites.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Coulston, F. (1966) Proceedings. Conference on non-human primate
toxicology. Session 3. Qualitative and quantitative relationships
between toxicity of drugs in man, lower mammals and non-human
primates. F.D.A., Arlie House, Warrenton, Virginia, 12-14 June 1966,
23 pages
Coulston, F. (1967) Unpublished information on the toxicity of
carbaryl to rats and monkeys. Institute of Experimental Pathology and
Toxicology. Albany Medical College, Albany, N.Y.
Coulston, F. (1969) Unpublished information on the effect of carbaryl
upon reproduction in monkeys. Institute of Experimental Pathology and
Toxicology, Albany Medical College, Albany, N.Y.
Dorough, H.W. and Wiggins, O.G. (1969) Nature of the water soluble
metabolites of carbaryl in bean plants and their fate on rats. J.
Econ. Entnmol. 62:49-53
FAO/WHO (1967b) Evaluation of some pesticide residues in food
FAO/PL:CP/15: WHO/Food Add./67.32
FAO/WHO (1968b) 1967 Evaluations of some pesticide residues in food
FAO/PL:1967/M/11/1; WHO/Food Add./68.30
Ghadiri, W. and Greenwood, D.A. (1966) Toxicity and biologic effects
of malathion, Phosdrin, and Sevin in the chick embryo. Toxicol. appl.
Pharmacol. 8:342
Herrett, R.A. and Bagley, W.P. (1965) and metabolism of Sevin in
plants. Unpublished report prepared and submitted by Union Carbide
Corporation
Imming, R.J., Shaffer, B.C. and Woodard, G. (1969) Sevin Safety
evaluation by feeding to female beagles from day one of gestation
through weaning of the offspring. Unpublished report of Woodard
Research Corporation submitted by Union Carbide Corporation.
Khera, K.S. (1966) Toxic and teratogenic effects of insecticides in
duck and chick embryos Toxicol. appl. Pharmacol. 8:345
Knaak, J.B. and Sullivan, L.J. (1967) Carbaryl - C14
(1-naphthyl-N-methyl carbamate) Excretion and metabolism by the dog.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.J. (1967)
The metabolism of carbaryl in man, monkey, pig and sheep. Unpublished
report of the Mellon Institute submitted by Union Carbide Corporation
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.J. (1968)
The Metabolism of carbaryl in man, monkey, pig and sheep. J. Agr.
Fond Chem. 16:465-70
Kuhr, R.J. and Casida, J.E. (1967) Persistent glycosides of metabolism
of methylcarbamate insecticide chemicals formed by hydroxylation in
bean plants. J. Agr. Food Chem. 15:814-24
Marliac, J.P. (1964) Toxicity and teratogenic effects of 12 pesticides
in the chick embryo Fed. Free. 23:105
Olefir, A.I. and Vinogradova, V.K.E. (1968) Embryotoxic effects of the
pesticides Sevin and cyram (Russian). Vroch. Delo. 11:103-06 (Chem.
Abstr. 70:46402f, (1969)
Orlova, N.V. and Zhalbe, E.P. (1968) Maximum permissible mounts of
Sevin in food products (Russian). Vop. Pitan. 27(6): 49-55 (Chem.
Abstr. 70: 46402f., (1969)
Panciera, R.J. (1967) Determination of teratogenic properties of
orally administered 1-naphthyl-N-methylcarbamate in sheep. Unpublished
report from the Department of Veterinary Pathology, Oklahoma State
University, Stillwater submitted by Union Carbide Corporation
Robens, J.P. (1969) Teratologic studies of carbaryl, diazinon, Norea,
and thiram in small laboratory animals. Toxicol. appl. Pharmacol.
15:152-163
Rybakova, M.N. (1966) Toxic effect of Sevin on animals (Russian).
Gig.Sanit, 31(9):42-47
Serrone, D. (1966) Unpublished information on the effect of carbaryl
on liver microsomal enzymes. Institute of Experimental Pathology and
Toxicolgy, Albany Medical College, Albany, N.Y.
Shaffer, B.C. and Levy, A.C. (1968) Evaluation of the teratogenic
potential of rabbits fed the compound from day 9 to day 16 of the
gestation period. Unpublished report of Woodard Research Corporation
submitted by Union Carbide Corporation
Shtenberg, A.I. and Rybakova, M.N. (1968) Effect of carbaryl on the
neuroendocrine system of rats. Food Cosmet. Toxicol. 6:461-467
Smalley, H.E., Curtis, J.M. and Earl, F.L. (1968) Teratogenic action of
carbaryl in beagle dogs. Toxicol. appl. Pharmacol. 13:392-403
Vashakidze, Y.I. (1965) Effect of Sevin Intoxication on sexual
function of experimental animals (Russian). Soobsch. Akad. Nauk. Gruz.
SSR, 39(2):471-474[(Chem. Abstr. 64:2689h (1966)]
Vashakidze, V.I. (1967) Mechanism of action of pesticides (granosan,
Sevin, dinoc) on the reproductive cycle of experimental animals
(Russian). Soobsch, Akad. Nauk. Gruz. SSR, 48(1):219-224[(Chem. Abstr.
68:28750x, (1968)]
Weil, C.S. and Palm, P.E. (1958) Chronic toxicity of Sevin for dogs.
Unpublished report of Mellon Institute submitted by Union Carbide
Corporation
Weil, C.S. (1968) 4-Hydroxy Sevin and 5-Hydroxy Sevin; results of
feeding in the diets of rats for 7 to 9 days. Unpublished report of
the Mellon Institute submitted by Union Carbide Corporation
Weil, C.S. (1969a) 1-Naphthyl N-hydroxymethylcarbamate; results of
feeding in the diets of rats for one week. Unpublished report of the
Mellon Institute submitted by Union Carbide Corporation
Weil, C.S. (1969b) 4-Hydroxy-1-naphthyl N-methylcarbamate,
5-hydroxy-1-naphthyl N-methyl carbamate, and 1-naphthyl
N-hydroxymethyl carbamate; results of feeding in the diets of rats for
one week. Unpublished report of the Mellon Institute submitted by
Union Carbide Corporation
Weil, C.S. (1969c) Alpha naphthol; results of feeding in the diets of
rate for one week Unpublished report of the Mellon Institute
submitted by Union Carbide Corporation
Weil, C.S. (1969d) 1-Naphthyl hydroxymethylcarbamate. Results of
feeding in the diets of rats for three months. Unpublished report of
the Mellon Institute submitted by Union Carbide Corporation
Wiggins, O.G. (1969) Fate of Sevin applied to the surface of bean
leaves under field conditions. Unpublished report prepared and
submitted by Union Carbide Corporation
Wilhelm, E. and Vandekar, M. (1966) Studies in the toxicology of
N-Methylcarbarnates. 1. Comparative toxicity tests and estimation of
persistence of inhibiters in the body. Proc. Int. Congr. Occ. Hlth.,
Vienna, P. 517-20
Wills, J.H., Jameson, E., Stein, A., Serrone, D. and Coulston, F.
(1967) Toxicol. appl. Pharmacol 10:390
Wills, J.H., Jameson, E., Coulston, F. (1968) Clin. Toxicol.,
1(3):265-71
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
In the light of the above considerations, it was decided to make
recommendations based on practical periods between treatments and
harvesting as follows:
TEMPORARY TOLERANCES
Pre-harvest use Temporary tolerance
Commodity limit (days) (ppm) Comments
Apples 14 5
Bananas (pulp) 3 5
Cane berries 7 10
(raspberries,
blackberries,
boysenberries,)
Citrus 5 7
Grapes 3 5
Rice 14 2.5
Stone fruit 1-3 10
Strawberries/
blueberries 1 7
Asparagus and okra 1 10
Beans and peas (pod) 1-3 5
Brassica 3 5
Corn, sweet (kernels) 1 1
Cotton seed (whole) 0 1
Cucurbits 1-3 3
Leaf vegetables 7-14 10
except brassica
(continued)
Pre-harvest use Temporary tolerance
Commodity limit (days) (ppm) Comments
Nuts (whole) - 10 )
) These figures are
Nuts (shelled) - 1 ) amendments to those
) made in 1968 and are
Olives (fresh) 14 10 ) based on new data.
Olives (processed) 14 1
Potatoes 0 0.2
Tomatoes, peppers, 0-1 5
egg plant
Poultry (use against 7 5 Calculated on the
external parasites) whole of the edible
parts.
Meat of cattle, goats
and sheep 1
Milk Recommendation made in
1968 withdrawn.
These Temporary Tolerances will be subject to review in 1973.
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Clarification of the effect upon reproductive physiology in several
species of animal.
2. Further studies to establish a no-effect level with respect to
kidney dysfunction in animals and/or man.
REFERENCES
Balba, M.H. (1967) Synthesis of possible metabolites of
methylcarbamate insecticide chemicals. Ph.D Thesis, University of
California, Berkeley, Diss. Abstr. B28(7):2761 (1968); Univ.
Microfilms Order No.68-28, 141 pages
Balba, M.H. and Casida, J.E. (1968) Synthesis of possible metabolites
of methylcarbamate insecticide chemicals. Hydroxyaryl and
hydroxyalkylphenyl methylcarbamates J. Agr. Food Chem. 16:561-67
Balba, M.H., Singer, M.S., Slade, M. and Casida, J.E. (1968) Synthesis
of possible metabolites of methylcarbamate insecticide chemicals.
Substituted-aryl N-hydroxy methylcarbamates. J. Agr. Food Chem.
16:821-25
Baron, R.C., Sphon, J.A., Chen, J.T., Lustig, E., Doherty, J.D.,
Hansen, E.A. and Kolbye, S.M. (1969) Confirmatory isolation and
identification of a metabolite of carbaryl in wine and milk. J. Agr.
Food Chem. 17:883-87
Carpenter, C.P., Weil, C.S., Palm, P.E., Woodside, M.H., Nair, J.H.,
and Smyth, H.F. (1961) Mammalian toxicity of
1-naphthyl-N-methylcarbamate (Sevin Insecticide). J. Agr. Food Chem.
9:30-39
Carpenter, C.P. (1969a) Carbaryl; data on plant metabolites.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Carpenter, C.P. (1969b) Carbaryl; data on Plant metabolites.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Coulston, F. (1966) Proceedings. Conference on non-human primate
toxicology. Session 3. Qualitative and quantitative relationships
between toxicity of drugs in man, lower mammals and non-human
primates. F.D.A., Arlie House, Warrenton, Virginia, 12-14 June 1966,
23 pages
Coulston, F. (1967) Unpublished information on the toxicity of
carbaryl to rats and monkeys. Institute of Experimental Pathology and
Toxicology. Albany Medical College, Albany, N.Y.
Coulston, F. (1969) Unpublished information on the effect of carbaryl
upon reproduction in monkeys. Institute of Experimental Pathology and
Toxicology, Albany Medical College, Albany, N.Y.
Dorough, H.W. and Wiggins, O.G. (1969) Nature of the water soluble
metabolites of carbaryl in bean plants and their fate on rats. J.
Econ. Entnmol. 62:49-53
FAO/WHO (1967b) Evaluation of some pesticide residues in food
FAO/PL:CP/15: WHO/Food Add./67.32
FAO/WHO (1968b) 1967 Evaluations of some pesticide residues in food
FAO/PL:1967/M/11/1; WHO/Food Add./68.30
Ghadiri, W. and Greenwood, D.A. (1966) Toxicity and biologic effects
of malathion, Phosdrin, and Sevin in the chick embryo. Toxicol. appl.
Pharmacol. 8:342
Herrett, R.A. and Bagley, W.P. (1965) and metabolism of Sevin in
plants. Unpublished report prepared and submitted by Union Carbide
Corporation
Imming, R.J., Shaffer, B.C. and Woodard, G. (1969) Sevin Safety
evaluation by feeding to female beagles from day one of gestation
through weaning of the offspring. Unpublished report of Woodard
Research Corporation submitted by Union Carbide Corporation.
Khera, K.S. (1966) Toxic and teratogenic effects of insecticides in
duck and chick embryos Toxicol. appl. Pharmacol. 8:345
Knaak, J.B. and Sullivan, L.J. (1967) Carbaryl - C14
(1-naphthyl-N-methyl carbamate) Excretion and metabolism by the dog.
Unpublished report of the Mellon Institute submitted by Union Carbide
Corporation
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.J. (1967)
The metabolism of carbaryl in man, monkey, pig and sheep. Unpublished
report of the Mellon Institute submitted by Union Carbide Corporation
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.J. (1968)
The Metabolism of carbaryl in man, monkey, pig and sheep. J. Agr.
Fond Chem. 16:465-70
Kuhr, R.J. and Casida, J.E. (1967) Persistent glycosides of metabolism
of methylcarbamate insecticide chemicals formed by hydroxylation in
bean plants. J. Agr. Food Chem. 15:814-24
Marliac, J.P. (1964) Toxicity and teratogenic effects of 12 pesticides
in the chick embryo Fed. Free. 23:105
Olefir, A.I. and Vinogradova, V.K.E. (1968) Embryotoxic effects of the
pesticides Sevin and cyram (Russian). Vroch. Delo. 11:103-06 (Chem.
Abstr. 70:46402f, (1969)
Orlova, N.V. and Zhalbe, E.P. (1968) Maximum permissible mounts of
Sevin in food products (Russian). Vop. Pitan. 27(6): 49-55 (Chem.
Abstr. 70: 46402f., (1969)
Panciera, R.J. (1967) Determination of teratogenic properties of
orally administered 1-naphthyl-N-methylcarbamate in sheep. Unpublished
report from the Department of Veterinary Pathology, Oklahoma State
University, Stillwater submitted by Union Carbide Corporation
Robens, J.P. (1969) Teratologic studies of carbaryl, diazinon, Norea,
and thiram in small laboratory animals. Toxicol. appl. Pharmacol.
15:152-163
Rybakova, M.N. (1966) Toxic effect of Sevin on animals (Russian).
Gig.Sanit, 31(9):42-47
Serrone, D. (1966) Unpublished information on the effect of carbaryl
on liver microsomal enzymes. Institute of Experimental Pathology and
Toxicolgy, Albany Medical College, Albany, N.Y.
Shaffer, B.C. and Levy, A.C. (1968) Evaluation of the teratogenic
potential of rabbits fed the compound from day 9 to day 16 of the
gestation period. Unpublished report of Woodard Research Corporation
submitted by Union Carbide Corporation
Shtenberg, A.I. and Rybakova, M.N. (1968) Effect of carbaryl on the
neuroendocrine system of rats. Food Cosmet. Toxicol. 6:461-467
Smalley, H.E., Curtis, J.M. and Earl, F.L. (1968) Teratogenic action of
carbaryl in beagle dogs. Toxicol. appl. Pharmacol. 13:392-403
Vashakidze, Y.I. (1965) Effect of Sevin Intoxication on sexual
function of experimental animals (Russian). Soobsch. Akad. Nauk. Gruz.
SSR, 39(2):471-474[(Chem. Abstr. 64:2689h (1966)]
Vashakidze, V.I. (1967) Mechanism of action of pesticides (granosan,
Sevin, dinoc) on the reproductive cycle of experimental animals
(Russian). Soobsch, Akad. Nauk. Gruz. SSR, 48(1):219-224[(Chem. Abstr.
68:28750x, (1968)]
Weil, C.S. and Palm, P.E. (1958) Chronic toxicity of Sevin for dogs.
Unpublished report of Mellon Institute submitted by Union Carbide
Corporation
Weil, C.S. (1968) 4-Hydroxy Sevin and 5-Hydroxy Sevin; results of
feeding in the diets of rats for 7 to 9 days. Unpublished report of
the Mellon Institute submitted by Union Carbide Corporation
Weil, C.S. (1969a) 1-Naphthyl N-hydroxymethylcarbamate; results of
feeding in the diets of rats for one week. Unpublished report of the
Mellon Institute submitted by Union Carbide Corporation
Weil, C.S. (1969b) 4-Hydroxy-1-naphthyl N-methylcarbamate,
5-hydroxy-1-naphthyl N-methyl carbamate, and 1-naphthyl
N-hydroxymethyl carbamate; results of feeding in the diets of rats for
one week. Unpublished report of the Mellon Institute submitted by
Union Carbide Corporation
Weil, C.S. (1969c) Alpha naphthol; results of feeding in the diets of
rate for one week Unpublished report of the Mellon Institute
submitted by Union Carbide Corporation
Weil, C.S. (1969d) 1-Naphthyl hydroxymethylcarbamate. Results of
feeding in the diets of rats for three months. Unpublished report of
the Mellon Institute submitted by Union Carbide Corporation
Wiggins, O.G. (1969) Fate of Sevin applied to the surface of bean
leaves under field conditions. Unpublished report prepared and
submitted by Union Carbide Corporation
Wilhelm, E. and Vandekar, M. (1966) Studies in the toxicology of
N-Methylcarbarnates. 1. Comparative toxicity tests and estimation of
persistence of inhibiters in the body. Proc. Int. Congr. Occ. Hlth.,
Vienna, P. 517-20
Wills, J.H., Jameson, E., Stein, A., Serrone, D. and Coulston, F.
(1967) Toxicol. appl. Pharmacol 10:390
Wills, J.H., Jameson, E., Coulston, F. (1968) Clin. Toxicol.,
1(3):265-71
