
CARBARYL
First draft prepared by
P.H. van Hoeven-Arentzen
National Institute of Public Health and Environmental Protection,
Bilthoven, Netherlands
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Enzyme induction and effects on the liver
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Neurotoxicity
Immunotoxicity
Haematological effects
Effects on the endocrine system
Studies with N-nitrosocarbaryl
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Carbaryl was evaluated for toxicological effects by the Joint
Meeting in 1963, 1965, 1966, 1967, 1969, and 1973 (Annex 1, references
2, 3, 6, 8, 12, and 20). An ADI of 0-0.02 mg/kg bw was established
in 1963 on the basis of a one-year study in dogs, and this ADI was
confirmed in 1965, 1966, and 1967. In 1969, a temporary ADI of
0-0.01 mg/kg bw was established, using an extra safety factor because
of concern about effects on the male reproductive system seen in a
one-year study by gavage in rats with an NOAEL of 2 mg/kg bw, and
because a dose of 0.12 mg/kg bw per day may have affected renal
function in a six-week study in volunteers. In 1973, the Meeting
established a full ADI of 0-0.01 mg/kg bw.
The compound was reviewed by the present Meeting within the CCPR
periodic review programme. The evaluation is based on a recent
Environmental Health Criteria monograph (EHC 153) on carbaryl (WHO,
1994) and is supplemented by newly received studies on metabolism,
dermal absorption, long-term toxicity and/or oncogenicity in rats and
mice, mechanistic studies, and a report of an epidemiological study on
exposed workers.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Sprague-Dawley rats, four to eight weeks old, received
14C-carbaryl (radiolabelled in the naphthalene ring) in 1% aqueous
methylcellulose by gavage or in 5% ethanol in sodium phosphate buffer
solution by intravenous injection. In a preliminary test, two rats of
each sex were given a single oral dose of 1 mg/kg bw by gavage. In the
main study, 14C-carbaryl was administered to four groups of five
animals of each sex as a single intravenous dose of 1 mg/kg bw, a
single dose by gavage of 1 mg/kg bw, 14 daily oral doses of
non-radiolabelled compound followed by a single radiolabelled dose of
1 mg/kg bw, or a single oral dose of 50 mg/kg bw (reduced from
100 mg/kg bw because of severe toxicological effects and with the
addition of 10 animals). Volatile organic compounds and expired carbon
dioxide were collected in the preliminary test but not in the
definitive study. Urine and faeces were collected from all animals at
6, 12, and 24 h after dosing and daily thereafter for seven days.
Animals were killed seven days after administration of the
radiolabelled dose, and blood and selected tissue samples were
collected and analysed for radiolabel. Selected samples of excreta
were analysed for the parent compound and for labelled metabolites.
In the preliminary test, < 0.01% of the administered dose was
found in traps for organic volatile compounds, and no radiolabel was
found in expired carbon dioxide. A total of 96-104% of the
administered dose was recovered in all treated animals. The labelled
compound was rapidly absorbed and excreted, > 95% of the total
urinary radiolabel and > 87% of total faecal radiolabel being
eliminated within 24 h after treatment with the low dose and within
48 h after treatment with the high dose. Absorption and elimination
were independent of dose, length of administration, or sex. Urine was
the primary route of elimination (Table 1). Comparison of the results
of intravenous and oral administration indicate that absorption was
nearly complete. Radiolabel was not accumulated in any tissue, with
tissue concentrations of < 0.01 ppm after the low dose and
< 0.06 ppm after the high dose, except in the carcass (0.26 ppm in
males and 0.44 ppm in females), kidneys (0.19 ppm in males and
0.33 ppm in females), and blood (0.10 ppm in males and 0.17 ppm in
females) (Strubble, 1994).
Table 1. Recovery (%) of radiolabel in various matrices in rats treated with 14C-carbaryl
Matrix 1 mg/kg bw 1 mg/kg bw 14 x 1 mg/kg bw 50 mg/kg bw
intravenously by gavage by gavage by gavage
Male Female Male Female Male Female Male Female
Urinea 90.0 88.6 92.1 91.5 95.0 95.0 84.5 88.2
Faeces 10.2 8.7 9.1 8.4 8.6 7.7 12.5 7.0
Tissuesb 0.02 0.02 < 0.01 0.01 < 0.01 < 0.01 < 0.01 0.01
Carcass 0.13 0.34 0.10 0.23 0.15 0.21 0.60 0.90
a Includes cage rinse, cage wash (with 1% trisodium phosphate), and cage wipe
b Includes blood
Absorption was monitored in groups of four male Charles River
Crl:CD-BR rats, weighing 185-220 g, after dermal application on a site
measuring about 12.5 cm2 of 0.444 mg (36 µg/cm2), 5.03 mg
(400 µg/cm2), or 43.1 mg (3450 µg/cm2) carbaryl per animal at
0.5-, 1-, 2-, 4-, 10-, and 24-h intervals. The suspensions were
prepared with known amounts of 14C-carbaryl, carbaryl XLR plus (43.9%
pure), and 1% carboxymethylcellulose. A control group of two rats was
treated with the carrier and were killed 0.5 and 24 h after treatment.
The skin at the test site was washed just before sacrifice, and urine
and faeces were collected throughout the test.
The overall mean recoveries were 95.8, 94.0, and 97.4% of the
total dose for the three groups, respectively. Most of the radiolabel
was washed off the application site, accounting for 86.3, 90.9, and
97.5% at 0.5 h and 60.9, 64.6, and 90.5% at 24 h in rats at the low,
middle, and high doses, respectively. The amount of radiolabel
found in excreta (including cage wipe and cage wash) accounted for
0.26-22.1% of the total administered at the low dose, 0.05-21.5% at
the middle dose, and 0.01-2.5% at the high dose, the largest amounts
being detected in samples collected 24 h after treatment. Most was
found in urine. A trend of increasing direct and indirect absorption
with length of exposure was seen at all doses. Direct absorption was
considered to be represented by the total amount of radiolabel in
blood, cage wash, cage wipe, excreta, and carcass; indirect absorption
was calculated from the sum of direct absorption and the amounts left
on or in the skin at the test site after washing. By 24 h after
treatment, the directly and indirectly absorbed radiolabel represented
24.9 and 34.0%, 24.7 and 27.8%, and 3.2 and 4.0% (equivalent to 0.11
and 0.15 mg, 1.2 and 1.4 mg, and 1.4 and 1.7 mg) of the administered
dose in rats at the low, middle, and high doses. These results
indicate that absorption was linear at the two lower doses but reached
a plateau at the highest dose (Cheng, 1995).
In an experiment of identical design, groups of four seven-week-
old male Charles River Crl:CD-BR rats were treated with suspensions
prepared with known amounts of 14C-carbaryl, the formulation Sevin
80S (80.1% carbaryl), and 1% carboxymethylcellulose at doses of
0.793 mg (63 µg/cm2), 7.83 mg (626 µg/cm2), or 42.7 mg (3410 µg/cm2)
carbaryl. The overall mean recoveries were 96.1-99.6%. Most of the
radiolabel was washed off the application site, accounting for 93.6,
97.0, and 94.4% at 0.5 h and 77.4, 95.2, and 94.7% at 24 h for animals
at the low, middle, and high doses, respectively. The radiolabel found
in excreta (including cage wipe and cage wash) accounted for 0.66-16.1%
of the total administered at the low dose, < 0.01-1.3% at the middle
dose, and 0.07-1.2% at the high dose, the largest amounts being
detected in samples collected 24 h after treatment. The radiolabel
retained in the carcass represented 0.58-2.4% in animals at the low
dose and a mean of < 0.2% in the groups at the two higher doses. A
trend of increasing direct and indirect absorption with length of
exposure was seen at all doses. By 24 h after treatment, directly and
indirectly absorbed radiolabel represented 16.1 and 19.8%, 1.3 and
2.1%, and 1.2 and 1.9% (equivalent to 0.13 and 0.16 mg, 0.10 and
0.16 mg, and 0.51 and 0.80 mg) of the administered dose for rats at
the low, middle, and high doses, respectively (Cheng, 1994).
(b) Biotransformation
In the study of Strubble (1994), the profiles of radiolabel
excretion in composite samples of urine and faeces were compared
in males and females in each group by two-dimensional thin-layer
chromatography and high-performance liquid chromatography. The results
indicated that the metabolism of carbaryl is similar regardless of the
route of administration, dose, or sex. Metabolites were therefore
isolated only from the excreta of animals at the high dose and
identified and quantified. The main metabolite identified in the
faeces (0.82% of the administered dose) was dihydrodihydroxy carbaryl;
a small amount (0.15%) of apparently unabsorbed 14C-carbaryl was also
found. In urine, identified metabolites accounted for about 75%
of the urinary radioactivity; five unidentified polar metabolites,
individually accounting for 0.3-2.3% of the administered dose, were
also found. The predominant urinary metabolites were 1-naphthol
(accounting for 14.5% of the administered dose), 5-hydroxycarbaryl
(12.8%), 5,6-dihydro-5,6-dihydroxycarbaryl (8.2%), 4-hydroxycarbaryl
(6.3%), and N-(hydroxy-methyl)hydroxycarbaryl (5.7%). Carbaryl
accounted for 2.9% of the administered dose. Other metabolites found
were 3,4-dihydro-3,4-dihydroxycarbaryl, alpha-naphthyl sulfate,
1,5-dihydroxy-naphthalene, hydroxydemethylcarbaryl, and a dimer of
1,4-naphthoquinone. The three main metabolic pathways observed
were: (i) arene oxide formation with subsequent metabolism to
dihydrodihydroxy-carbaryl and conjugation with glutathione via the
mercapturic acid pathway; (ii) carbamate hydrolysis to form
1-naphthol; and (iii) oxidation of the N-methyl moiety (alkyl
oxidation). The metabolites formed via these pathways formed
conjugates with sulfate or glucuronic acid.
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) also noted that carbaryl is rapidly absorbed in the lungs and
digestive tract. In human volunteers, 45% of an applied dermal dose
in acetone was absorbed within 8 h, although studies of dermal
penetration in vitro and of toxicity indicate that dermal absorption
usually occurs at a much lower rate.
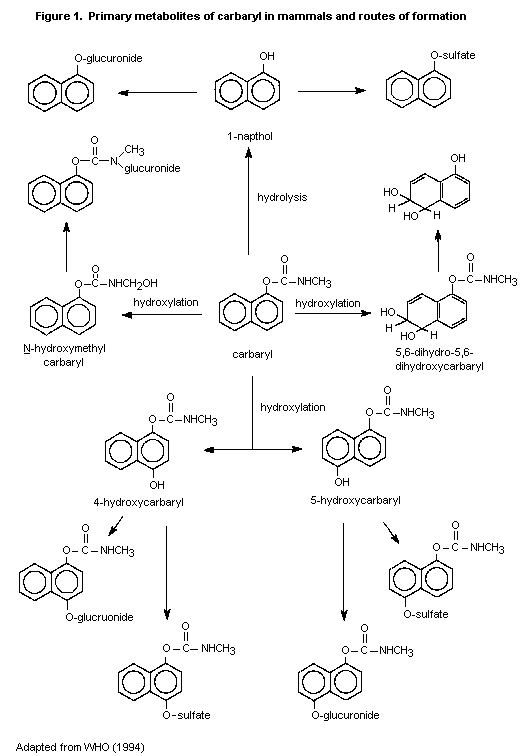
The metabolism of carbaryl has been studied in a variety of
mammals, including rats, rabbits, guinea-pigs, monkeys, sheep, cows,
pigs, dogs, and humans, all of which have essentially similar
metabolic pathways (Figure 1). As the principal pathways are ring
hydroxylation and hydrolysis, numerous metabolites are found, which
conjugate to form water-soluble sulfates, glucuronides, and
mercapturates and are excreted in the urine. Hydrolysis results
in the formation of 1-naphthol, carbon dioxide, and methylamine.
Hydroxylation produces 4-hydroxycarbaryl, 5-hydroxycarbaryl,
N-hydroxy-methylcarbaryl, 5,6-dihydro-5,6-dyhydroxycarbaryl, and
1,4-naphthalendiol. The principal metabolite in humans is 1-naphthol.
Under normal conditions of exposure, carbaryl is unlikely to
accumulate in animals. It is excreted primarily in urine, since
the product of its hydrolysis, 1-naphthol, is detoxified mainly
to water-soluble conjugates. Enterohepatic cycling of carbaryl
metabolites is also considerable, especially after oral administration.
The hydrolysis product, N-methylcarbamic acid, decomposes
spontaneously to methylamine and carbon dioxide; the methylamine
moiety is subsequently demethylated to carbon dioxide and formate,
and the latter is excreted mainly in urine. Carbaryl metabolites also
represent a small percentage of the absorbed dose in saliva and milk.
 (c) Enzyme induction and effects on the liver
Six male CD1 mice were fed a diet designed to provide carbaryl
(purity, 99.6%) at a dose of 8000 ppm, equal to 1154 mg/kg bw per day,
for 14 days. Five animals received control diet. Food consumption was
markedly reduced during the first three days of treatment, accompanied
by body weight loss. At the end of treatment, body weights were
decreased to 85%, and the relative liver weight had increased to 135%
relative to controls. Protein fractions were determined in the
microsomal and cytosolic fractions: a 1.3-fold increase (per gram of
liver) in microsomal protein was found, with a similar increase in
microsomal cytochrome P450 content. The ethoxylation of ethoxy-
resorufin and the depentylation of pentoxyresorufin were increased by
1.9- and 3.1-fold, respectively. Total testosterone hydroxylation was
increased by 1.5-fold relative to controls; while some forms of
testosterone hydroxylation were minimally altered, the 6 alpha, 11
alpha, 11ß, and 16ß forms were increased by three- to fourfold.
Hepatic glutathione levels were only slightly increased. The author of
the report concluded that carbaryl is a low-potency barbiturate-type
inducer (Thomas, 1994); however, in view of the magnitude of the
effects found in relation to the dose, there is no clear evidence that
the compound should be considered an inducer.
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that disturbances in carbohydrate metabolism, protein
synthesis, and detoxification have been reported in the livers of
mammals treated with carbaryl. It concluded that carbaryl is a weak
inducer of hepatic microsomal drug metabolizing activity, hepatic
levels of cytochrome P450 and b5 are increased, and phenobarbital
sleeping time is shortened. The changes in hepatic metabolism may
account in part for the three-fold increase in the LD50 for
carbaryl-pretreated rats.
2. Toxicological studies
(a) Acute toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that the compound is
moderately toxic after acute oral administration. The LD50 for rats
was 225-721 mg/kg bw. Interspecies difference were found, cats being
the most sensitive and guinea-pigs, rats, mice, and rabbits showing
more resistance, in that order. The LD50 was increased threefold
by pretreating animals with small doses of carbaryl. The compound
is slightly toxic after acute dermal administration, the LD50
being > 2000 mg/kg bw. No LC50 for acute exposure by inhalation was
available, but the effects observed in dogs, cats, and rats exposed to
carbaryl dust or to formulations of carbaryl were typical of those of
cholinesterase inhibition. In cats, exposure for 6 h to a dust
concentration of only 20 mg/m3 inhibited serum and erythrocyte
cholinesterase activity. Many of the known metabolites of carbaryl
are much less toxic than the parent compound. None of the metabolites
with a methylcarbamate moiety was appreciably more active as a
cholinesterase inhibitor than carbaryl itself.
(b) Short-term toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that in a study of
cats the NOAEC was 16 mg/m3 after exposure for 120 days, on the basis
of cholinergic reactions at 30 mg/m3 after exposure for 30 days. In a
study in rats, no effects were observed after 90 days' exposure to
10 mg/m3.
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female CD1 mice were given diets
designed to provide carbaryl (purity, 99.3%) at concentrations of 0,
100, 1000, or 8000 ppm, equal to 0, 15, 150, and 1200 mg/kg bw per day
for males and 0, 18, 180, and 1400 mg/kg bw per day for females, for
two years. Haematological parameters and plasma, erythrocyte, and
brain cholinesterase activities were determined in 10 mice of each sex
per group at week 53 and at termination.
There were no treatment-related effects on mortality. A thin
appearance and hunched posture were noted in many females and some
males at the high dose during the first three to six weeks and the
last six months of the study. Some animals at this dose also had a
languid appearance, urine stains, rough coats, and opaque eyes, the
latter in females during the last three months. Body-weight gain was
impaired during the first two weeks of the study in mice at the high
dose, and the weights remained significantly lower than that of
controls throughout the study. At the end of the study, the body
weights of males and females at the high dose were 88 and 87% of those
of the respective controls, but the weight gain over the whole period
was only 62 and 68% of that of controls. Food consumption was markedly
depressed in animals of each sex at the high dose during the first
months of the study. It remained low in females throughout the study
and was again lower in males from week 78 of the study. Erythrocyte
counts, haemoglobin, and packed cell volume were decreased in females
at the high dose at week 53 and in males at the end of the study.
The platelet count was increased in females at the high dose.
Cholinesterase activity was inhibited in animals at the middle and
high doses. Significant decreases in erythrocyte acetylcholinesterase
were seen only at week 53 in males at the middle and high doses (78
and 70% of the control value, respectively). Significant, dose-related
decreases in brain acetylcholinesterase activity were seen in animals
of each sex at the middle and high doses at interim and terminal
sacrifice. At the end of the study, brain acetylcholinesterase
activity was only 60 and 66% of the control levels in males and
females at the high dose, respectively.
Decreases in the absolute and relative weights of lungs and ovary
(at interim sacrifice only) were seen in females at the high dose. The
absolute and relative weights of the livers (with gall-bladder) and
the relative weight of the kidneys were increased in animals of each
sex at the high dose. On histopathological examination, a dose-related
increase in the incidence and severity of intracytoplasmic protein-
like droplets was found in the urinary bladder of animals of each sex
at the middle and high doses. An increased incidence of uni- and/or
bilateral cataracts was found in high-dose males and females, and a
slight increase in the severity of extramedullary haematopoiesis
and the presence of pigment in the spleen were found at terminal
sacrifice. The incidence of renal tubular-cell neoplasia was increased
in high-dose males, that of hepatocellular neoplasia in high-dose
females, and that of vascular tissue neoplasia in all treated males
and in high-dose females (Table 2). Carbaryl was thus found to be
carcinogenic in mice. The NOAEL for non-neoplastic findings was
100 ppm, equal to 15 mg/kg bw per day, on the basis of inhibition
of erythrocyte and brain acetylcholinesterase activity and
histopathological changes in the urinary bladder (Hamada, 1993a).
A re-examination of the histological slides of the liver and
kidneys from mice in the control and high-dose groups at interim
sacrifice, performed by two pathologists from the Rhône Poulenc
Company (Debruyne & Irisarri, 1996), showed no microscopic changes in
the liver or kidney. In a position paper, Klonne (1995) compared the
incidence of vascular tumours (mostly in the liver and spleen) in the
male mice in this study with those in historical controls in the
laboratory that performed the experiment and in several other
laboratories. The tumour incidences were clearly increased over
the mean values for historical controls; but, in the performing
laboratory, data were available only from 18-month studies. When the
incidences were compared with the ranges in historical controls in
studies of up to two years' duration, the incidences of vascular
tumours in the livers of animals at the low and middle doses were just
below the upper limit for the controls, and the incidences of vascular
tumours in the spleens of animals at the middle dose were outside the
range. The incidences among animals at the high dose were outside the
range for vascular tumours in the liver and within the range for
tumours in the spleen.
Table 2. Incidences of tumours in groups of 80 mice fed diets containing carbaryl
Tumour Males Females
0 ppm 100 ppm 1000 ppm 8000 ppm 0 ppm 100 ppm 1000 ppm 8000 ppm
Renal tubular-cell adenoma 0 0 0 3 0 0 0 1
Renal tubular-cell carcinoma 0 0 0 3 0 0 0 0
Hepatocellular adenoma 12 7 13 8 0 0 1 7
Hepatocellular carcinoma 6 7 3 8 1 1 1 3
Hepatoblastoma 0 0 0 0 0 0 0 1
Haemangioma 0 1 1 3 1 0 1 0
Haemangiosarcoma 2 5 9 7 2 3 3 9
No. of vascular 2 6 10 10 3 3 4 9
tumour-bearing animals
All vascular tumours 2 9 13 18 5 6 5 10
Rats
Groups of 80 male and 80 female Sprague-Dawley rats were given
diets designed to provide concentrations of carbaryl (purity, 99%) of
0, 250, 1500, or 7500 ppm, equal to 0, 10, 60, and 350 mg/kg bw per
day for males and 0, 13, 79, and 480 mg/kg bw per day for females, for
two years. Haematology, clinical chemistry, cholinesterase activity in
plasma and erythrocytes, and urinary parameters were studied in 10
rats of each sex at weeks 26, 52, 78, and 104. Brain acetylcholin-
esterase activity was determined at interim sacrifice and at the end
of study in 10 rats of each sex. Two additional groups of 10 rats of
each sex were treated with 0 or 7500 ppm for 52 weeks and were then
kept for a recovery period of four weeks, when haematology, clinical
chemistry, and cholinesterase activity were studied. There were no
treatment-related effects on mortality, and survival was increased in
females at the high dose; however, an increased incidence of alopecia
on the limbs was found in these animals, and an increased prevalence
of urine stains was seen in animals of each sex. The mean body weights
were significantly depressed in animals at the high dose at all
intervals analysed and in those at the middle dose at many intervals.
At the end of study, the body weights of males and females at the high
dose were only 5 and 55% of those of the respective controls, and
those of animals at the middle dose were 94% and 88% of the control
values, respectively. Food consumption was markedly lower in the
high-dose group. The numbers of animals with cataracts were increased
in the high-dose group, with incidences of 4, 6, 7, and 12 males and
3, 2, 4, and 10 females at the control, low, middle, and high doses,
respectively.
There were no treatment-related findings in haematological
parameters. Increased serum cholesterol and urea nitrogen were found
in high-dose females. Plasma cholinesterase activity was depressed in
animals of each sex at the high dose, to 58-73% of the control value
in males and 43-54% in females. Erythrocyte acetylcholinesterase
activity was also significantly decreased, to 63-81% of the control
value in males at the high dose and 62-75% in females; in females at
the middle dose, the activity was 74-81% of the control value, whereas
in males at this dose the depression was found only at weeks 52
(81%) and 78 (77%). Significant, dose-related decreases in brain
acetylcholinesterase activity were found at both 52 and 104 weeks in
high-dose males and middle- and high-dose females. Males at the middle
dose showed a significant depression only at the 52-week interim
sacrifice. Changes seen on urinalysis in animals at the high dose
included an increased incidence of dark urine, decreased urine volume
in females (at week 52), and erythrocytes in urine and occult blood in
males (at weeks 78 and 104). The relative weights of lungs, brain,
kidney, and liver were significantly increased in animals at the high
dose at the interim and terminal sacrifices; testicular weight was
also increased in those at the high dose. The increase in relative
liver weight was dose-related, but a significant increase at the
middle dose was found only in the females at interim sacrifice.
Gross pathological examination of animals at the high dose
revealed increased incidences of masses in the urinary bladder, pale
areas in the lungs, and dark areas in the glandular stomach (only in
males). A decreased incidence of mammary masses was noted in females
at this dose, with 65% in controls, 57% at the low dose, 64% at the
middle dose, and 41% at the high dose. Histopathological evaluation
of tissues taken from rats at interim sacrifice revealed hyaline
inclusions in the livers of males at the high dose. At the end of the
study, the following non-neoplastic findings were observed in animals
at the high dose: an increased incidence of thyroid hypertrophy (8/70
in males and 33/70 in females, in comparison with 1/70 and 4/70 in
controls, respectively); eosinophilic hepatocellular alterations and
increased pigment in females, hepatocyte hypertrophy in animals of
each sex, and intracytoplasmic hyaline inclusions in males; an
increased incidence of transitional epithelial hyperplasia in the
kidneys of males, and squamous metaplasia, a high mitotic index, and
cytological atypia in the urinary bladders of animals of each sex, all
associated with the carcinomas found; an increased incidence of
alveolar foamy macrophages and focal pneumonitis; and an increased
incidence of degeneration of the sciatic nerve and adjacent skeletal
muscles. The neoplastic findings in thyroid, liver, and urinary
bladder are summarized in Table 3. Nearly all of the animals with
transitional-cell neoplasms in the bladder also had extensive
hyperplasia, indicating the preneoplastic nature of this change. Males
at the high dose also had an increased incidence of transitional-cell
hyperplasia in the kidney; a single carcinoma was found. The incidence
of benign interstitial-cell tumours in the testis was increased in
animals at the high dose (5/70; 2/70 in controls).
After one year of exposure and the recovery period of four weeks,
the food consumption of animals at the high dose improved and the
animals gained more weight. The effects on cholinesterase activity
were reversible, as were the histological changes in the liver. The
relative weights of the lungs, brain, kidney, and liver, however,
remained increased. Carbaryl thus induced tumours at 7500 ppm, a
dose that exceeds the maximum tolerated dose (MTD). The NOAEL for
non-neoplastic findings in this study was 250 ppm, equal to 10 mg/kg
bw for males per day, on the basis of inhibition of erythrocyte and
brain acetylcholinesterase and the decrease in mean body weight
(Hamada, 1993b).
A re-examination of the histological slides of the liver, kidney,
urinary bladder, and thyroid of rats of all doses at interim sacrifice
and of the controls and those at the high dose after recovery,
performed by two pathologists from Rhône Poulenc Company (Debruyne &
Irisarri, 1996), revealed the presence of further microscopic changes
in animals at the high dose in the bladder (irreversible epithelial
hyperplasia in males and females), kidney (reversible pelvic
urothelial hyperplasia in males), thyroid (reversible follicular
hypertrophy in males), and liver (reversible hepatocellular
hypertrophy in males and females).
Table 3. Incidences of tumours in groups of 70 rats fed diets containing carbaryl
Tumour Males Females
0 ppm 250 ppm 1500 ppm 7500 ppm 0 ppm 250 ppm 1500 ppm 7500 ppm
Thyroid follicular-cell adenoma 0 2 0 8 0 0 0 1
Thyroid follicular-cell carcinoma 0 0 0 1 1 0 0 0
Hepatocellular adenoma 1 1 2 1 1 0 3 7
Hepatocellular carcinoma 0 2 2 1 0 0 0 0
Transitional-cell hyperplasia 9 8 10 54 6 6 6 56
Transitional-cell papilloma 0 à 0 13a 1 0 0 7
Transitional-cell carcinoma 0 0 0 11 0 0 0 6
a Includes one squamous-cell papilloma
In the Environmental Health Criteria monograph on carbaryl (WHO,
1994), the results of several long-term studies in mice were
summarized, one of which was a report of the data obtained at interim
sacrifice in the study of Hamada (1993a). In another study, no effects
were observed at the highest level tested (400 ppm). Seven further
studies of carcinogenicity in mice involving different strains, routes
of administration, doses, dose schedules, and lengths of exposure and
observation were considered unsuitable for evaluation of carcinogenic
potential; only one showed a marginal response.
Several short- and long-term studies in rats were available. The
most recent report presented the interim results of the long-term
study of Hamada (1993b). In two older dietary studies, lasting 96 days
and two years, the most obvious effects were on the kidney. The NOAEL
in these studies was 200 ppm, equal to 7.9 mg/kg bw per day, with
effects on the kidney at 400 ppm. In two one-year studies of gavage
(for which only summaries were available), effects were seen on the
thyroid and on male and female reproductive organs and/or function at
doses > 5 mg/kg bw per day. The NOAEL was 2 mg/kg bw per day. Two
studies of carcinogenicity in rats were available. In a study in which
carbaryl was administered orally or by a single subcutaneous
implantation, fibrosarcomas of the skin were observed; the other
(dietary) study gave negative results. Neither was considered suitable
for evaluation of carcinogenic potential.
Three one-year studies in dogs were available. In a study in
which carbaryl was given in capsules, effects on the kidney were found
at 7.2 mg/kg bw per day, but no effects were observed at 1.8 mg/kg bw
per day (approximately 100 ppm in the diet). In the two dietary
studies, the NOAEL was 125 ppm, equivalent to 3.1 mg/kg bw per day, on
the basis of effects on liver weight and inhibition of erythrocyte and
brain acetylcholinesterase activity at 400 ppm.
(d) Reproductive toxicity
No new information had become available. The summaries in the
Environmental Health Criteria monograph on carbaryl (WHO, 1994),
although extensive, could not always be used to derive NOAELs for
various aspects of the reproductive toxicity of carbaryl. Additional
information was therefore obtained for some studies, from either the
original papers or reviews.
In two five-day studies of male mice exposed orally, no effects
were found on the testis or accessory glands at a dose of 34 mg/kg bw
per day; at 68 mg/kg bw per day, androstenedione synthesis was
decreased.
Three three-generation studies of reproductive toxicity in rats
were summarized. In the first, in which 0, 2000, 5000, or 10 000 ppm
carbaryl were administered in the diet, impaired fertility, reduced
postnatal survival, and reduced postnatal growth were observed at
5000 ppm; no effects were seen at 2000 ppm, equivalent to 125 mg/kg bw
per day. There was no clear NOAEL in this study, since the parameters
were wrongly defined. In a second dietary study, at doses of 0, 7, 25,
100, and 200 mg/kg bw per day, the NOAEL was 100 mg/kg bw per day, on
the basis of decreased maternal weight at 200 mg/kg bw per day. There
were no reproductive effects. In a study in which rats were treated by
gavage at doses of 0, 3, 7, 25, or 100 mg/kg bw per day, the NOAEL was
25 mg/kg bw per day, on the basis of decreased maternal weight and
mortality and effects on litter size and viability at the high dose.
In a study in which special attention was paid to effects on
reproductive parameters, male and female rats were exposed by gavage
for one month to doses of 1-50 mg/kg bw per day. Adverse effects were
observed on male reproductive cells; furthermore, increased embryonic
and fetal deaths, decreased numbers of implantations, and a prolonged
oestrus cycle were observed. This study was also described by Cranmer
(1986), who reported that the effects on reproductive parameters were
seen only at 1, 5, 10, and 20 mg/kg bw per day. He also noted that
only the change in the number of tubules containing spermatogonia
was seen at all doses, and most of the other treatment-related
histological and functional changes in the testis occurred at doses
> 5 mg/kg bw per day. Dose-response relationships were not seen for
the other effects on litter parameters or for the prolonged oestrus
cycle, limiting interpretation of the study. This and other studies of
reproductive toxicity were considered of dubious value for risk
assessment as they suffered from various shortcomings in study design.
A three-generation study of reproductive toxicity in which
gerbils were exposed daily via the diet to concentrations of
2000-10 000 ppm was not considered suitable for establishing a NOAEL;
however, adverse effects on various reproductive parameters were seen.
(e) Developmental toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) was supplemented by
additional information from the original papers and reviews. All of
the studies suffered from small group sizes and some deficiencies in
comparison with currently acceptable scientific standards.
Two of five studies in mice were used for evaluation, in which
animals were exposed by gavage to carbaryl on days 6-15 of gestation;
in the second study, groups were also exposed only on day 8 or 12. In
the first study, with doses of 0, 100, and 150 mg/kg bw per day, the
NOAEL was 100 mg/kg bw per day on the basis of maternal toxicity
(ataxia, lethality) and litter resorption at 150 mg/kg bw. In one
group exposed via the diet to 5560 ppm, a decrease in fetal weight was
observed. In the second study, with doses of 0, 100, 150, and
200 mg/kg bw per day, the NOAEL was 150 mg/kg bw per day, on the
basis of maternal death and fetal toxicity (weight reductions and
indications of growth retardation) at 200 mg/kg bw per day.
Three of six studies in rats were used for evaluation. In the
first study, groups of six rats were fed diets containing carbaryl on
days 1-5, 5-15, or 1-21 of gestation. The NOAEL for maternal toxicity
was 100 mg/kg bw per day, on the basis of reduced weight gain in dams
at 500 mg/kg bw per day when exposed on gestation days 5-15 or 1-21.
No adverse fetal effects were seen. The original papers (Weil &
Carpenter 1965, 1966) reported that the doses used were 0, 2.5, 10,
20, 100, and 500 mg/kg bw per day and that fetuses underwent only
skeletal examination. In addition to the effects summarized above, the
authors reported reduced postnatal survival in the pups of dams
exposed to 500 mg/kg bw per day on days 1-21. In the second study,
groups of 10 rats received oral doses of 200 or 300 mg/kg bw or an
intraperitoneal dose of 40 mg/kg bw on single or multiple days of
gestation. The only effect seen was a reduction in fetal weight in
some groups. In the third study, groups of six or seven rats received
diets containing carbaryl at doses of 0, 1, 10, or 100 mg/kg bw per
day three months before and during gestation. The NOAEL for maternal
toxicity was 10 mg/kg bw per day, on the basis of reduced weight gain
at 100 mg/kg bw per day. No adverse fetal effects were seen. This
study was also described by Cranmer (1986), who reported a slight
reduction in the numbers of implantations and live fetuses at 100
mg/kg bw per day.
One of two studies in guinea-pigs was used for evaluation. Groups
of four to nine animals were exposed to 0, 100, 200, or 300 mg/kg bw
per day in the diet or 0, 50, 100, or 200 mg/kg bw per day orally.
Reduced maternal weight gain was observed at 200 mg/kg bw per day, but
there were no effects on embryos or fetuses.
One of three studies in rabbits was used for evaluation. Groups
of 15-20 animals were given oral doses of 0, 150, or 200 mg/kg bw per
day on days 6-18 of gestation. There was no NOAEL for maternal
toxicity since a reduction in weight gain was observed in both treated
groups. A significant increase in umbilical hernia was observed in
fetuses at 200 mg/kg bw per day.
In two studies in dogs, animals were fed diets containing
carbaryl at doses of 3.1-50 mg/kg bw per day or 2-12.5 mg/kg bw per
day during gestation. Various birth defects were observed in the pups
at doses > 5 mg/kg bw per day. Maternal toxicity was observed at
all doses.
In pigs fed doses of 4-32 mg/kg bw per day in the diet, the
effects noted (prenatal lethality and malformations) were not
consistent across the studies. Another study in pigs was considered
unsuitable for evaluation.
One of two studies in monkeys showed an increased rate of
abortions after oral treatment with 2 or 20 mg/kg bw per day
throughout gestation. No adverse effects were noted in the second
study in which the doses were 0.2-32 mg.kg bw per day orally on days
20-38 of gestation.
(f) Genotoxicity
The results of studies of chromosomal aberrations in rats and
micronucleus formation in mice treated with carbaryl in vivo are
summarized in Table 4. Negative results were obtained in both studies
at doses at which limited signs of toxicity were seen; however, there
was no evidence that the test compound had reached the target organ,
as no cytotoxicity was found.
In a study of DNA binding, two groups of four male CD mice were
treated by oral intubation with 14C-carbaryl (labelled in the
naphthyl group) in 0.5% aqueous carboxymethylcellulose at a dose of
75 mg/kg bw (8 mCi/kg bw). One group was pretreated with carbaryl
(purity, 99.6%) at 8000 ppm (equal to 1900 mg/kg bw per day) in the
diet for 14 days. Four mice served as controls. Urinary excretion and
exhalation of carbon dioxide were measured over 24 h in one pretreated,
one non-pretreated, and one control mouse. Animals were sacrificed 24 h
after administration of radiolabelled material, and the liver, kidneys,
and urinary bladder were removed; however, only the liver was used
for determining DNA and protein binding. There was no significant
exhalation of 14C-carbon dioxide, and about 30% of the administered
dose was excreted in the urine of both treated groups. The pretreated
group had lower body weights and increased relative liver weights at
the end of treatment. Binding of 14C-carbaryl to chromatin protein
was found in both treated groups, resulting in specific radioactivities
of 340-537 dpm/mg protein (7-11 pmol/mg protein). As there was no
significant radioactivity in purified DNA, in the presence or absence
of pretreatment, covalent binding of the 14C-naphthyl label does not
seem to have occurred (Sagelsdorff, 1994).
Table 4. Results of tests for the genotoxicity of carbaryl in vivo
End-point Test system Concentration Purity Results Reference
(mg/kg bw) (%)
Micronucleus formation Male and female 50, 100, 200 orally for 99.9 Negativea Marshall (1996)
CD-1 mice (5 per 2 daysb; killed at 24 or
dose), bone-marrow 48 h; vehicle, 0.5%
cells carboxymethylcellulose
Chromosomal aberration Male and female Single dose of 30, 60, 99.7 Negativea McEnaney (1993)
Sprague-Dawley 120c; killed at 6, 24,
rats (5 per dose), 48 h; vehicle, 0.25%
bone-marrow cells carboxymethylcellulose
a Positive controls yielded positive results
b Clinical signs of toxicity (lethargy, slight weight loss) at highest dose; no change in polychromatic:normochromatic
erythrocyte ratio. Doses based on the results of a preliminary test in which one of six animals at 300 mg/kg bw died.
c Clinical signs of toxicity (lethargy, tremors) observed at the highest dose; no depression of mitotic index. Doses based on
the results of a preliminary test in which the LD50 was 231 mg/kg bw
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that carbaryl has been evaluated for mutagenicity in a
number of tests in vitro and in vivo, in bacterial, yeast, plant,
insect, and mammalian systems, with a variety of end-points. It
concluded that carbaryl does not damage DNA. Reports of induction of
mitotic recombination, gene conversion, and unscheduled DNA synthesis
in prokaryotes (Haemophilus influenzae, Bacillus subtilis) and
eukaryotes ( Saccharomyces cerevisiae, Aspergillus nidulans, cultured
human lymphocytes, and rat hepatocytes) in vitro have not been
confirmed. Negative results were obtained in tests for gene mutations
in all but two bacterial assays. Although several studies of gene
mutation were conducted in mammalian cells in vitro, only one
equivocally positive result was obtained, in a study that had several
shortcomings and which has not been confirmed. Chromosomal damage has
been reported in human, rat, and hamster cells and in plants treated
in vitro with high doses of carbaryl. No such effects have been
observed in mammalian tests in vivo, even at doses as high as
1000 mg/kg bw. Carbaryl induces disturbances in the spindle fibre
mechanism in plant and mammalian cells in vitro, but the relevance
of the assays in plants for humans is unclear. The Environmental
Health Criteria monograph concluded that the available database did
not indicate that carbaryl induces genetic changes in somatic or
germinal tissues of humans.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported that carbaryl is
not or only weakly irritating to the skin and is weakly irritating to
the eye. The available studies indicated that carbaryl has little or
no sensitizing potential.
(ii) Neurotoxicity
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported that the effects
of carbaryl on the central nervous system had been studied in rats
and monkeys treated by intraperitoneal, intramuscular, or oral
administration or by inhalation. Changes in motor activity, working
memory, and behaviour were seen. Dietary exposure to doses of
10-20 mg/kg bw per day for 50 days was reported to disrupt learning
and performance in rats. In a small study on pigs, administration of
carbaryl in the diet at 150 mg/kg bw per day for 72-82 days was
reported to produce a number of neuromuscular effects. Reversible leg
weakness was noted in chickens given high doses of carbaryl, but no
evidence of demyelination was observed in the brain, sciatic nerve, or
spinal cord sections examined microscopically. Similar effects were
not observed in long-term studies in rodents. The effects of carbaryl
on the nervous system are primarily related to inhibition of
cholinesterase activity and are usually transitory.
(iii) Immunotoxicity
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that several studies
in mice, rats, rabbits, and guinea-pigs, mostly treated orally, showed
that carbaryl administered at doses that do not cause overt clinical
signs has a variety of non-life-threatening effects on both cellular
and humoral immunity. Many of the effects were detected at doses close
to the LD50. A lack of consistency and sometimes overt contradiction
between the results of several of these studies precludes definition
of the immunotoxic mechanism. Life-time exposure to carbaryl did not
increase the occurrence of disease in rats or mice, and no enhancement
of viral infections was found, even at doses close to the LD50. Most
of the studies on mice and rabbits at doses that permitted survival
did not show significant effects on the immune system. Vital
enhancement has been demonstrated in vitro in a number of studies
including prior incubation with carbaryl. Inhibition of human serum
complement activity and interleukin-2-driven proliferation of large
granular lymphocytes have also been found in vitro.
(iv) Haematological effects
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported effects on
coagulation in rats, rabbits, and dogs in vivo and in studies in
vitro, but the direction of the effect is unclear. In glucose-
6-phosphate dehydrogenase-deficient sheep erythrocytes, carbaryl
produced a dose-dependent increase in methaemoglobin formation. Human
serum albumin reacted in vitro with the ester group of carbaryl.
Carbaryl binds free blood amino acids.
(v) Effects on the endocrine system
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) concluded from several
experiments in vivo and in vitro that carbaryl increases the
gonadotropic function of the hypophysis of rats.
(vi) Studies with N-nitrosocarbaryl
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that carbaryl is a secondary amine and is therefore
capable of nitrosation in the presence of nitro donor groups, such as
sodium nitrate, to give a nitrosamide. A condition of such nitrosation
is an acidic pH (< 2), such as that found in the human stomach;
however, N-nitrosocarbaryl is not stable at this pH; its maximal
stability is at pH 3-5, at which no significant amount of carbaryl can
be nitrosated. Carbaryl was nitrosated in several studies, in vitro
as well as in vivo, in guinea-pigs, in which the gastric acidity is
similar to that of humans.
N-Nitrosocarbaryl induced local tumours in rats, consisting of
sarcomas at the site of injection and forestomach squamous-cell
carcinomas, after oral administration. This local carcinogenic effect
and the lack of systemic carcinogenicity characterize the compound as
a directly acting alkylating agent. Given the human chemistry of
carbaryl, the risk of carcinogenic effects of N-nitrosocarbaryl for
humans after exposure to carbaryl can be considered negligible.
N-Nitrosocarbaryl can induce mitotic recombination and gene
conversion in prokaryotes (H. influenzae and B. subtilis) and
eukaryotes (S. cerevisiae) in vitro and gives positive results in
Escherichia coli spot tests. It also binds to DNA, causing
alkali-sensitive bonds and single-strand breakage. It is not
clastogenic in vivo in bone marrow and germ cells, even at highly
toxic doses.
3. Observations in humans
An epidemiological study of total and cause-specific mortality
among employees exposed to carbaryl at a production plant was based on
information obtained in 1988 on the vital status and cause of death of
all individuals who were first hired between the start-up of the
carbaryl unit in 1960 through 1978. Employees hired after 1978 were
not included in the study. Three categories of workers exposed to
carbaryl were defined: those involved in production, maintenance
employees, and those working in packaging and distribution. A total of
448 employees contributing 7532 person-years to the analysis were
available, representing the combined number of years in which these
employees were followed through 1988. Mortality was measured in terms
of standardized mortality ratios (SMRs), which are the ratios of the
observed numbers of death among members of a cohort to the number
expected, on a year-specific and age-adjusted basis. The 25 deaths
identified in this cohort resulted in elevated SMRs for cancer of the
pancreas, unspecified cancer, and cancers of the brain and other parts
of the nervous system. In the first two categories, the excess was
slight and based on only one death. In the last category, the SMR was
based on two deaths due to tumours of different histological origins,
reducing the possibility that the two malignancies were caused by the
same exposure. Furthermore, in all three categories the confidence
intervals were wide, indicating a relatively imprecise SMR estimate
and reflecting the small sample size on which it is based (Pastides,
1993).
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) summarized several cases of poisoning. The clinical picture is
dominated by symptoms of inhibition of cholinesterase activity. Signs
of poisoning develop quickly after absorption and disappear rapidly
after exposure ends.
In controlled studies of human volunteers, single doses of
< 2 mg/kg bw were well tolerated. A single dose of 250 mg (about
2.8 mg/kg bw) produced moderate symptoms of cholinesterase inhibition
(epigastric pains and sweating) within 20 min. Complete recovery was
seen within 2 h of treatment with atropine sulfate. Two groups of
human volunteers given carbaryl at doses of 0.06 or 0.13 mg/kg bw for
six weeks underwent physical examinations, removal of bromosulphthalein
from blood, electroencephalography, routine blood and urinalysis, and
measurement of cholinesterase in plasma and erythrocytes. No inhibition
of cholinesterase activity was observed, and no changes were seen in
the group receiving the low dose. The only finding at the high dose was
an increase in the ratio of amino acid nitrogen to creatinine in the
urine, which may represent a decrease in the ability of the proximal
convoluted tubule to reabsorb amino acids; this change was reversible.
The NOAEL was 0.06 mg/kg bw.
In cases of occupational overexposure to carbaryl, mild symptoms
are observed long before a dangerous dose is absorbed. No local
irritating effect is usually seen, although skin rash after accidental
splashing with carbaryl formulations has been described.
Reports of the effects of carbaryl on sperm count and changes in
sperm morphology in plant workers are conflicting. No adverse effects
on reproduction have been described.
Comments
Carbaryl is rapidly and almost completely absorbed after oral
administration. Excretion is rapid and occurs predominantly via the
urine; enterohepatic cycling of carbaryl metabolites is also
considerable. There were no significant dose-related or sex-specific
differences in elimination patterns, and there was no evidence of
bioaccumulation. Dermal absorption in rats was slow; after 24 h,
16-34% of the administered radiolabel had been absorbed. Higher doses
were less readily absorbed. In volunteers, 45% of a dose applied to
the skin in acetone was absorbed within 8 h. Carbaryl was rapidly
absorbed in the lungs.
The metabolism of carbaryl has been studied in various mammals,
including humans. The principal metabolic pathways are ring
hydroxylation, hydrolysis, and conjugation. There were no species
differences. The main metabolite in humans is 1-naphthol. The
hydrolysis product, N-methyl carbamic acid, spontaneously decomposes
to methylamine and carbon dioxide. The methylamine is later converted
to carbon dioxide and formate, the latter being excreted mainly in the
urine. Carbaryl metabolites are also found at small percentages of the
absorbed doses in saliva and milk.
Carbaryl is moderately toxic after acute oral administration, the
LD50 in rats being 225-721 mg/kg bw. Interspecies differences in
toxicity were found, cats (LD50, 150 mg/kg bw) being the most
sensitive species. The LD50 was increased threefold when animals were
pretreated with small doses of carbaryl. The compound is slightly
toxic after acute dermal administration, with an LD50 > 2000 mg/kg
bw. No LC50 for acute exposure by inhalation was available, but the
effects observed in dogs, cats, and rats exposed to dusts or
formulations of carbaryl were typical of those resulting from
inhibition of cholinesterase activity. In cats exposed to carbaryl
dust for 6 h, a concentration of 20 mg/m3 inhibited cholinesterase
activity in plasma and erythrocytes. Carbaryl was weakly irritating to
the eye but not the skin and was not considered to be a sensitizer.
WHO has classified carbaryl as 'moderately toxic' (WHO, 1996).
After oral administration of carbaryl in capsules to dogs at a
dose of 0.45, 1.8, or 7.2 mg/kg bw per day for one year, slight
effects were observed on the kidney at 7.2 mg/kg bw per day; the NOAEL
was 1.8 mg/kg bw per day. In two studies in which dogs were fed diets
containing carbaryl at 20-125 ppm for five weeks and 125-1250 ppm for
one year, the NOAEL was 125 ppm, equivalent to 3.1 mg/kg bw per
day, on the basis of effects on liver weight and inhibition of
acetylcholinesterase activity in erythrocytes and brain at 400 ppm.
In cats exposed to carbaryl by inhalation, cholinergic signs were
observed at 30 mg/m3 after exposure for 30 days. The NOAEL was
16 mg/m3 for 120 days. In a study in rats, no effects were observed
after exposure to 10 mg/m3 for 90 days.
Several studies of long-term toxicity or carcinogenicity in mice
cited in EHC 153 were considered to be unsuitable for evaluation of
carcinogenicity by either the Environmental Health Criteria Task Force
or the present Meeting, although they were suitable for assessing
long-term toxicity. In a recent study of carcinogenicity, mice were
given diets providing 0, 100, 1000, or 8000 ppm carbaryl for
104 weeks. Tumours were observed in the liver in females and the
kidney in males, and vascular tumours were found in animals of each
sex at the highest dose, which exceeded the maximum tolerated dose
(MTD). In male mice, increases in the incidences of vascular tumours
were also seen at the two lower doses; after considering all of the
available data, the Meeting could not identify an NOAEL for this
neoplastic lesion. The NOAEL for non-neoplastic lesions was 100 ppm
(equal to 14.7 mg/kg bw per day), on the basis of inhibition
of erythrocyte and brain acetylcholinesterase activity and
histopathological changes in the urinary bladder at 1000 ppm. This
NOAEL is consistent with the results of the earlier studies. The
Meeting concluded that the compound is carcinogenic in mice.
In several studies cited in EHC 153, carbaryl was administered in
the diet of rats for 96 days to two years. The most obvious effects
were in the kidney at doses > 400 ppm. In two one-year studies in
rats treated by gavage, effects on the thyroid and on male and
female reproductive organs and/or function were observed at doses
> 5 mg/kg bw per day; the NOAEL was 2 mg/kg bw per day. None of
these studies was considered suitable for evaluating carcinogenicity.
In a recent study of long-term toxicity and carcinogenicity, rats
were fed diets containing 0, 250, 1500, or 7500 ppm carbaryl for 104
weeks. In animals at the highest dose, which exceeded the MTD, tumours
were found in the thyroid in males, in the liver in females, and in
the urinary bladder in animals of each sex. The NOAEL for non-
neoplastic findings in this study was 250 ppm, equal to 10 mg/kg bw
per day, on the basis of inhibition of erythrocyte and brain
acetylcholinesterase activity and a decrease in mean body weight at
1500 ppm. This NOAEL is consistent with the results of earlier dietary
studies. The Committee concluded that carbaryl is carcinogenic in rats
only at levels that exceed the MTD.
The available studies on reproductive toxicity were conducted
some time ago and had some deficiencies in relation to currently
acceptable scientific standards. In three-generation studies, dietary
administration of carbaryl to rats induced reproductive effects
(impaired fertility and reduced postnatal survival and growth) at
doses > 2000 ppm (equal to 125 mg/kg bw per day); a dose of 100 mg/kg
bw per day did not induce maternal toxicity. When carbaryl was
administered by gavage, maternal toxicity was not observed at 25 mg/kg
bw per day, but both maternal and reproductive toxicity (reduced
litter size and viability) were found at 100 mg/kg bw per day. The
Meeting recommended that a new two-generation study of reproductive
toxicity be carried out in rats, with special attention to the male
reproductive system since effects on this system were observed in some
long-term studies of toxicity at gavage doses significantly lower than
those evaluated in the dietary studies of reproductive toxicity.
The available studies on developmental toxicity suffered from
small group size and had some deficiencies in relation to currently
acceptable scientific standards. In two studies in mice, the NOAEL for
maternal toxicity was 100 mg/kg per day; at 150 mg/kg bw per day,
increased litter resorption was found. In rats, administration of
carbaryl in the diet for part or all of the gestation period resulted
in maternal toxicity at 100 mg/kg bw per day. No overt signs of
fetotoxicity were seen at this dose. In a study in which rats
were exposed to carbaryl by gavage and then mated, maternal and
embryotoxicity were observed at 100 mg/kg bw per day; no effects were
seen at 10 mg/kg bw per day. In guinea-pigs, administration of
carbaryl during gestation in the diet or by gavage resulted in an
NOAEL for maternal toxicity of 100 mg/kg bw per day. No embryo- or
fetotoxicity was observed at 300 mg/kg bw, the highest dose tested. In
rabbits, teratogenic effects were reported after administration of
200 mg/kg bw per day orally; maternal toxicity was also seen at this
dose. In two studies in dogs, maternal toxicity (dystocia, at
parturition only) was observed at a dose of 3.1 mg/kg bw per day.
A variety of birth defects was found after exposure to doses
> 5 mg/kg bw per day. Thus, the LOAEL for maternal toxicity was
3.1 mg/kg bw per day, and this was the NOAEL for birth defects in the
offspring.
The Meeting concluded that carbaryl induces developmental
toxicity, manifested as deaths in utero, reduced fetal weight, and
malformations, but only at doses that cause overt maternal toxicity.
The shortcomings of these studies made them inadequate for identifying
NOAELs for developmental toxicity that could be used for assessing
risk under conditions of exposure other than in the diet.
Carbaryl has been adequately tested for genotoxicity in a series
of assays in vitro and in vivo. While chromosomal aberrations have
been induced in vitro and carbaryl has been shown to disturb spindle
fibre mechanisms in vitro, there is no evidence from well-conducted
experiments that carbaryl is clastogenic in vivo. The Meeting
concluded that carbaryl is not genotoxic.
The effects of carbaryl on the nervous system are primarily
related to cholinesterase inhibition and are usually transitory.
Dietary exposure to doses of 10-20 mg/kg bw per day for 50 days was
reported to disrupt learning and performance in rats. In chickens
given high doses of carbaryl, there was no histological evidence of
neurotoxicity.
In controlled studies in volunteers, single oral doses of
< 2 mg/kg bw were well tolerated. A single oral dose of 250 mg (about
2.8 mg/kg bw) produced moderate cholinergic symptoms. In volunteers
given repeated daily oral doses over six weeks, the NOAEL was
0.06 mg/kg bw per day, on the basis of an increased ratio of amino
acid nitrogen to creatinine in the urine at a dose of 0.13 mg/kg bw
per day. This effect may represent a decrease in the ability of the
proximal convoluted tubule to reabsorb amino acids. The change was
reversible. No inhibition of plasma or erythrocyte acetylcholin-
esterase activity was observed.
An epidemiological study on carbaryl production workers employed
between 1960 and 1978 showed no increase in cancer mortality.
An ADI of 0-0.003 mg/kg bw was established on the basis of the
LOAEL of 15 mg/kg bw per day in the study of carcinogenicity in mice,
using a safety factor of 5000, which includes an extra safety factor
of 50 to account for the presence of vascular tumours in male mice at
all doses tested. The resulting ADI provides an adequate margin of
safety, taking into account the LOAEL in the study of developmental
toxicity in dogs and the uncertainties about the effects on the male
reproductive system.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: NOAEL not identified. Lowest effective dose: 100 ppm,
equal to 14.7 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Rat: 250 ppm, equal to 10 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
2 mg/kg bw per day (one-year study of toxicity)
Dog: NOAEL not identified. Lowest effective dose: 3.1 mg/kg
bw per day (study of developmental toxicity)
1.8 mg/kg bw per day (one-year study of toxicity)
Human: 0.06 mg/kg bw per day (six-week study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Study of reproductive toxicity with special attention to the
male reproductive system
2. Studies of teratogenicity in rats and rabbits
3. Completion of on-going studies to elucidate the mechanism of
tumour formation
4. Study of developmental neurotoxicity and/or screening for
acute or subchronic neurotoxicity
5. Follow-up of the epidemiological study in workers, taking
into consideration the latent period before development of
cancer
References
Cheng, T. (1994) Dermal absorption of 14C-carbaryl (80S) in male rats
(preliminary and definitive phases). Guideline No. 85-2. Unpublished
report No. HWI 6224-207, dated July 1994 from Hazleton Wisconsin,
Inc., USA. Supplied to WHO by Rhône Poulenc, Research Triangle Park,
North Carolina, USA.
Cheng, T. (1995) Dermal absorption of 14C-carbaryl (XLR Plus) in
male rats (preliminary and definitive phases). Guideline No. 85-2.
Unpublished report No. HWI 6224-206, dated January 1995, from Hazleton
Wisconsin, Inc., USA. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Cranmer, M.F. (1986) Carbaryl; a toxicological review and risk
analysis. Neurotoxicology, 7, 247-332.
Debruyne, E. & Irisarri, E. (1996) Carbaryl technical-chronic toxicity
studies in the rat (HWA Study No. 656-139) and the mouse (HWA Study
No. 656-138): evaluation of histological slides. Unpublished report
No. R&D/CRSA/TOX-HPA-4, dated March 1996 from Rhône Poulenc Agrochimie.
Supplied to WHO by Rhône Poulenc, Research Triangle Park, North
Carolina, USA.
Hamada, N.N. (1993a) Oncogenicity study with carbaryl technical in
CD-1 mice. Unpublished report No. HWA 656-138, dated May 1993 from
Hazleton Washington, Inc., USA. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Hamada, N.N. (1993b) Combined chronic toxicity and oncogenicity study
with carbaryl technical in Sprague Dawley rats. Unpublished report No.
HWA 656-139, dated August 1993 from Hazleton Washington, Inc., USA.
Supplied to WHO by Rhône Poulenc, Research Triangle Park, North
Carolina, USA.
Klonne, D.R. (1995) Carbaryl mouse historical control data. Position
paper dated November 1995. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Marshall, R. (1996) Carbaryl: induction of micronuclei in the bone
marrow of treated mice. Unpublished report No. CH 198/89-1052, dated
March 1996 from Corning Hazleton, United Kingdom. Supplied to WHO by
Rhône Poulenc, Research Triangle Park, North Carolina, USA.
McEnaney, S. (1993) Technical study to evaluate the chromosome
damaging potential of carbaryl by its effects on the bone marrow cells
of treated rats. Unpublished report No. 198/64, dated September 1993
from Hazleton Microtest/Hazleton UK. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Pastides H. (1993) Standardized mortality ratio analysis of employees
exposed to carbaryl at the Rhône Poulenc Institute, West Virginia
Plant. Report dated January 1993. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Sagelsdorff, P. (1994) Investigation of the potential of protein- and
DNA-binding of carbaryl. Unpublished report No. CB93/52, dated April
1994 from Ciba-Geigy Ltd. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Strubble, C.B. (1994) Metabolism of 14C-carbaryl in rats (preliminary
and definitive phases). Unpublished report No: HWI 6224-184, dated
August 1994, from Hazleton Wisconsin, Inc., USA. Supplied to WHO by
Rhône Poulenc, Research Triangle Park, North Carolina, USA.
Thomas, H. (1994) Liver cytochrome P-450 inducer phenotyping in the
male CD-1 mouse. Unpublished report No. CB94/23, dated October 1994,
from Ciba-Geigy Ltd. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Weil C.S. & Carpenter, C.P. (1965) Results of a three generation
reproduction study on rats fed Sevin in their diet. Unpublished report
No. 28-53, dated April 1965, from Mellon Institute, USA. Supplied to
WHO by Rhône Poulenc, Research Triangle Park, North Carolina, USA.
Weil C.S. & Carpenter, C.P. (1966) Evaluation of the teratogenic
potential of insecticide Sevin in rats. Unpublished report No. 29-49,
dated June 1966, from Mellon Institute, USA. Supplied to WHO by Rhône
Poulenc, Research Triangle Park, North Carolina, USA.
WHO (1994) Carbaryl (Environmental Health Criteria 153), International
Programme on Chemical Safety, Geneva.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
(c) Enzyme induction and effects on the liver
Six male CD1 mice were fed a diet designed to provide carbaryl
(purity, 99.6%) at a dose of 8000 ppm, equal to 1154 mg/kg bw per day,
for 14 days. Five animals received control diet. Food consumption was
markedly reduced during the first three days of treatment, accompanied
by body weight loss. At the end of treatment, body weights were
decreased to 85%, and the relative liver weight had increased to 135%
relative to controls. Protein fractions were determined in the
microsomal and cytosolic fractions: a 1.3-fold increase (per gram of
liver) in microsomal protein was found, with a similar increase in
microsomal cytochrome P450 content. The ethoxylation of ethoxy-
resorufin and the depentylation of pentoxyresorufin were increased by
1.9- and 3.1-fold, respectively. Total testosterone hydroxylation was
increased by 1.5-fold relative to controls; while some forms of
testosterone hydroxylation were minimally altered, the 6 alpha, 11
alpha, 11ß, and 16ß forms were increased by three- to fourfold.
Hepatic glutathione levels were only slightly increased. The author of
the report concluded that carbaryl is a low-potency barbiturate-type
inducer (Thomas, 1994); however, in view of the magnitude of the
effects found in relation to the dose, there is no clear evidence that
the compound should be considered an inducer.
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that disturbances in carbohydrate metabolism, protein
synthesis, and detoxification have been reported in the livers of
mammals treated with carbaryl. It concluded that carbaryl is a weak
inducer of hepatic microsomal drug metabolizing activity, hepatic
levels of cytochrome P450 and b5 are increased, and phenobarbital
sleeping time is shortened. The changes in hepatic metabolism may
account in part for the three-fold increase in the LD50 for
carbaryl-pretreated rats.
2. Toxicological studies
(a) Acute toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that the compound is
moderately toxic after acute oral administration. The LD50 for rats
was 225-721 mg/kg bw. Interspecies difference were found, cats being
the most sensitive and guinea-pigs, rats, mice, and rabbits showing
more resistance, in that order. The LD50 was increased threefold
by pretreating animals with small doses of carbaryl. The compound
is slightly toxic after acute dermal administration, the LD50
being > 2000 mg/kg bw. No LC50 for acute exposure by inhalation was
available, but the effects observed in dogs, cats, and rats exposed to
carbaryl dust or to formulations of carbaryl were typical of those of
cholinesterase inhibition. In cats, exposure for 6 h to a dust
concentration of only 20 mg/m3 inhibited serum and erythrocyte
cholinesterase activity. Many of the known metabolites of carbaryl
are much less toxic than the parent compound. None of the metabolites
with a methylcarbamate moiety was appreciably more active as a
cholinesterase inhibitor than carbaryl itself.
(b) Short-term toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that in a study of
cats the NOAEC was 16 mg/m3 after exposure for 120 days, on the basis
of cholinergic reactions at 30 mg/m3 after exposure for 30 days. In a
study in rats, no effects were observed after 90 days' exposure to
10 mg/m3.
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female CD1 mice were given diets
designed to provide carbaryl (purity, 99.3%) at concentrations of 0,
100, 1000, or 8000 ppm, equal to 0, 15, 150, and 1200 mg/kg bw per day
for males and 0, 18, 180, and 1400 mg/kg bw per day for females, for
two years. Haematological parameters and plasma, erythrocyte, and
brain cholinesterase activities were determined in 10 mice of each sex
per group at week 53 and at termination.
There were no treatment-related effects on mortality. A thin
appearance and hunched posture were noted in many females and some
males at the high dose during the first three to six weeks and the
last six months of the study. Some animals at this dose also had a
languid appearance, urine stains, rough coats, and opaque eyes, the
latter in females during the last three months. Body-weight gain was
impaired during the first two weeks of the study in mice at the high
dose, and the weights remained significantly lower than that of
controls throughout the study. At the end of the study, the body
weights of males and females at the high dose were 88 and 87% of those
of the respective controls, but the weight gain over the whole period
was only 62 and 68% of that of controls. Food consumption was markedly
depressed in animals of each sex at the high dose during the first
months of the study. It remained low in females throughout the study
and was again lower in males from week 78 of the study. Erythrocyte
counts, haemoglobin, and packed cell volume were decreased in females
at the high dose at week 53 and in males at the end of the study.
The platelet count was increased in females at the high dose.
Cholinesterase activity was inhibited in animals at the middle and
high doses. Significant decreases in erythrocyte acetylcholinesterase
were seen only at week 53 in males at the middle and high doses (78
and 70% of the control value, respectively). Significant, dose-related
decreases in brain acetylcholinesterase activity were seen in animals
of each sex at the middle and high doses at interim and terminal
sacrifice. At the end of the study, brain acetylcholinesterase
activity was only 60 and 66% of the control levels in males and
females at the high dose, respectively.
Decreases in the absolute and relative weights of lungs and ovary
(at interim sacrifice only) were seen in females at the high dose. The
absolute and relative weights of the livers (with gall-bladder) and
the relative weight of the kidneys were increased in animals of each
sex at the high dose. On histopathological examination, a dose-related
increase in the incidence and severity of intracytoplasmic protein-
like droplets was found in the urinary bladder of animals of each sex
at the middle and high doses. An increased incidence of uni- and/or
bilateral cataracts was found in high-dose males and females, and a
slight increase in the severity of extramedullary haematopoiesis
and the presence of pigment in the spleen were found at terminal
sacrifice. The incidence of renal tubular-cell neoplasia was increased
in high-dose males, that of hepatocellular neoplasia in high-dose
females, and that of vascular tissue neoplasia in all treated males
and in high-dose females (Table 2). Carbaryl was thus found to be
carcinogenic in mice. The NOAEL for non-neoplastic findings was
100 ppm, equal to 15 mg/kg bw per day, on the basis of inhibition
of erythrocyte and brain acetylcholinesterase activity and
histopathological changes in the urinary bladder (Hamada, 1993a).
A re-examination of the histological slides of the liver and
kidneys from mice in the control and high-dose groups at interim
sacrifice, performed by two pathologists from the Rhône Poulenc
Company (Debruyne & Irisarri, 1996), showed no microscopic changes in
the liver or kidney. In a position paper, Klonne (1995) compared the
incidence of vascular tumours (mostly in the liver and spleen) in the
male mice in this study with those in historical controls in the
laboratory that performed the experiment and in several other
laboratories. The tumour incidences were clearly increased over
the mean values for historical controls; but, in the performing
laboratory, data were available only from 18-month studies. When the
incidences were compared with the ranges in historical controls in
studies of up to two years' duration, the incidences of vascular
tumours in the livers of animals at the low and middle doses were just
below the upper limit for the controls, and the incidences of vascular
tumours in the spleens of animals at the middle dose were outside the
range. The incidences among animals at the high dose were outside the
range for vascular tumours in the liver and within the range for
tumours in the spleen.
Table 2. Incidences of tumours in groups of 80 mice fed diets containing carbaryl
Tumour Males Females
0 ppm 100 ppm 1000 ppm 8000 ppm 0 ppm 100 ppm 1000 ppm 8000 ppm
Renal tubular-cell adenoma 0 0 0 3 0 0 0 1
Renal tubular-cell carcinoma 0 0 0 3 0 0 0 0
Hepatocellular adenoma 12 7 13 8 0 0 1 7
Hepatocellular carcinoma 6 7 3 8 1 1 1 3
Hepatoblastoma 0 0 0 0 0 0 0 1
Haemangioma 0 1 1 3 1 0 1 0
Haemangiosarcoma 2 5 9 7 2 3 3 9
No. of vascular 2 6 10 10 3 3 4 9
tumour-bearing animals
All vascular tumours 2 9 13 18 5 6 5 10
Rats
Groups of 80 male and 80 female Sprague-Dawley rats were given
diets designed to provide concentrations of carbaryl (purity, 99%) of
0, 250, 1500, or 7500 ppm, equal to 0, 10, 60, and 350 mg/kg bw per
day for males and 0, 13, 79, and 480 mg/kg bw per day for females, for
two years. Haematology, clinical chemistry, cholinesterase activity in
plasma and erythrocytes, and urinary parameters were studied in 10
rats of each sex at weeks 26, 52, 78, and 104. Brain acetylcholin-
esterase activity was determined at interim sacrifice and at the end
of study in 10 rats of each sex. Two additional groups of 10 rats of
each sex were treated with 0 or 7500 ppm for 52 weeks and were then
kept for a recovery period of four weeks, when haematology, clinical
chemistry, and cholinesterase activity were studied. There were no
treatment-related effects on mortality, and survival was increased in
females at the high dose; however, an increased incidence of alopecia
on the limbs was found in these animals, and an increased prevalence
of urine stains was seen in animals of each sex. The mean body weights
were significantly depressed in animals at the high dose at all
intervals analysed and in those at the middle dose at many intervals.
At the end of study, the body weights of males and females at the high
dose were only 5 and 55% of those of the respective controls, and
those of animals at the middle dose were 94% and 88% of the control
values, respectively. Food consumption was markedly lower in the
high-dose group. The numbers of animals with cataracts were increased
in the high-dose group, with incidences of 4, 6, 7, and 12 males and
3, 2, 4, and 10 females at the control, low, middle, and high doses,
respectively.
There were no treatment-related findings in haematological
parameters. Increased serum cholesterol and urea nitrogen were found
in high-dose females. Plasma cholinesterase activity was depressed in
animals of each sex at the high dose, to 58-73% of the control value
in males and 43-54% in females. Erythrocyte acetylcholinesterase
activity was also significantly decreased, to 63-81% of the control
value in males at the high dose and 62-75% in females; in females at
the middle dose, the activity was 74-81% of the control value, whereas
in males at this dose the depression was found only at weeks 52
(81%) and 78 (77%). Significant, dose-related decreases in brain
acetylcholinesterase activity were found at both 52 and 104 weeks in
high-dose males and middle- and high-dose females. Males at the middle
dose showed a significant depression only at the 52-week interim
sacrifice. Changes seen on urinalysis in animals at the high dose
included an increased incidence of dark urine, decreased urine volume
in females (at week 52), and erythrocytes in urine and occult blood in
males (at weeks 78 and 104). The relative weights of lungs, brain,
kidney, and liver were significantly increased in animals at the high
dose at the interim and terminal sacrifices; testicular weight was
also increased in those at the high dose. The increase in relative
liver weight was dose-related, but a significant increase at the
middle dose was found only in the females at interim sacrifice.
Gross pathological examination of animals at the high dose
revealed increased incidences of masses in the urinary bladder, pale
areas in the lungs, and dark areas in the glandular stomach (only in
males). A decreased incidence of mammary masses was noted in females
at this dose, with 65% in controls, 57% at the low dose, 64% at the
middle dose, and 41% at the high dose. Histopathological evaluation
of tissues taken from rats at interim sacrifice revealed hyaline
inclusions in the livers of males at the high dose. At the end of the
study, the following non-neoplastic findings were observed in animals
at the high dose: an increased incidence of thyroid hypertrophy (8/70
in males and 33/70 in females, in comparison with 1/70 and 4/70 in
controls, respectively); eosinophilic hepatocellular alterations and
increased pigment in females, hepatocyte hypertrophy in animals of
each sex, and intracytoplasmic hyaline inclusions in males; an
increased incidence of transitional epithelial hyperplasia in the
kidneys of males, and squamous metaplasia, a high mitotic index, and
cytological atypia in the urinary bladders of animals of each sex, all
associated with the carcinomas found; an increased incidence of
alveolar foamy macrophages and focal pneumonitis; and an increased
incidence of degeneration of the sciatic nerve and adjacent skeletal
muscles. The neoplastic findings in thyroid, liver, and urinary
bladder are summarized in Table 3. Nearly all of the animals with
transitional-cell neoplasms in the bladder also had extensive
hyperplasia, indicating the preneoplastic nature of this change. Males
at the high dose also had an increased incidence of transitional-cell
hyperplasia in the kidney; a single carcinoma was found. The incidence
of benign interstitial-cell tumours in the testis was increased in
animals at the high dose (5/70; 2/70 in controls).
After one year of exposure and the recovery period of four weeks,
the food consumption of animals at the high dose improved and the
animals gained more weight. The effects on cholinesterase activity
were reversible, as were the histological changes in the liver. The
relative weights of the lungs, brain, kidney, and liver, however,
remained increased. Carbaryl thus induced tumours at 7500 ppm, a
dose that exceeds the maximum tolerated dose (MTD). The NOAEL for
non-neoplastic findings in this study was 250 ppm, equal to 10 mg/kg
bw for males per day, on the basis of inhibition of erythrocyte and
brain acetylcholinesterase and the decrease in mean body weight
(Hamada, 1993b).
A re-examination of the histological slides of the liver, kidney,
urinary bladder, and thyroid of rats of all doses at interim sacrifice
and of the controls and those at the high dose after recovery,
performed by two pathologists from Rhône Poulenc Company (Debruyne &
Irisarri, 1996), revealed the presence of further microscopic changes
in animals at the high dose in the bladder (irreversible epithelial
hyperplasia in males and females), kidney (reversible pelvic
urothelial hyperplasia in males), thyroid (reversible follicular
hypertrophy in males), and liver (reversible hepatocellular
hypertrophy in males and females).
Table 3. Incidences of tumours in groups of 70 rats fed diets containing carbaryl
Tumour Males Females
0 ppm 250 ppm 1500 ppm 7500 ppm 0 ppm 250 ppm 1500 ppm 7500 ppm
Thyroid follicular-cell adenoma 0 2 0 8 0 0 0 1
Thyroid follicular-cell carcinoma 0 0 0 1 1 0 0 0
Hepatocellular adenoma 1 1 2 1 1 0 3 7
Hepatocellular carcinoma 0 2 2 1 0 0 0 0
Transitional-cell hyperplasia 9 8 10 54 6 6 6 56
Transitional-cell papilloma 0 à 0 13a 1 0 0 7
Transitional-cell carcinoma 0 0 0 11 0 0 0 6
a Includes one squamous-cell papilloma
In the Environmental Health Criteria monograph on carbaryl (WHO,
1994), the results of several long-term studies in mice were
summarized, one of which was a report of the data obtained at interim
sacrifice in the study of Hamada (1993a). In another study, no effects
were observed at the highest level tested (400 ppm). Seven further
studies of carcinogenicity in mice involving different strains, routes
of administration, doses, dose schedules, and lengths of exposure and
observation were considered unsuitable for evaluation of carcinogenic
potential; only one showed a marginal response.
Several short- and long-term studies in rats were available. The
most recent report presented the interim results of the long-term
study of Hamada (1993b). In two older dietary studies, lasting 96 days
and two years, the most obvious effects were on the kidney. The NOAEL
in these studies was 200 ppm, equal to 7.9 mg/kg bw per day, with
effects on the kidney at 400 ppm. In two one-year studies of gavage
(for which only summaries were available), effects were seen on the
thyroid and on male and female reproductive organs and/or function at
doses > 5 mg/kg bw per day. The NOAEL was 2 mg/kg bw per day. Two
studies of carcinogenicity in rats were available. In a study in which
carbaryl was administered orally or by a single subcutaneous
implantation, fibrosarcomas of the skin were observed; the other
(dietary) study gave negative results. Neither was considered suitable
for evaluation of carcinogenic potential.
Three one-year studies in dogs were available. In a study in
which carbaryl was given in capsules, effects on the kidney were found
at 7.2 mg/kg bw per day, but no effects were observed at 1.8 mg/kg bw
per day (approximately 100 ppm in the diet). In the two dietary
studies, the NOAEL was 125 ppm, equivalent to 3.1 mg/kg bw per day, on
the basis of effects on liver weight and inhibition of erythrocyte and
brain acetylcholinesterase activity at 400 ppm.
(d) Reproductive toxicity
No new information had become available. The summaries in the
Environmental Health Criteria monograph on carbaryl (WHO, 1994),
although extensive, could not always be used to derive NOAELs for
various aspects of the reproductive toxicity of carbaryl. Additional
information was therefore obtained for some studies, from either the
original papers or reviews.
In two five-day studies of male mice exposed orally, no effects
were found on the testis or accessory glands at a dose of 34 mg/kg bw
per day; at 68 mg/kg bw per day, androstenedione synthesis was
decreased.
Three three-generation studies of reproductive toxicity in rats
were summarized. In the first, in which 0, 2000, 5000, or 10 000 ppm
carbaryl were administered in the diet, impaired fertility, reduced
postnatal survival, and reduced postnatal growth were observed at
5000 ppm; no effects were seen at 2000 ppm, equivalent to 125 mg/kg bw
per day. There was no clear NOAEL in this study, since the parameters
were wrongly defined. In a second dietary study, at doses of 0, 7, 25,
100, and 200 mg/kg bw per day, the NOAEL was 100 mg/kg bw per day, on
the basis of decreased maternal weight at 200 mg/kg bw per day. There
were no reproductive effects. In a study in which rats were treated by
gavage at doses of 0, 3, 7, 25, or 100 mg/kg bw per day, the NOAEL was
25 mg/kg bw per day, on the basis of decreased maternal weight and
mortality and effects on litter size and viability at the high dose.
In a study in which special attention was paid to effects on
reproductive parameters, male and female rats were exposed by gavage
for one month to doses of 1-50 mg/kg bw per day. Adverse effects were
observed on male reproductive cells; furthermore, increased embryonic
and fetal deaths, decreased numbers of implantations, and a prolonged
oestrus cycle were observed. This study was also described by Cranmer
(1986), who reported that the effects on reproductive parameters were
seen only at 1, 5, 10, and 20 mg/kg bw per day. He also noted that
only the change in the number of tubules containing spermatogonia
was seen at all doses, and most of the other treatment-related
histological and functional changes in the testis occurred at doses
> 5 mg/kg bw per day. Dose-response relationships were not seen for
the other effects on litter parameters or for the prolonged oestrus
cycle, limiting interpretation of the study. This and other studies of
reproductive toxicity were considered of dubious value for risk
assessment as they suffered from various shortcomings in study design.
A three-generation study of reproductive toxicity in which
gerbils were exposed daily via the diet to concentrations of
2000-10 000 ppm was not considered suitable for establishing a NOAEL;
however, adverse effects on various reproductive parameters were seen.
(e) Developmental toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) was supplemented by
additional information from the original papers and reviews. All of
the studies suffered from small group sizes and some deficiencies in
comparison with currently acceptable scientific standards.
Two of five studies in mice were used for evaluation, in which
animals were exposed by gavage to carbaryl on days 6-15 of gestation;
in the second study, groups were also exposed only on day 8 or 12. In
the first study, with doses of 0, 100, and 150 mg/kg bw per day, the
NOAEL was 100 mg/kg bw per day on the basis of maternal toxicity
(ataxia, lethality) and litter resorption at 150 mg/kg bw. In one
group exposed via the diet to 5560 ppm, a decrease in fetal weight was
observed. In the second study, with doses of 0, 100, 150, and
200 mg/kg bw per day, the NOAEL was 150 mg/kg bw per day, on the
basis of maternal death and fetal toxicity (weight reductions and
indications of growth retardation) at 200 mg/kg bw per day.
Three of six studies in rats were used for evaluation. In the
first study, groups of six rats were fed diets containing carbaryl on
days 1-5, 5-15, or 1-21 of gestation. The NOAEL for maternal toxicity
was 100 mg/kg bw per day, on the basis of reduced weight gain in dams
at 500 mg/kg bw per day when exposed on gestation days 5-15 or 1-21.
No adverse fetal effects were seen. The original papers (Weil &
Carpenter 1965, 1966) reported that the doses used were 0, 2.5, 10,
20, 100, and 500 mg/kg bw per day and that fetuses underwent only
skeletal examination. In addition to the effects summarized above, the
authors reported reduced postnatal survival in the pups of dams
exposed to 500 mg/kg bw per day on days 1-21. In the second study,
groups of 10 rats received oral doses of 200 or 300 mg/kg bw or an
intraperitoneal dose of 40 mg/kg bw on single or multiple days of
gestation. The only effect seen was a reduction in fetal weight in
some groups. In the third study, groups of six or seven rats received
diets containing carbaryl at doses of 0, 1, 10, or 100 mg/kg bw per
day three months before and during gestation. The NOAEL for maternal
toxicity was 10 mg/kg bw per day, on the basis of reduced weight gain
at 100 mg/kg bw per day. No adverse fetal effects were seen. This
study was also described by Cranmer (1986), who reported a slight
reduction in the numbers of implantations and live fetuses at 100
mg/kg bw per day.
One of two studies in guinea-pigs was used for evaluation. Groups
of four to nine animals were exposed to 0, 100, 200, or 300 mg/kg bw
per day in the diet or 0, 50, 100, or 200 mg/kg bw per day orally.
Reduced maternal weight gain was observed at 200 mg/kg bw per day, but
there were no effects on embryos or fetuses.
One of three studies in rabbits was used for evaluation. Groups
of 15-20 animals were given oral doses of 0, 150, or 200 mg/kg bw per
day on days 6-18 of gestation. There was no NOAEL for maternal
toxicity since a reduction in weight gain was observed in both treated
groups. A significant increase in umbilical hernia was observed in
fetuses at 200 mg/kg bw per day.
In two studies in dogs, animals were fed diets containing
carbaryl at doses of 3.1-50 mg/kg bw per day or 2-12.5 mg/kg bw per
day during gestation. Various birth defects were observed in the pups
at doses > 5 mg/kg bw per day. Maternal toxicity was observed at
all doses.
In pigs fed doses of 4-32 mg/kg bw per day in the diet, the
effects noted (prenatal lethality and malformations) were not
consistent across the studies. Another study in pigs was considered
unsuitable for evaluation.
One of two studies in monkeys showed an increased rate of
abortions after oral treatment with 2 or 20 mg/kg bw per day
throughout gestation. No adverse effects were noted in the second
study in which the doses were 0.2-32 mg.kg bw per day orally on days
20-38 of gestation.
(f) Genotoxicity
The results of studies of chromosomal aberrations in rats and
micronucleus formation in mice treated with carbaryl in vivo are
summarized in Table 4. Negative results were obtained in both studies
at doses at which limited signs of toxicity were seen; however, there
was no evidence that the test compound had reached the target organ,
as no cytotoxicity was found.
In a study of DNA binding, two groups of four male CD mice were
treated by oral intubation with 14C-carbaryl (labelled in the
naphthyl group) in 0.5% aqueous carboxymethylcellulose at a dose of
75 mg/kg bw (8 mCi/kg bw). One group was pretreated with carbaryl
(purity, 99.6%) at 8000 ppm (equal to 1900 mg/kg bw per day) in the
diet for 14 days. Four mice served as controls. Urinary excretion and
exhalation of carbon dioxide were measured over 24 h in one pretreated,
one non-pretreated, and one control mouse. Animals were sacrificed 24 h
after administration of radiolabelled material, and the liver, kidneys,
and urinary bladder were removed; however, only the liver was used
for determining DNA and protein binding. There was no significant
exhalation of 14C-carbon dioxide, and about 30% of the administered
dose was excreted in the urine of both treated groups. The pretreated
group had lower body weights and increased relative liver weights at
the end of treatment. Binding of 14C-carbaryl to chromatin protein
was found in both treated groups, resulting in specific radioactivities
of 340-537 dpm/mg protein (7-11 pmol/mg protein). As there was no
significant radioactivity in purified DNA, in the presence or absence
of pretreatment, covalent binding of the 14C-naphthyl label does not
seem to have occurred (Sagelsdorff, 1994).
Table 4. Results of tests for the genotoxicity of carbaryl in vivo
End-point Test system Concentration Purity Results Reference
(mg/kg bw) (%)
Micronucleus formation Male and female 50, 100, 200 orally for 99.9 Negativea Marshall (1996)
CD-1 mice (5 per 2 daysb; killed at 24 or
dose), bone-marrow 48 h; vehicle, 0.5%
cells carboxymethylcellulose
Chromosomal aberration Male and female Single dose of 30, 60, 99.7 Negativea McEnaney (1993)
Sprague-Dawley 120c; killed at 6, 24,
rats (5 per dose), 48 h; vehicle, 0.25%
bone-marrow cells carboxymethylcellulose
a Positive controls yielded positive results
b Clinical signs of toxicity (lethargy, slight weight loss) at highest dose; no change in polychromatic:normochromatic
erythrocyte ratio. Doses based on the results of a preliminary test in which one of six animals at 300 mg/kg bw died.
c Clinical signs of toxicity (lethargy, tremors) observed at the highest dose; no depression of mitotic index. Doses based on
the results of a preliminary test in which the LD50 was 231 mg/kg bw
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that carbaryl has been evaluated for mutagenicity in a
number of tests in vitro and in vivo, in bacterial, yeast, plant,
insect, and mammalian systems, with a variety of end-points. It
concluded that carbaryl does not damage DNA. Reports of induction of
mitotic recombination, gene conversion, and unscheduled DNA synthesis
in prokaryotes (Haemophilus influenzae, Bacillus subtilis) and
eukaryotes ( Saccharomyces cerevisiae, Aspergillus nidulans, cultured
human lymphocytes, and rat hepatocytes) in vitro have not been
confirmed. Negative results were obtained in tests for gene mutations
in all but two bacterial assays. Although several studies of gene
mutation were conducted in mammalian cells in vitro, only one
equivocally positive result was obtained, in a study that had several
shortcomings and which has not been confirmed. Chromosomal damage has
been reported in human, rat, and hamster cells and in plants treated
in vitro with high doses of carbaryl. No such effects have been
observed in mammalian tests in vivo, even at doses as high as
1000 mg/kg bw. Carbaryl induces disturbances in the spindle fibre
mechanism in plant and mammalian cells in vitro, but the relevance
of the assays in plants for humans is unclear. The Environmental
Health Criteria monograph concluded that the available database did
not indicate that carbaryl induces genetic changes in somatic or
germinal tissues of humans.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported that carbaryl is
not or only weakly irritating to the skin and is weakly irritating to
the eye. The available studies indicated that carbaryl has little or
no sensitizing potential.
(ii) Neurotoxicity
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported that the effects
of carbaryl on the central nervous system had been studied in rats
and monkeys treated by intraperitoneal, intramuscular, or oral
administration or by inhalation. Changes in motor activity, working
memory, and behaviour were seen. Dietary exposure to doses of
10-20 mg/kg bw per day for 50 days was reported to disrupt learning
and performance in rats. In a small study on pigs, administration of
carbaryl in the diet at 150 mg/kg bw per day for 72-82 days was
reported to produce a number of neuromuscular effects. Reversible leg
weakness was noted in chickens given high doses of carbaryl, but no
evidence of demyelination was observed in the brain, sciatic nerve, or
spinal cord sections examined microscopically. Similar effects were
not observed in long-term studies in rodents. The effects of carbaryl
on the nervous system are primarily related to inhibition of
cholinesterase activity and are usually transitory.
(iii) Immunotoxicity
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that several studies
in mice, rats, rabbits, and guinea-pigs, mostly treated orally, showed
that carbaryl administered at doses that do not cause overt clinical
signs has a variety of non-life-threatening effects on both cellular
and humoral immunity. Many of the effects were detected at doses close
to the LD50. A lack of consistency and sometimes overt contradiction
between the results of several of these studies precludes definition
of the immunotoxic mechanism. Life-time exposure to carbaryl did not
increase the occurrence of disease in rats or mice, and no enhancement
of viral infections was found, even at doses close to the LD50. Most
of the studies on mice and rabbits at doses that permitted survival
did not show significant effects on the immune system. Vital
enhancement has been demonstrated in vitro in a number of studies
including prior incubation with carbaryl. Inhibition of human serum
complement activity and interleukin-2-driven proliferation of large
granular lymphocytes have also been found in vitro.
(iv) Haematological effects
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported effects on
coagulation in rats, rabbits, and dogs in vivo and in studies in
vitro, but the direction of the effect is unclear. In glucose-
6-phosphate dehydrogenase-deficient sheep erythrocytes, carbaryl
produced a dose-dependent increase in methaemoglobin formation. Human
serum albumin reacted in vitro with the ester group of carbaryl.
Carbaryl binds free blood amino acids.
(v) Effects on the endocrine system
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) concluded from several
experiments in vivo and in vitro that carbaryl increases the
gonadotropic function of the hypophysis of rats.
(vi) Studies with N-nitrosocarbaryl
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that carbaryl is a secondary amine and is therefore
capable of nitrosation in the presence of nitro donor groups, such as
sodium nitrate, to give a nitrosamide. A condition of such nitrosation
is an acidic pH (< 2), such as that found in the human stomach;
however, N-nitrosocarbaryl is not stable at this pH; its maximal
stability is at pH 3-5, at which no significant amount of carbaryl can
be nitrosated. Carbaryl was nitrosated in several studies, in vitro
as well as in vivo, in guinea-pigs, in which the gastric acidity is
similar to that of humans.
N-Nitrosocarbaryl induced local tumours in rats, consisting of
sarcomas at the site of injection and forestomach squamous-cell
carcinomas, after oral administration. This local carcinogenic effect
and the lack of systemic carcinogenicity characterize the compound as
a directly acting alkylating agent. Given the human chemistry of
carbaryl, the risk of carcinogenic effects of N-nitrosocarbaryl for
humans after exposure to carbaryl can be considered negligible.
N-Nitrosocarbaryl can induce mitotic recombination and gene
conversion in prokaryotes (H. influenzae and B. subtilis) and
eukaryotes (S. cerevisiae) in vitro and gives positive results in
Escherichia coli spot tests. It also binds to DNA, causing
alkali-sensitive bonds and single-strand breakage. It is not
clastogenic in vivo in bone marrow and germ cells, even at highly
toxic doses.
3. Observations in humans
An epidemiological study of total and cause-specific mortality
among employees exposed to carbaryl at a production plant was based on
information obtained in 1988 on the vital status and cause of death of
all individuals who were first hired between the start-up of the
carbaryl unit in 1960 through 1978. Employees hired after 1978 were
not included in the study. Three categories of workers exposed to
carbaryl were defined: those involved in production, maintenance
employees, and those working in packaging and distribution. A total of
448 employees contributing 7532 person-years to the analysis were
available, representing the combined number of years in which these
employees were followed through 1988. Mortality was measured in terms
of standardized mortality ratios (SMRs), which are the ratios of the
observed numbers of death among members of a cohort to the number
expected, on a year-specific and age-adjusted basis. The 25 deaths
identified in this cohort resulted in elevated SMRs for cancer of the
pancreas, unspecified cancer, and cancers of the brain and other parts
of the nervous system. In the first two categories, the excess was
slight and based on only one death. In the last category, the SMR was
based on two deaths due to tumours of different histological origins,
reducing the possibility that the two malignancies were caused by the
same exposure. Furthermore, in all three categories the confidence
intervals were wide, indicating a relatively imprecise SMR estimate
and reflecting the small sample size on which it is based (Pastides,
1993).
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) summarized several cases of poisoning. The clinical picture is
dominated by symptoms of inhibition of cholinesterase activity. Signs
of poisoning develop quickly after absorption and disappear rapidly
after exposure ends.
In controlled studies of human volunteers, single doses of
< 2 mg/kg bw were well tolerated. A single dose of 250 mg (about
2.8 mg/kg bw) produced moderate symptoms of cholinesterase inhibition
(epigastric pains and sweating) within 20 min. Complete recovery was
seen within 2 h of treatment with atropine sulfate. Two groups of
human volunteers given carbaryl at doses of 0.06 or 0.13 mg/kg bw for
six weeks underwent physical examinations, removal of bromosulphthalein
from blood, electroencephalography, routine blood and urinalysis, and
measurement of cholinesterase in plasma and erythrocytes. No inhibition
of cholinesterase activity was observed, and no changes were seen in
the group receiving the low dose. The only finding at the high dose was
an increase in the ratio of amino acid nitrogen to creatinine in the
urine, which may represent a decrease in the ability of the proximal
convoluted tubule to reabsorb amino acids; this change was reversible.
The NOAEL was 0.06 mg/kg bw.
In cases of occupational overexposure to carbaryl, mild symptoms
are observed long before a dangerous dose is absorbed. No local
irritating effect is usually seen, although skin rash after accidental
splashing with carbaryl formulations has been described.
Reports of the effects of carbaryl on sperm count and changes in
sperm morphology in plant workers are conflicting. No adverse effects
on reproduction have been described.
Comments
Carbaryl is rapidly and almost completely absorbed after oral
administration. Excretion is rapid and occurs predominantly via the
urine; enterohepatic cycling of carbaryl metabolites is also
considerable. There were no significant dose-related or sex-specific
differences in elimination patterns, and there was no evidence of
bioaccumulation. Dermal absorption in rats was slow; after 24 h,
16-34% of the administered radiolabel had been absorbed. Higher doses
were less readily absorbed. In volunteers, 45% of a dose applied to
the skin in acetone was absorbed within 8 h. Carbaryl was rapidly
absorbed in the lungs.
The metabolism of carbaryl has been studied in various mammals,
including humans. The principal metabolic pathways are ring
hydroxylation, hydrolysis, and conjugation. There were no species
differences. The main metabolite in humans is 1-naphthol. The
hydrolysis product, N-methyl carbamic acid, spontaneously decomposes
to methylamine and carbon dioxide. The methylamine is later converted
to carbon dioxide and formate, the latter being excreted mainly in the
urine. Carbaryl metabolites are also found at small percentages of the
absorbed doses in saliva and milk.
Carbaryl is moderately toxic after acute oral administration, the
LD50 in rats being 225-721 mg/kg bw. Interspecies differences in
toxicity were found, cats (LD50, 150 mg/kg bw) being the most
sensitive species. The LD50 was increased threefold when animals were
pretreated with small doses of carbaryl. The compound is slightly
toxic after acute dermal administration, with an LD50 > 2000 mg/kg
bw. No LC50 for acute exposure by inhalation was available, but the
effects observed in dogs, cats, and rats exposed to dusts or
formulations of carbaryl were typical of those resulting from
inhibition of cholinesterase activity. In cats exposed to carbaryl
dust for 6 h, a concentration of 20 mg/m3 inhibited cholinesterase
activity in plasma and erythrocytes. Carbaryl was weakly irritating to
the eye but not the skin and was not considered to be a sensitizer.
WHO has classified carbaryl as 'moderately toxic' (WHO, 1996).
After oral administration of carbaryl in capsules to dogs at a
dose of 0.45, 1.8, or 7.2 mg/kg bw per day for one year, slight
effects were observed on the kidney at 7.2 mg/kg bw per day; the NOAEL
was 1.8 mg/kg bw per day. In two studies in which dogs were fed diets
containing carbaryl at 20-125 ppm for five weeks and 125-1250 ppm for
one year, the NOAEL was 125 ppm, equivalent to 3.1 mg/kg bw per
day, on the basis of effects on liver weight and inhibition of
acetylcholinesterase activity in erythrocytes and brain at 400 ppm.
In cats exposed to carbaryl by inhalation, cholinergic signs were
observed at 30 mg/m3 after exposure for 30 days. The NOAEL was
16 mg/m3 for 120 days. In a study in rats, no effects were observed
after exposure to 10 mg/m3 for 90 days.
Several studies of long-term toxicity or carcinogenicity in mice
cited in EHC 153 were considered to be unsuitable for evaluation of
carcinogenicity by either the Environmental Health Criteria Task Force
or the present Meeting, although they were suitable for assessing
long-term toxicity. In a recent study of carcinogenicity, mice were
given diets providing 0, 100, 1000, or 8000 ppm carbaryl for
104 weeks. Tumours were observed in the liver in females and the
kidney in males, and vascular tumours were found in animals of each
sex at the highest dose, which exceeded the maximum tolerated dose
(MTD). In male mice, increases in the incidences of vascular tumours
were also seen at the two lower doses; after considering all of the
available data, the Meeting could not identify an NOAEL for this
neoplastic lesion. The NOAEL for non-neoplastic lesions was 100 ppm
(equal to 14.7 mg/kg bw per day), on the basis of inhibition
of erythrocyte and brain acetylcholinesterase activity and
histopathological changes in the urinary bladder at 1000 ppm. This
NOAEL is consistent with the results of the earlier studies. The
Meeting concluded that the compound is carcinogenic in mice.
In several studies cited in EHC 153, carbaryl was administered in
the diet of rats for 96 days to two years. The most obvious effects
were in the kidney at doses > 400 ppm. In two one-year studies in
rats treated by gavage, effects on the thyroid and on male and
female reproductive organs and/or function were observed at doses
> 5 mg/kg bw per day; the NOAEL was 2 mg/kg bw per day. None of
these studies was considered suitable for evaluating carcinogenicity.
In a recent study of long-term toxicity and carcinogenicity, rats
were fed diets containing 0, 250, 1500, or 7500 ppm carbaryl for 104
weeks. In animals at the highest dose, which exceeded the MTD, tumours
were found in the thyroid in males, in the liver in females, and in
the urinary bladder in animals of each sex. The NOAEL for non-
neoplastic findings in this study was 250 ppm, equal to 10 mg/kg bw
per day, on the basis of inhibition of erythrocyte and brain
acetylcholinesterase activity and a decrease in mean body weight at
1500 ppm. This NOAEL is consistent with the results of earlier dietary
studies. The Committee concluded that carbaryl is carcinogenic in rats
only at levels that exceed the MTD.
The available studies on reproductive toxicity were conducted
some time ago and had some deficiencies in relation to currently
acceptable scientific standards. In three-generation studies, dietary
administration of carbaryl to rats induced reproductive effects
(impaired fertility and reduced postnatal survival and growth) at
doses > 2000 ppm (equal to 125 mg/kg bw per day); a dose of 100 mg/kg
bw per day did not induce maternal toxicity. When carbaryl was
administered by gavage, maternal toxicity was not observed at 25 mg/kg
bw per day, but both maternal and reproductive toxicity (reduced
litter size and viability) were found at 100 mg/kg bw per day. The
Meeting recommended that a new two-generation study of reproductive
toxicity be carried out in rats, with special attention to the male
reproductive system since effects on this system were observed in some
long-term studies of toxicity at gavage doses significantly lower than
those evaluated in the dietary studies of reproductive toxicity.
The available studies on developmental toxicity suffered from
small group size and had some deficiencies in relation to currently
acceptable scientific standards. In two studies in mice, the NOAEL for
maternal toxicity was 100 mg/kg per day; at 150 mg/kg bw per day,
increased litter resorption was found. In rats, administration of
carbaryl in the diet for part or all of the gestation period resulted
in maternal toxicity at 100 mg/kg bw per day. No overt signs of
fetotoxicity were seen at this dose. In a study in which rats
were exposed to carbaryl by gavage and then mated, maternal and
embryotoxicity were observed at 100 mg/kg bw per day; no effects were
seen at 10 mg/kg bw per day. In guinea-pigs, administration of
carbaryl during gestation in the diet or by gavage resulted in an
NOAEL for maternal toxicity of 100 mg/kg bw per day. No embryo- or
fetotoxicity was observed at 300 mg/kg bw, the highest dose tested. In
rabbits, teratogenic effects were reported after administration of
200 mg/kg bw per day orally; maternal toxicity was also seen at this
dose. In two studies in dogs, maternal toxicity (dystocia, at
parturition only) was observed at a dose of 3.1 mg/kg bw per day.
A variety of birth defects was found after exposure to doses
> 5 mg/kg bw per day. Thus, the LOAEL for maternal toxicity was
3.1 mg/kg bw per day, and this was the NOAEL for birth defects in the
offspring.
The Meeting concluded that carbaryl induces developmental
toxicity, manifested as deaths in utero, reduced fetal weight, and
malformations, but only at doses that cause overt maternal toxicity.
The shortcomings of these studies made them inadequate for identifying
NOAELs for developmental toxicity that could be used for assessing
risk under conditions of exposure other than in the diet.
Carbaryl has been adequately tested for genotoxicity in a series
of assays in vitro and in vivo. While chromosomal aberrations have
been induced in vitro and carbaryl has been shown to disturb spindle
fibre mechanisms in vitro, there is no evidence from well-conducted
experiments that carbaryl is clastogenic in vivo. The Meeting
concluded that carbaryl is not genotoxic.
The effects of carbaryl on the nervous system are primarily
related to cholinesterase inhibition and are usually transitory.
Dietary exposure to doses of 10-20 mg/kg bw per day for 50 days was
reported to disrupt learning and performance in rats. In chickens
given high doses of carbaryl, there was no histological evidence of
neurotoxicity.
In controlled studies in volunteers, single oral doses of
< 2 mg/kg bw were well tolerated. A single oral dose of 250 mg (about
2.8 mg/kg bw) produced moderate cholinergic symptoms. In volunteers
given repeated daily oral doses over six weeks, the NOAEL was
0.06 mg/kg bw per day, on the basis of an increased ratio of amino
acid nitrogen to creatinine in the urine at a dose of 0.13 mg/kg bw
per day. This effect may represent a decrease in the ability of the
proximal convoluted tubule to reabsorb amino acids. The change was
reversible. No inhibition of plasma or erythrocyte acetylcholin-
esterase activity was observed.
An epidemiological study on carbaryl production workers employed
between 1960 and 1978 showed no increase in cancer mortality.
An ADI of 0-0.003 mg/kg bw was established on the basis of the
LOAEL of 15 mg/kg bw per day in the study of carcinogenicity in mice,
using a safety factor of 5000, which includes an extra safety factor
of 50 to account for the presence of vascular tumours in male mice at
all doses tested. The resulting ADI provides an adequate margin of
safety, taking into account the LOAEL in the study of developmental
toxicity in dogs and the uncertainties about the effects on the male
reproductive system.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: NOAEL not identified. Lowest effective dose: 100 ppm,
equal to 14.7 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Rat: 250 ppm, equal to 10 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
2 mg/kg bw per day (one-year study of toxicity)
Dog: NOAEL not identified. Lowest effective dose: 3.1 mg/kg
bw per day (study of developmental toxicity)
1.8 mg/kg bw per day (one-year study of toxicity)
Human: 0.06 mg/kg bw per day (six-week study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Study of reproductive toxicity with special attention to the
male reproductive system
2. Studies of teratogenicity in rats and rabbits
3. Completion of on-going studies to elucidate the mechanism of
tumour formation
4. Study of developmental neurotoxicity and/or screening for
acute or subchronic neurotoxicity
5. Follow-up of the epidemiological study in workers, taking
into consideration the latent period before development of
cancer
References
Cheng, T. (1994) Dermal absorption of 14C-carbaryl (80S) in male rats
(preliminary and definitive phases). Guideline No. 85-2. Unpublished
report No. HWI 6224-207, dated July 1994 from Hazleton Wisconsin,
Inc., USA. Supplied to WHO by Rhône Poulenc, Research Triangle Park,
North Carolina, USA.
Cheng, T. (1995) Dermal absorption of 14C-carbaryl (XLR Plus) in
male rats (preliminary and definitive phases). Guideline No. 85-2.
Unpublished report No. HWI 6224-206, dated January 1995, from Hazleton
Wisconsin, Inc., USA. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Cranmer, M.F. (1986) Carbaryl; a toxicological review and risk
analysis. Neurotoxicology, 7, 247-332.
Debruyne, E. & Irisarri, E. (1996) Carbaryl technical-chronic toxicity
studies in the rat (HWA Study No. 656-139) and the mouse (HWA Study
No. 656-138): evaluation of histological slides. Unpublished report
No. R&D/CRSA/TOX-HPA-4, dated March 1996 from Rhône Poulenc Agrochimie.
Supplied to WHO by Rhône Poulenc, Research Triangle Park, North
Carolina, USA.
Hamada, N.N. (1993a) Oncogenicity study with carbaryl technical in
CD-1 mice. Unpublished report No. HWA 656-138, dated May 1993 from
Hazleton Washington, Inc., USA. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Hamada, N.N. (1993b) Combined chronic toxicity and oncogenicity study
with carbaryl technical in Sprague Dawley rats. Unpublished report No.
HWA 656-139, dated August 1993 from Hazleton Washington, Inc., USA.
Supplied to WHO by Rhône Poulenc, Research Triangle Park, North
Carolina, USA.
Klonne, D.R. (1995) Carbaryl mouse historical control data. Position
paper dated November 1995. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Marshall, R. (1996) Carbaryl: induction of micronuclei in the bone
marrow of treated mice. Unpublished report No. CH 198/89-1052, dated
March 1996 from Corning Hazleton, United Kingdom. Supplied to WHO by
Rhône Poulenc, Research Triangle Park, North Carolina, USA.
McEnaney, S. (1993) Technical study to evaluate the chromosome
damaging potential of carbaryl by its effects on the bone marrow cells
of treated rats. Unpublished report No. 198/64, dated September 1993
from Hazleton Microtest/Hazleton UK. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Pastides H. (1993) Standardized mortality ratio analysis of employees
exposed to carbaryl at the Rhône Poulenc Institute, West Virginia
Plant. Report dated January 1993. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Sagelsdorff, P. (1994) Investigation of the potential of protein- and
DNA-binding of carbaryl. Unpublished report No. CB93/52, dated April
1994 from Ciba-Geigy Ltd. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Strubble, C.B. (1994) Metabolism of 14C-carbaryl in rats (preliminary
and definitive phases). Unpublished report No: HWI 6224-184, dated
August 1994, from Hazleton Wisconsin, Inc., USA. Supplied to WHO by
Rhône Poulenc, Research Triangle Park, North Carolina, USA.
Thomas, H. (1994) Liver cytochrome P-450 inducer phenotyping in the
male CD-1 mouse. Unpublished report No. CB94/23, dated October 1994,
from Ciba-Geigy Ltd. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Weil C.S. & Carpenter, C.P. (1965) Results of a three generation
reproduction study on rats fed Sevin in their diet. Unpublished report
No. 28-53, dated April 1965, from Mellon Institute, USA. Supplied to
WHO by Rhône Poulenc, Research Triangle Park, North Carolina, USA.
Weil C.S. & Carpenter, C.P. (1966) Evaluation of the teratogenic
potential of insecticide Sevin in rats. Unpublished report No. 29-49,
dated June 1966, from Mellon Institute, USA. Supplied to WHO by Rhône
Poulenc, Research Triangle Park, North Carolina, USA.
WHO (1994) Carbaryl (Environmental Health Criteria 153), International
Programme on Chemical Safety, Geneva.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
 (c) Enzyme induction and effects on the liver
Six male CD1 mice were fed a diet designed to provide carbaryl
(purity, 99.6%) at a dose of 8000 ppm, equal to 1154 mg/kg bw per day,
for 14 days. Five animals received control diet. Food consumption was
markedly reduced during the first three days of treatment, accompanied
by body weight loss. At the end of treatment, body weights were
decreased to 85%, and the relative liver weight had increased to 135%
relative to controls. Protein fractions were determined in the
microsomal and cytosolic fractions: a 1.3-fold increase (per gram of
liver) in microsomal protein was found, with a similar increase in
microsomal cytochrome P450 content. The ethoxylation of ethoxy-
resorufin and the depentylation of pentoxyresorufin were increased by
1.9- and 3.1-fold, respectively. Total testosterone hydroxylation was
increased by 1.5-fold relative to controls; while some forms of
testosterone hydroxylation were minimally altered, the 6 alpha, 11
alpha, 11ß, and 16ß forms were increased by three- to fourfold.
Hepatic glutathione levels were only slightly increased. The author of
the report concluded that carbaryl is a low-potency barbiturate-type
inducer (Thomas, 1994); however, in view of the magnitude of the
effects found in relation to the dose, there is no clear evidence that
the compound should be considered an inducer.
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that disturbances in carbohydrate metabolism, protein
synthesis, and detoxification have been reported in the livers of
mammals treated with carbaryl. It concluded that carbaryl is a weak
inducer of hepatic microsomal drug metabolizing activity, hepatic
levels of cytochrome P450 and b5 are increased, and phenobarbital
sleeping time is shortened. The changes in hepatic metabolism may
account in part for the three-fold increase in the LD50 for
carbaryl-pretreated rats.
2. Toxicological studies
(a) Acute toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that the compound is
moderately toxic after acute oral administration. The LD50 for rats
was 225-721 mg/kg bw. Interspecies difference were found, cats being
the most sensitive and guinea-pigs, rats, mice, and rabbits showing
more resistance, in that order. The LD50 was increased threefold
by pretreating animals with small doses of carbaryl. The compound
is slightly toxic after acute dermal administration, the LD50
being > 2000 mg/kg bw. No LC50 for acute exposure by inhalation was
available, but the effects observed in dogs, cats, and rats exposed to
carbaryl dust or to formulations of carbaryl were typical of those of
cholinesterase inhibition. In cats, exposure for 6 h to a dust
concentration of only 20 mg/m3 inhibited serum and erythrocyte
cholinesterase activity. Many of the known metabolites of carbaryl
are much less toxic than the parent compound. None of the metabolites
with a methylcarbamate moiety was appreciably more active as a
cholinesterase inhibitor than carbaryl itself.
(b) Short-term toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that in a study of
cats the NOAEC was 16 mg/m3 after exposure for 120 days, on the basis
of cholinergic reactions at 30 mg/m3 after exposure for 30 days. In a
study in rats, no effects were observed after 90 days' exposure to
10 mg/m3.
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female CD1 mice were given diets
designed to provide carbaryl (purity, 99.3%) at concentrations of 0,
100, 1000, or 8000 ppm, equal to 0, 15, 150, and 1200 mg/kg bw per day
for males and 0, 18, 180, and 1400 mg/kg bw per day for females, for
two years. Haematological parameters and plasma, erythrocyte, and
brain cholinesterase activities were determined in 10 mice of each sex
per group at week 53 and at termination.
There were no treatment-related effects on mortality. A thin
appearance and hunched posture were noted in many females and some
males at the high dose during the first three to six weeks and the
last six months of the study. Some animals at this dose also had a
languid appearance, urine stains, rough coats, and opaque eyes, the
latter in females during the last three months. Body-weight gain was
impaired during the first two weeks of the study in mice at the high
dose, and the weights remained significantly lower than that of
controls throughout the study. At the end of the study, the body
weights of males and females at the high dose were 88 and 87% of those
of the respective controls, but the weight gain over the whole period
was only 62 and 68% of that of controls. Food consumption was markedly
depressed in animals of each sex at the high dose during the first
months of the study. It remained low in females throughout the study
and was again lower in males from week 78 of the study. Erythrocyte
counts, haemoglobin, and packed cell volume were decreased in females
at the high dose at week 53 and in males at the end of the study.
The platelet count was increased in females at the high dose.
Cholinesterase activity was inhibited in animals at the middle and
high doses. Significant decreases in erythrocyte acetylcholinesterase
were seen only at week 53 in males at the middle and high doses (78
and 70% of the control value, respectively). Significant, dose-related
decreases in brain acetylcholinesterase activity were seen in animals
of each sex at the middle and high doses at interim and terminal
sacrifice. At the end of the study, brain acetylcholinesterase
activity was only 60 and 66% of the control levels in males and
females at the high dose, respectively.
Decreases in the absolute and relative weights of lungs and ovary
(at interim sacrifice only) were seen in females at the high dose. The
absolute and relative weights of the livers (with gall-bladder) and
the relative weight of the kidneys were increased in animals of each
sex at the high dose. On histopathological examination, a dose-related
increase in the incidence and severity of intracytoplasmic protein-
like droplets was found in the urinary bladder of animals of each sex
at the middle and high doses. An increased incidence of uni- and/or
bilateral cataracts was found in high-dose males and females, and a
slight increase in the severity of extramedullary haematopoiesis
and the presence of pigment in the spleen were found at terminal
sacrifice. The incidence of renal tubular-cell neoplasia was increased
in high-dose males, that of hepatocellular neoplasia in high-dose
females, and that of vascular tissue neoplasia in all treated males
and in high-dose females (Table 2). Carbaryl was thus found to be
carcinogenic in mice. The NOAEL for non-neoplastic findings was
100 ppm, equal to 15 mg/kg bw per day, on the basis of inhibition
of erythrocyte and brain acetylcholinesterase activity and
histopathological changes in the urinary bladder (Hamada, 1993a).
A re-examination of the histological slides of the liver and
kidneys from mice in the control and high-dose groups at interim
sacrifice, performed by two pathologists from the Rhône Poulenc
Company (Debruyne & Irisarri, 1996), showed no microscopic changes in
the liver or kidney. In a position paper, Klonne (1995) compared the
incidence of vascular tumours (mostly in the liver and spleen) in the
male mice in this study with those in historical controls in the
laboratory that performed the experiment and in several other
laboratories. The tumour incidences were clearly increased over
the mean values for historical controls; but, in the performing
laboratory, data were available only from 18-month studies. When the
incidences were compared with the ranges in historical controls in
studies of up to two years' duration, the incidences of vascular
tumours in the livers of animals at the low and middle doses were just
below the upper limit for the controls, and the incidences of vascular
tumours in the spleens of animals at the middle dose were outside the
range. The incidences among animals at the high dose were outside the
range for vascular tumours in the liver and within the range for
tumours in the spleen.
Table 2. Incidences of tumours in groups of 80 mice fed diets containing carbaryl
Tumour Males Females
0 ppm 100 ppm 1000 ppm 8000 ppm 0 ppm 100 ppm 1000 ppm 8000 ppm
Renal tubular-cell adenoma 0 0 0 3 0 0 0 1
Renal tubular-cell carcinoma 0 0 0 3 0 0 0 0
Hepatocellular adenoma 12 7 13 8 0 0 1 7
Hepatocellular carcinoma 6 7 3 8 1 1 1 3
Hepatoblastoma 0 0 0 0 0 0 0 1
Haemangioma 0 1 1 3 1 0 1 0
Haemangiosarcoma 2 5 9 7 2 3 3 9
No. of vascular 2 6 10 10 3 3 4 9
tumour-bearing animals
All vascular tumours 2 9 13 18 5 6 5 10
Rats
Groups of 80 male and 80 female Sprague-Dawley rats were given
diets designed to provide concentrations of carbaryl (purity, 99%) of
0, 250, 1500, or 7500 ppm, equal to 0, 10, 60, and 350 mg/kg bw per
day for males and 0, 13, 79, and 480 mg/kg bw per day for females, for
two years. Haematology, clinical chemistry, cholinesterase activity in
plasma and erythrocytes, and urinary parameters were studied in 10
rats of each sex at weeks 26, 52, 78, and 104. Brain acetylcholin-
esterase activity was determined at interim sacrifice and at the end
of study in 10 rats of each sex. Two additional groups of 10 rats of
each sex were treated with 0 or 7500 ppm for 52 weeks and were then
kept for a recovery period of four weeks, when haematology, clinical
chemistry, and cholinesterase activity were studied. There were no
treatment-related effects on mortality, and survival was increased in
females at the high dose; however, an increased incidence of alopecia
on the limbs was found in these animals, and an increased prevalence
of urine stains was seen in animals of each sex. The mean body weights
were significantly depressed in animals at the high dose at all
intervals analysed and in those at the middle dose at many intervals.
At the end of study, the body weights of males and females at the high
dose were only 5 and 55% of those of the respective controls, and
those of animals at the middle dose were 94% and 88% of the control
values, respectively. Food consumption was markedly lower in the
high-dose group. The numbers of animals with cataracts were increased
in the high-dose group, with incidences of 4, 6, 7, and 12 males and
3, 2, 4, and 10 females at the control, low, middle, and high doses,
respectively.
There were no treatment-related findings in haematological
parameters. Increased serum cholesterol and urea nitrogen were found
in high-dose females. Plasma cholinesterase activity was depressed in
animals of each sex at the high dose, to 58-73% of the control value
in males and 43-54% in females. Erythrocyte acetylcholinesterase
activity was also significantly decreased, to 63-81% of the control
value in males at the high dose and 62-75% in females; in females at
the middle dose, the activity was 74-81% of the control value, whereas
in males at this dose the depression was found only at weeks 52
(81%) and 78 (77%). Significant, dose-related decreases in brain
acetylcholinesterase activity were found at both 52 and 104 weeks in
high-dose males and middle- and high-dose females. Males at the middle
dose showed a significant depression only at the 52-week interim
sacrifice. Changes seen on urinalysis in animals at the high dose
included an increased incidence of dark urine, decreased urine volume
in females (at week 52), and erythrocytes in urine and occult blood in
males (at weeks 78 and 104). The relative weights of lungs, brain,
kidney, and liver were significantly increased in animals at the high
dose at the interim and terminal sacrifices; testicular weight was
also increased in those at the high dose. The increase in relative
liver weight was dose-related, but a significant increase at the
middle dose was found only in the females at interim sacrifice.
Gross pathological examination of animals at the high dose
revealed increased incidences of masses in the urinary bladder, pale
areas in the lungs, and dark areas in the glandular stomach (only in
males). A decreased incidence of mammary masses was noted in females
at this dose, with 65% in controls, 57% at the low dose, 64% at the
middle dose, and 41% at the high dose. Histopathological evaluation
of tissues taken from rats at interim sacrifice revealed hyaline
inclusions in the livers of males at the high dose. At the end of the
study, the following non-neoplastic findings were observed in animals
at the high dose: an increased incidence of thyroid hypertrophy (8/70
in males and 33/70 in females, in comparison with 1/70 and 4/70 in
controls, respectively); eosinophilic hepatocellular alterations and
increased pigment in females, hepatocyte hypertrophy in animals of
each sex, and intracytoplasmic hyaline inclusions in males; an
increased incidence of transitional epithelial hyperplasia in the
kidneys of males, and squamous metaplasia, a high mitotic index, and
cytological atypia in the urinary bladders of animals of each sex, all
associated with the carcinomas found; an increased incidence of
alveolar foamy macrophages and focal pneumonitis; and an increased
incidence of degeneration of the sciatic nerve and adjacent skeletal
muscles. The neoplastic findings in thyroid, liver, and urinary
bladder are summarized in Table 3. Nearly all of the animals with
transitional-cell neoplasms in the bladder also had extensive
hyperplasia, indicating the preneoplastic nature of this change. Males
at the high dose also had an increased incidence of transitional-cell
hyperplasia in the kidney; a single carcinoma was found. The incidence
of benign interstitial-cell tumours in the testis was increased in
animals at the high dose (5/70; 2/70 in controls).
After one year of exposure and the recovery period of four weeks,
the food consumption of animals at the high dose improved and the
animals gained more weight. The effects on cholinesterase activity
were reversible, as were the histological changes in the liver. The
relative weights of the lungs, brain, kidney, and liver, however,
remained increased. Carbaryl thus induced tumours at 7500 ppm, a
dose that exceeds the maximum tolerated dose (MTD). The NOAEL for
non-neoplastic findings in this study was 250 ppm, equal to 10 mg/kg
bw for males per day, on the basis of inhibition of erythrocyte and
brain acetylcholinesterase and the decrease in mean body weight
(Hamada, 1993b).
A re-examination of the histological slides of the liver, kidney,
urinary bladder, and thyroid of rats of all doses at interim sacrifice
and of the controls and those at the high dose after recovery,
performed by two pathologists from Rhône Poulenc Company (Debruyne &
Irisarri, 1996), revealed the presence of further microscopic changes
in animals at the high dose in the bladder (irreversible epithelial
hyperplasia in males and females), kidney (reversible pelvic
urothelial hyperplasia in males), thyroid (reversible follicular
hypertrophy in males), and liver (reversible hepatocellular
hypertrophy in males and females).
Table 3. Incidences of tumours in groups of 70 rats fed diets containing carbaryl
Tumour Males Females
0 ppm 250 ppm 1500 ppm 7500 ppm 0 ppm 250 ppm 1500 ppm 7500 ppm
Thyroid follicular-cell adenoma 0 2 0 8 0 0 0 1
Thyroid follicular-cell carcinoma 0 0 0 1 1 0 0 0
Hepatocellular adenoma 1 1 2 1 1 0 3 7
Hepatocellular carcinoma 0 2 2 1 0 0 0 0
Transitional-cell hyperplasia 9 8 10 54 6 6 6 56
Transitional-cell papilloma 0 à 0 13a 1 0 0 7
Transitional-cell carcinoma 0 0 0 11 0 0 0 6
a Includes one squamous-cell papilloma
In the Environmental Health Criteria monograph on carbaryl (WHO,
1994), the results of several long-term studies in mice were
summarized, one of which was a report of the data obtained at interim
sacrifice in the study of Hamada (1993a). In another study, no effects
were observed at the highest level tested (400 ppm). Seven further
studies of carcinogenicity in mice involving different strains, routes
of administration, doses, dose schedules, and lengths of exposure and
observation were considered unsuitable for evaluation of carcinogenic
potential; only one showed a marginal response.
Several short- and long-term studies in rats were available. The
most recent report presented the interim results of the long-term
study of Hamada (1993b). In two older dietary studies, lasting 96 days
and two years, the most obvious effects were on the kidney. The NOAEL
in these studies was 200 ppm, equal to 7.9 mg/kg bw per day, with
effects on the kidney at 400 ppm. In two one-year studies of gavage
(for which only summaries were available), effects were seen on the
thyroid and on male and female reproductive organs and/or function at
doses > 5 mg/kg bw per day. The NOAEL was 2 mg/kg bw per day. Two
studies of carcinogenicity in rats were available. In a study in which
carbaryl was administered orally or by a single subcutaneous
implantation, fibrosarcomas of the skin were observed; the other
(dietary) study gave negative results. Neither was considered suitable
for evaluation of carcinogenic potential.
Three one-year studies in dogs were available. In a study in
which carbaryl was given in capsules, effects on the kidney were found
at 7.2 mg/kg bw per day, but no effects were observed at 1.8 mg/kg bw
per day (approximately 100 ppm in the diet). In the two dietary
studies, the NOAEL was 125 ppm, equivalent to 3.1 mg/kg bw per day, on
the basis of effects on liver weight and inhibition of erythrocyte and
brain acetylcholinesterase activity at 400 ppm.
(d) Reproductive toxicity
No new information had become available. The summaries in the
Environmental Health Criteria monograph on carbaryl (WHO, 1994),
although extensive, could not always be used to derive NOAELs for
various aspects of the reproductive toxicity of carbaryl. Additional
information was therefore obtained for some studies, from either the
original papers or reviews.
In two five-day studies of male mice exposed orally, no effects
were found on the testis or accessory glands at a dose of 34 mg/kg bw
per day; at 68 mg/kg bw per day, androstenedione synthesis was
decreased.
Three three-generation studies of reproductive toxicity in rats
were summarized. In the first, in which 0, 2000, 5000, or 10 000 ppm
carbaryl were administered in the diet, impaired fertility, reduced
postnatal survival, and reduced postnatal growth were observed at
5000 ppm; no effects were seen at 2000 ppm, equivalent to 125 mg/kg bw
per day. There was no clear NOAEL in this study, since the parameters
were wrongly defined. In a second dietary study, at doses of 0, 7, 25,
100, and 200 mg/kg bw per day, the NOAEL was 100 mg/kg bw per day, on
the basis of decreased maternal weight at 200 mg/kg bw per day. There
were no reproductive effects. In a study in which rats were treated by
gavage at doses of 0, 3, 7, 25, or 100 mg/kg bw per day, the NOAEL was
25 mg/kg bw per day, on the basis of decreased maternal weight and
mortality and effects on litter size and viability at the high dose.
In a study in which special attention was paid to effects on
reproductive parameters, male and female rats were exposed by gavage
for one month to doses of 1-50 mg/kg bw per day. Adverse effects were
observed on male reproductive cells; furthermore, increased embryonic
and fetal deaths, decreased numbers of implantations, and a prolonged
oestrus cycle were observed. This study was also described by Cranmer
(1986), who reported that the effects on reproductive parameters were
seen only at 1, 5, 10, and 20 mg/kg bw per day. He also noted that
only the change in the number of tubules containing spermatogonia
was seen at all doses, and most of the other treatment-related
histological and functional changes in the testis occurred at doses
> 5 mg/kg bw per day. Dose-response relationships were not seen for
the other effects on litter parameters or for the prolonged oestrus
cycle, limiting interpretation of the study. This and other studies of
reproductive toxicity were considered of dubious value for risk
assessment as they suffered from various shortcomings in study design.
A three-generation study of reproductive toxicity in which
gerbils were exposed daily via the diet to concentrations of
2000-10 000 ppm was not considered suitable for establishing a NOAEL;
however, adverse effects on various reproductive parameters were seen.
(e) Developmental toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) was supplemented by
additional information from the original papers and reviews. All of
the studies suffered from small group sizes and some deficiencies in
comparison with currently acceptable scientific standards.
Two of five studies in mice were used for evaluation, in which
animals were exposed by gavage to carbaryl on days 6-15 of gestation;
in the second study, groups were also exposed only on day 8 or 12. In
the first study, with doses of 0, 100, and 150 mg/kg bw per day, the
NOAEL was 100 mg/kg bw per day on the basis of maternal toxicity
(ataxia, lethality) and litter resorption at 150 mg/kg bw. In one
group exposed via the diet to 5560 ppm, a decrease in fetal weight was
observed. In the second study, with doses of 0, 100, 150, and
200 mg/kg bw per day, the NOAEL was 150 mg/kg bw per day, on the
basis of maternal death and fetal toxicity (weight reductions and
indications of growth retardation) at 200 mg/kg bw per day.
Three of six studies in rats were used for evaluation. In the
first study, groups of six rats were fed diets containing carbaryl on
days 1-5, 5-15, or 1-21 of gestation. The NOAEL for maternal toxicity
was 100 mg/kg bw per day, on the basis of reduced weight gain in dams
at 500 mg/kg bw per day when exposed on gestation days 5-15 or 1-21.
No adverse fetal effects were seen. The original papers (Weil &
Carpenter 1965, 1966) reported that the doses used were 0, 2.5, 10,
20, 100, and 500 mg/kg bw per day and that fetuses underwent only
skeletal examination. In addition to the effects summarized above, the
authors reported reduced postnatal survival in the pups of dams
exposed to 500 mg/kg bw per day on days 1-21. In the second study,
groups of 10 rats received oral doses of 200 or 300 mg/kg bw or an
intraperitoneal dose of 40 mg/kg bw on single or multiple days of
gestation. The only effect seen was a reduction in fetal weight in
some groups. In the third study, groups of six or seven rats received
diets containing carbaryl at doses of 0, 1, 10, or 100 mg/kg bw per
day three months before and during gestation. The NOAEL for maternal
toxicity was 10 mg/kg bw per day, on the basis of reduced weight gain
at 100 mg/kg bw per day. No adverse fetal effects were seen. This
study was also described by Cranmer (1986), who reported a slight
reduction in the numbers of implantations and live fetuses at 100
mg/kg bw per day.
One of two studies in guinea-pigs was used for evaluation. Groups
of four to nine animals were exposed to 0, 100, 200, or 300 mg/kg bw
per day in the diet or 0, 50, 100, or 200 mg/kg bw per day orally.
Reduced maternal weight gain was observed at 200 mg/kg bw per day, but
there were no effects on embryos or fetuses.
One of three studies in rabbits was used for evaluation. Groups
of 15-20 animals were given oral doses of 0, 150, or 200 mg/kg bw per
day on days 6-18 of gestation. There was no NOAEL for maternal
toxicity since a reduction in weight gain was observed in both treated
groups. A significant increase in umbilical hernia was observed in
fetuses at 200 mg/kg bw per day.
In two studies in dogs, animals were fed diets containing
carbaryl at doses of 3.1-50 mg/kg bw per day or 2-12.5 mg/kg bw per
day during gestation. Various birth defects were observed in the pups
at doses > 5 mg/kg bw per day. Maternal toxicity was observed at
all doses.
In pigs fed doses of 4-32 mg/kg bw per day in the diet, the
effects noted (prenatal lethality and malformations) were not
consistent across the studies. Another study in pigs was considered
unsuitable for evaluation.
One of two studies in monkeys showed an increased rate of
abortions after oral treatment with 2 or 20 mg/kg bw per day
throughout gestation. No adverse effects were noted in the second
study in which the doses were 0.2-32 mg.kg bw per day orally on days
20-38 of gestation.
(f) Genotoxicity
The results of studies of chromosomal aberrations in rats and
micronucleus formation in mice treated with carbaryl in vivo are
summarized in Table 4. Negative results were obtained in both studies
at doses at which limited signs of toxicity were seen; however, there
was no evidence that the test compound had reached the target organ,
as no cytotoxicity was found.
In a study of DNA binding, two groups of four male CD mice were
treated by oral intubation with 14C-carbaryl (labelled in the
naphthyl group) in 0.5% aqueous carboxymethylcellulose at a dose of
75 mg/kg bw (8 mCi/kg bw). One group was pretreated with carbaryl
(purity, 99.6%) at 8000 ppm (equal to 1900 mg/kg bw per day) in the
diet for 14 days. Four mice served as controls. Urinary excretion and
exhalation of carbon dioxide were measured over 24 h in one pretreated,
one non-pretreated, and one control mouse. Animals were sacrificed 24 h
after administration of radiolabelled material, and the liver, kidneys,
and urinary bladder were removed; however, only the liver was used
for determining DNA and protein binding. There was no significant
exhalation of 14C-carbon dioxide, and about 30% of the administered
dose was excreted in the urine of both treated groups. The pretreated
group had lower body weights and increased relative liver weights at
the end of treatment. Binding of 14C-carbaryl to chromatin protein
was found in both treated groups, resulting in specific radioactivities
of 340-537 dpm/mg protein (7-11 pmol/mg protein). As there was no
significant radioactivity in purified DNA, in the presence or absence
of pretreatment, covalent binding of the 14C-naphthyl label does not
seem to have occurred (Sagelsdorff, 1994).
Table 4. Results of tests for the genotoxicity of carbaryl in vivo
End-point Test system Concentration Purity Results Reference
(mg/kg bw) (%)
Micronucleus formation Male and female 50, 100, 200 orally for 99.9 Negativea Marshall (1996)
CD-1 mice (5 per 2 daysb; killed at 24 or
dose), bone-marrow 48 h; vehicle, 0.5%
cells carboxymethylcellulose
Chromosomal aberration Male and female Single dose of 30, 60, 99.7 Negativea McEnaney (1993)
Sprague-Dawley 120c; killed at 6, 24,
rats (5 per dose), 48 h; vehicle, 0.25%
bone-marrow cells carboxymethylcellulose
a Positive controls yielded positive results
b Clinical signs of toxicity (lethargy, slight weight loss) at highest dose; no change in polychromatic:normochromatic
erythrocyte ratio. Doses based on the results of a preliminary test in which one of six animals at 300 mg/kg bw died.
c Clinical signs of toxicity (lethargy, tremors) observed at the highest dose; no depression of mitotic index. Doses based on
the results of a preliminary test in which the LD50 was 231 mg/kg bw
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that carbaryl has been evaluated for mutagenicity in a
number of tests in vitro and in vivo, in bacterial, yeast, plant,
insect, and mammalian systems, with a variety of end-points. It
concluded that carbaryl does not damage DNA. Reports of induction of
mitotic recombination, gene conversion, and unscheduled DNA synthesis
in prokaryotes (Haemophilus influenzae, Bacillus subtilis) and
eukaryotes ( Saccharomyces cerevisiae, Aspergillus nidulans, cultured
human lymphocytes, and rat hepatocytes) in vitro have not been
confirmed. Negative results were obtained in tests for gene mutations
in all but two bacterial assays. Although several studies of gene
mutation were conducted in mammalian cells in vitro, only one
equivocally positive result was obtained, in a study that had several
shortcomings and which has not been confirmed. Chromosomal damage has
been reported in human, rat, and hamster cells and in plants treated
in vitro with high doses of carbaryl. No such effects have been
observed in mammalian tests in vivo, even at doses as high as
1000 mg/kg bw. Carbaryl induces disturbances in the spindle fibre
mechanism in plant and mammalian cells in vitro, but the relevance
of the assays in plants for humans is unclear. The Environmental
Health Criteria monograph concluded that the available database did
not indicate that carbaryl induces genetic changes in somatic or
germinal tissues of humans.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported that carbaryl is
not or only weakly irritating to the skin and is weakly irritating to
the eye. The available studies indicated that carbaryl has little or
no sensitizing potential.
(ii) Neurotoxicity
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported that the effects
of carbaryl on the central nervous system had been studied in rats
and monkeys treated by intraperitoneal, intramuscular, or oral
administration or by inhalation. Changes in motor activity, working
memory, and behaviour were seen. Dietary exposure to doses of
10-20 mg/kg bw per day for 50 days was reported to disrupt learning
and performance in rats. In a small study on pigs, administration of
carbaryl in the diet at 150 mg/kg bw per day for 72-82 days was
reported to produce a number of neuromuscular effects. Reversible leg
weakness was noted in chickens given high doses of carbaryl, but no
evidence of demyelination was observed in the brain, sciatic nerve, or
spinal cord sections examined microscopically. Similar effects were
not observed in long-term studies in rodents. The effects of carbaryl
on the nervous system are primarily related to inhibition of
cholinesterase activity and are usually transitory.
(iii) Immunotoxicity
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that several studies
in mice, rats, rabbits, and guinea-pigs, mostly treated orally, showed
that carbaryl administered at doses that do not cause overt clinical
signs has a variety of non-life-threatening effects on both cellular
and humoral immunity. Many of the effects were detected at doses close
to the LD50. A lack of consistency and sometimes overt contradiction
between the results of several of these studies precludes definition
of the immunotoxic mechanism. Life-time exposure to carbaryl did not
increase the occurrence of disease in rats or mice, and no enhancement
of viral infections was found, even at doses close to the LD50. Most
of the studies on mice and rabbits at doses that permitted survival
did not show significant effects on the immune system. Vital
enhancement has been demonstrated in vitro in a number of studies
including prior incubation with carbaryl. Inhibition of human serum
complement activity and interleukin-2-driven proliferation of large
granular lymphocytes have also been found in vitro.
(iv) Haematological effects
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported effects on
coagulation in rats, rabbits, and dogs in vivo and in studies in
vitro, but the direction of the effect is unclear. In glucose-
6-phosphate dehydrogenase-deficient sheep erythrocytes, carbaryl
produced a dose-dependent increase in methaemoglobin formation. Human
serum albumin reacted in vitro with the ester group of carbaryl.
Carbaryl binds free blood amino acids.
(v) Effects on the endocrine system
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) concluded from several
experiments in vivo and in vitro that carbaryl increases the
gonadotropic function of the hypophysis of rats.
(vi) Studies with N-nitrosocarbaryl
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that carbaryl is a secondary amine and is therefore
capable of nitrosation in the presence of nitro donor groups, such as
sodium nitrate, to give a nitrosamide. A condition of such nitrosation
is an acidic pH (< 2), such as that found in the human stomach;
however, N-nitrosocarbaryl is not stable at this pH; its maximal
stability is at pH 3-5, at which no significant amount of carbaryl can
be nitrosated. Carbaryl was nitrosated in several studies, in vitro
as well as in vivo, in guinea-pigs, in which the gastric acidity is
similar to that of humans.
N-Nitrosocarbaryl induced local tumours in rats, consisting of
sarcomas at the site of injection and forestomach squamous-cell
carcinomas, after oral administration. This local carcinogenic effect
and the lack of systemic carcinogenicity characterize the compound as
a directly acting alkylating agent. Given the human chemistry of
carbaryl, the risk of carcinogenic effects of N-nitrosocarbaryl for
humans after exposure to carbaryl can be considered negligible.
N-Nitrosocarbaryl can induce mitotic recombination and gene
conversion in prokaryotes (H. influenzae and B. subtilis) and
eukaryotes (S. cerevisiae) in vitro and gives positive results in
Escherichia coli spot tests. It also binds to DNA, causing
alkali-sensitive bonds and single-strand breakage. It is not
clastogenic in vivo in bone marrow and germ cells, even at highly
toxic doses.
3. Observations in humans
An epidemiological study of total and cause-specific mortality
among employees exposed to carbaryl at a production plant was based on
information obtained in 1988 on the vital status and cause of death of
all individuals who were first hired between the start-up of the
carbaryl unit in 1960 through 1978. Employees hired after 1978 were
not included in the study. Three categories of workers exposed to
carbaryl were defined: those involved in production, maintenance
employees, and those working in packaging and distribution. A total of
448 employees contributing 7532 person-years to the analysis were
available, representing the combined number of years in which these
employees were followed through 1988. Mortality was measured in terms
of standardized mortality ratios (SMRs), which are the ratios of the
observed numbers of death among members of a cohort to the number
expected, on a year-specific and age-adjusted basis. The 25 deaths
identified in this cohort resulted in elevated SMRs for cancer of the
pancreas, unspecified cancer, and cancers of the brain and other parts
of the nervous system. In the first two categories, the excess was
slight and based on only one death. In the last category, the SMR was
based on two deaths due to tumours of different histological origins,
reducing the possibility that the two malignancies were caused by the
same exposure. Furthermore, in all three categories the confidence
intervals were wide, indicating a relatively imprecise SMR estimate
and reflecting the small sample size on which it is based (Pastides,
1993).
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) summarized several cases of poisoning. The clinical picture is
dominated by symptoms of inhibition of cholinesterase activity. Signs
of poisoning develop quickly after absorption and disappear rapidly
after exposure ends.
In controlled studies of human volunteers, single doses of
< 2 mg/kg bw were well tolerated. A single dose of 250 mg (about
2.8 mg/kg bw) produced moderate symptoms of cholinesterase inhibition
(epigastric pains and sweating) within 20 min. Complete recovery was
seen within 2 h of treatment with atropine sulfate. Two groups of
human volunteers given carbaryl at doses of 0.06 or 0.13 mg/kg bw for
six weeks underwent physical examinations, removal of bromosulphthalein
from blood, electroencephalography, routine blood and urinalysis, and
measurement of cholinesterase in plasma and erythrocytes. No inhibition
of cholinesterase activity was observed, and no changes were seen in
the group receiving the low dose. The only finding at the high dose was
an increase in the ratio of amino acid nitrogen to creatinine in the
urine, which may represent a decrease in the ability of the proximal
convoluted tubule to reabsorb amino acids; this change was reversible.
The NOAEL was 0.06 mg/kg bw.
In cases of occupational overexposure to carbaryl, mild symptoms
are observed long before a dangerous dose is absorbed. No local
irritating effect is usually seen, although skin rash after accidental
splashing with carbaryl formulations has been described.
Reports of the effects of carbaryl on sperm count and changes in
sperm morphology in plant workers are conflicting. No adverse effects
on reproduction have been described.
Comments
Carbaryl is rapidly and almost completely absorbed after oral
administration. Excretion is rapid and occurs predominantly via the
urine; enterohepatic cycling of carbaryl metabolites is also
considerable. There were no significant dose-related or sex-specific
differences in elimination patterns, and there was no evidence of
bioaccumulation. Dermal absorption in rats was slow; after 24 h,
16-34% of the administered radiolabel had been absorbed. Higher doses
were less readily absorbed. In volunteers, 45% of a dose applied to
the skin in acetone was absorbed within 8 h. Carbaryl was rapidly
absorbed in the lungs.
The metabolism of carbaryl has been studied in various mammals,
including humans. The principal metabolic pathways are ring
hydroxylation, hydrolysis, and conjugation. There were no species
differences. The main metabolite in humans is 1-naphthol. The
hydrolysis product, N-methyl carbamic acid, spontaneously decomposes
to methylamine and carbon dioxide. The methylamine is later converted
to carbon dioxide and formate, the latter being excreted mainly in the
urine. Carbaryl metabolites are also found at small percentages of the
absorbed doses in saliva and milk.
Carbaryl is moderately toxic after acute oral administration, the
LD50 in rats being 225-721 mg/kg bw. Interspecies differences in
toxicity were found, cats (LD50, 150 mg/kg bw) being the most
sensitive species. The LD50 was increased threefold when animals were
pretreated with small doses of carbaryl. The compound is slightly
toxic after acute dermal administration, with an LD50 > 2000 mg/kg
bw. No LC50 for acute exposure by inhalation was available, but the
effects observed in dogs, cats, and rats exposed to dusts or
formulations of carbaryl were typical of those resulting from
inhibition of cholinesterase activity. In cats exposed to carbaryl
dust for 6 h, a concentration of 20 mg/m3 inhibited cholinesterase
activity in plasma and erythrocytes. Carbaryl was weakly irritating to
the eye but not the skin and was not considered to be a sensitizer.
WHO has classified carbaryl as 'moderately toxic' (WHO, 1996).
After oral administration of carbaryl in capsules to dogs at a
dose of 0.45, 1.8, or 7.2 mg/kg bw per day for one year, slight
effects were observed on the kidney at 7.2 mg/kg bw per day; the NOAEL
was 1.8 mg/kg bw per day. In two studies in which dogs were fed diets
containing carbaryl at 20-125 ppm for five weeks and 125-1250 ppm for
one year, the NOAEL was 125 ppm, equivalent to 3.1 mg/kg bw per
day, on the basis of effects on liver weight and inhibition of
acetylcholinesterase activity in erythrocytes and brain at 400 ppm.
In cats exposed to carbaryl by inhalation, cholinergic signs were
observed at 30 mg/m3 after exposure for 30 days. The NOAEL was
16 mg/m3 for 120 days. In a study in rats, no effects were observed
after exposure to 10 mg/m3 for 90 days.
Several studies of long-term toxicity or carcinogenicity in mice
cited in EHC 153 were considered to be unsuitable for evaluation of
carcinogenicity by either the Environmental Health Criteria Task Force
or the present Meeting, although they were suitable for assessing
long-term toxicity. In a recent study of carcinogenicity, mice were
given diets providing 0, 100, 1000, or 8000 ppm carbaryl for
104 weeks. Tumours were observed in the liver in females and the
kidney in males, and vascular tumours were found in animals of each
sex at the highest dose, which exceeded the maximum tolerated dose
(MTD). In male mice, increases in the incidences of vascular tumours
were also seen at the two lower doses; after considering all of the
available data, the Meeting could not identify an NOAEL for this
neoplastic lesion. The NOAEL for non-neoplastic lesions was 100 ppm
(equal to 14.7 mg/kg bw per day), on the basis of inhibition
of erythrocyte and brain acetylcholinesterase activity and
histopathological changes in the urinary bladder at 1000 ppm. This
NOAEL is consistent with the results of the earlier studies. The
Meeting concluded that the compound is carcinogenic in mice.
In several studies cited in EHC 153, carbaryl was administered in
the diet of rats for 96 days to two years. The most obvious effects
were in the kidney at doses > 400 ppm. In two one-year studies in
rats treated by gavage, effects on the thyroid and on male and
female reproductive organs and/or function were observed at doses
> 5 mg/kg bw per day; the NOAEL was 2 mg/kg bw per day. None of
these studies was considered suitable for evaluating carcinogenicity.
In a recent study of long-term toxicity and carcinogenicity, rats
were fed diets containing 0, 250, 1500, or 7500 ppm carbaryl for 104
weeks. In animals at the highest dose, which exceeded the MTD, tumours
were found in the thyroid in males, in the liver in females, and in
the urinary bladder in animals of each sex. The NOAEL for non-
neoplastic findings in this study was 250 ppm, equal to 10 mg/kg bw
per day, on the basis of inhibition of erythrocyte and brain
acetylcholinesterase activity and a decrease in mean body weight at
1500 ppm. This NOAEL is consistent with the results of earlier dietary
studies. The Committee concluded that carbaryl is carcinogenic in rats
only at levels that exceed the MTD.
The available studies on reproductive toxicity were conducted
some time ago and had some deficiencies in relation to currently
acceptable scientific standards. In three-generation studies, dietary
administration of carbaryl to rats induced reproductive effects
(impaired fertility and reduced postnatal survival and growth) at
doses > 2000 ppm (equal to 125 mg/kg bw per day); a dose of 100 mg/kg
bw per day did not induce maternal toxicity. When carbaryl was
administered by gavage, maternal toxicity was not observed at 25 mg/kg
bw per day, but both maternal and reproductive toxicity (reduced
litter size and viability) were found at 100 mg/kg bw per day. The
Meeting recommended that a new two-generation study of reproductive
toxicity be carried out in rats, with special attention to the male
reproductive system since effects on this system were observed in some
long-term studies of toxicity at gavage doses significantly lower than
those evaluated in the dietary studies of reproductive toxicity.
The available studies on developmental toxicity suffered from
small group size and had some deficiencies in relation to currently
acceptable scientific standards. In two studies in mice, the NOAEL for
maternal toxicity was 100 mg/kg per day; at 150 mg/kg bw per day,
increased litter resorption was found. In rats, administration of
carbaryl in the diet for part or all of the gestation period resulted
in maternal toxicity at 100 mg/kg bw per day. No overt signs of
fetotoxicity were seen at this dose. In a study in which rats
were exposed to carbaryl by gavage and then mated, maternal and
embryotoxicity were observed at 100 mg/kg bw per day; no effects were
seen at 10 mg/kg bw per day. In guinea-pigs, administration of
carbaryl during gestation in the diet or by gavage resulted in an
NOAEL for maternal toxicity of 100 mg/kg bw per day. No embryo- or
fetotoxicity was observed at 300 mg/kg bw, the highest dose tested. In
rabbits, teratogenic effects were reported after administration of
200 mg/kg bw per day orally; maternal toxicity was also seen at this
dose. In two studies in dogs, maternal toxicity (dystocia, at
parturition only) was observed at a dose of 3.1 mg/kg bw per day.
A variety of birth defects was found after exposure to doses
> 5 mg/kg bw per day. Thus, the LOAEL for maternal toxicity was
3.1 mg/kg bw per day, and this was the NOAEL for birth defects in the
offspring.
The Meeting concluded that carbaryl induces developmental
toxicity, manifested as deaths in utero, reduced fetal weight, and
malformations, but only at doses that cause overt maternal toxicity.
The shortcomings of these studies made them inadequate for identifying
NOAELs for developmental toxicity that could be used for assessing
risk under conditions of exposure other than in the diet.
Carbaryl has been adequately tested for genotoxicity in a series
of assays in vitro and in vivo. While chromosomal aberrations have
been induced in vitro and carbaryl has been shown to disturb spindle
fibre mechanisms in vitro, there is no evidence from well-conducted
experiments that carbaryl is clastogenic in vivo. The Meeting
concluded that carbaryl is not genotoxic.
The effects of carbaryl on the nervous system are primarily
related to cholinesterase inhibition and are usually transitory.
Dietary exposure to doses of 10-20 mg/kg bw per day for 50 days was
reported to disrupt learning and performance in rats. In chickens
given high doses of carbaryl, there was no histological evidence of
neurotoxicity.
In controlled studies in volunteers, single oral doses of
< 2 mg/kg bw were well tolerated. A single oral dose of 250 mg (about
2.8 mg/kg bw) produced moderate cholinergic symptoms. In volunteers
given repeated daily oral doses over six weeks, the NOAEL was
0.06 mg/kg bw per day, on the basis of an increased ratio of amino
acid nitrogen to creatinine in the urine at a dose of 0.13 mg/kg bw
per day. This effect may represent a decrease in the ability of the
proximal convoluted tubule to reabsorb amino acids. The change was
reversible. No inhibition of plasma or erythrocyte acetylcholin-
esterase activity was observed.
An epidemiological study on carbaryl production workers employed
between 1960 and 1978 showed no increase in cancer mortality.
An ADI of 0-0.003 mg/kg bw was established on the basis of the
LOAEL of 15 mg/kg bw per day in the study of carcinogenicity in mice,
using a safety factor of 5000, which includes an extra safety factor
of 50 to account for the presence of vascular tumours in male mice at
all doses tested. The resulting ADI provides an adequate margin of
safety, taking into account the LOAEL in the study of developmental
toxicity in dogs and the uncertainties about the effects on the male
reproductive system.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: NOAEL not identified. Lowest effective dose: 100 ppm,
equal to 14.7 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Rat: 250 ppm, equal to 10 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
2 mg/kg bw per day (one-year study of toxicity)
Dog: NOAEL not identified. Lowest effective dose: 3.1 mg/kg
bw per day (study of developmental toxicity)
1.8 mg/kg bw per day (one-year study of toxicity)
Human: 0.06 mg/kg bw per day (six-week study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Study of reproductive toxicity with special attention to the
male reproductive system
2. Studies of teratogenicity in rats and rabbits
3. Completion of on-going studies to elucidate the mechanism of
tumour formation
4. Study of developmental neurotoxicity and/or screening for
acute or subchronic neurotoxicity
5. Follow-up of the epidemiological study in workers, taking
into consideration the latent period before development of
cancer
References
Cheng, T. (1994) Dermal absorption of 14C-carbaryl (80S) in male rats
(preliminary and definitive phases). Guideline No. 85-2. Unpublished
report No. HWI 6224-207, dated July 1994 from Hazleton Wisconsin,
Inc., USA. Supplied to WHO by Rhône Poulenc, Research Triangle Park,
North Carolina, USA.
Cheng, T. (1995) Dermal absorption of 14C-carbaryl (XLR Plus) in
male rats (preliminary and definitive phases). Guideline No. 85-2.
Unpublished report No. HWI 6224-206, dated January 1995, from Hazleton
Wisconsin, Inc., USA. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Cranmer, M.F. (1986) Carbaryl; a toxicological review and risk
analysis. Neurotoxicology, 7, 247-332.
Debruyne, E. & Irisarri, E. (1996) Carbaryl technical-chronic toxicity
studies in the rat (HWA Study No. 656-139) and the mouse (HWA Study
No. 656-138): evaluation of histological slides. Unpublished report
No. R&D/CRSA/TOX-HPA-4, dated March 1996 from Rhône Poulenc Agrochimie.
Supplied to WHO by Rhône Poulenc, Research Triangle Park, North
Carolina, USA.
Hamada, N.N. (1993a) Oncogenicity study with carbaryl technical in
CD-1 mice. Unpublished report No. HWA 656-138, dated May 1993 from
Hazleton Washington, Inc., USA. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Hamada, N.N. (1993b) Combined chronic toxicity and oncogenicity study
with carbaryl technical in Sprague Dawley rats. Unpublished report No.
HWA 656-139, dated August 1993 from Hazleton Washington, Inc., USA.
Supplied to WHO by Rhône Poulenc, Research Triangle Park, North
Carolina, USA.
Klonne, D.R. (1995) Carbaryl mouse historical control data. Position
paper dated November 1995. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Marshall, R. (1996) Carbaryl: induction of micronuclei in the bone
marrow of treated mice. Unpublished report No. CH 198/89-1052, dated
March 1996 from Corning Hazleton, United Kingdom. Supplied to WHO by
Rhône Poulenc, Research Triangle Park, North Carolina, USA.
McEnaney, S. (1993) Technical study to evaluate the chromosome
damaging potential of carbaryl by its effects on the bone marrow cells
of treated rats. Unpublished report No. 198/64, dated September 1993
from Hazleton Microtest/Hazleton UK. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Pastides H. (1993) Standardized mortality ratio analysis of employees
exposed to carbaryl at the Rhône Poulenc Institute, West Virginia
Plant. Report dated January 1993. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Sagelsdorff, P. (1994) Investigation of the potential of protein- and
DNA-binding of carbaryl. Unpublished report No. CB93/52, dated April
1994 from Ciba-Geigy Ltd. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Strubble, C.B. (1994) Metabolism of 14C-carbaryl in rats (preliminary
and definitive phases). Unpublished report No: HWI 6224-184, dated
August 1994, from Hazleton Wisconsin, Inc., USA. Supplied to WHO by
Rhône Poulenc, Research Triangle Park, North Carolina, USA.
Thomas, H. (1994) Liver cytochrome P-450 inducer phenotyping in the
male CD-1 mouse. Unpublished report No. CB94/23, dated October 1994,
from Ciba-Geigy Ltd. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Weil C.S. & Carpenter, C.P. (1965) Results of a three generation
reproduction study on rats fed Sevin in their diet. Unpublished report
No. 28-53, dated April 1965, from Mellon Institute, USA. Supplied to
WHO by Rhône Poulenc, Research Triangle Park, North Carolina, USA.
Weil C.S. & Carpenter, C.P. (1966) Evaluation of the teratogenic
potential of insecticide Sevin in rats. Unpublished report No. 29-49,
dated June 1966, from Mellon Institute, USA. Supplied to WHO by Rhône
Poulenc, Research Triangle Park, North Carolina, USA.
WHO (1994) Carbaryl (Environmental Health Criteria 153), International
Programme on Chemical Safety, Geneva.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
(c) Enzyme induction and effects on the liver
Six male CD1 mice were fed a diet designed to provide carbaryl
(purity, 99.6%) at a dose of 8000 ppm, equal to 1154 mg/kg bw per day,
for 14 days. Five animals received control diet. Food consumption was
markedly reduced during the first three days of treatment, accompanied
by body weight loss. At the end of treatment, body weights were
decreased to 85%, and the relative liver weight had increased to 135%
relative to controls. Protein fractions were determined in the
microsomal and cytosolic fractions: a 1.3-fold increase (per gram of
liver) in microsomal protein was found, with a similar increase in
microsomal cytochrome P450 content. The ethoxylation of ethoxy-
resorufin and the depentylation of pentoxyresorufin were increased by
1.9- and 3.1-fold, respectively. Total testosterone hydroxylation was
increased by 1.5-fold relative to controls; while some forms of
testosterone hydroxylation were minimally altered, the 6 alpha, 11
alpha, 11ß, and 16ß forms were increased by three- to fourfold.
Hepatic glutathione levels were only slightly increased. The author of
the report concluded that carbaryl is a low-potency barbiturate-type
inducer (Thomas, 1994); however, in view of the magnitude of the
effects found in relation to the dose, there is no clear evidence that
the compound should be considered an inducer.
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that disturbances in carbohydrate metabolism, protein
synthesis, and detoxification have been reported in the livers of
mammals treated with carbaryl. It concluded that carbaryl is a weak
inducer of hepatic microsomal drug metabolizing activity, hepatic
levels of cytochrome P450 and b5 are increased, and phenobarbital
sleeping time is shortened. The changes in hepatic metabolism may
account in part for the three-fold increase in the LD50 for
carbaryl-pretreated rats.
2. Toxicological studies
(a) Acute toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that the compound is
moderately toxic after acute oral administration. The LD50 for rats
was 225-721 mg/kg bw. Interspecies difference were found, cats being
the most sensitive and guinea-pigs, rats, mice, and rabbits showing
more resistance, in that order. The LD50 was increased threefold
by pretreating animals with small doses of carbaryl. The compound
is slightly toxic after acute dermal administration, the LD50
being > 2000 mg/kg bw. No LC50 for acute exposure by inhalation was
available, but the effects observed in dogs, cats, and rats exposed to
carbaryl dust or to formulations of carbaryl were typical of those of
cholinesterase inhibition. In cats, exposure for 6 h to a dust
concentration of only 20 mg/m3 inhibited serum and erythrocyte
cholinesterase activity. Many of the known metabolites of carbaryl
are much less toxic than the parent compound. None of the metabolites
with a methylcarbamate moiety was appreciably more active as a
cholinesterase inhibitor than carbaryl itself.
(b) Short-term toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that in a study of
cats the NOAEC was 16 mg/m3 after exposure for 120 days, on the basis
of cholinergic reactions at 30 mg/m3 after exposure for 30 days. In a
study in rats, no effects were observed after 90 days' exposure to
10 mg/m3.
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female CD1 mice were given diets
designed to provide carbaryl (purity, 99.3%) at concentrations of 0,
100, 1000, or 8000 ppm, equal to 0, 15, 150, and 1200 mg/kg bw per day
for males and 0, 18, 180, and 1400 mg/kg bw per day for females, for
two years. Haematological parameters and plasma, erythrocyte, and
brain cholinesterase activities were determined in 10 mice of each sex
per group at week 53 and at termination.
There were no treatment-related effects on mortality. A thin
appearance and hunched posture were noted in many females and some
males at the high dose during the first three to six weeks and the
last six months of the study. Some animals at this dose also had a
languid appearance, urine stains, rough coats, and opaque eyes, the
latter in females during the last three months. Body-weight gain was
impaired during the first two weeks of the study in mice at the high
dose, and the weights remained significantly lower than that of
controls throughout the study. At the end of the study, the body
weights of males and females at the high dose were 88 and 87% of those
of the respective controls, but the weight gain over the whole period
was only 62 and 68% of that of controls. Food consumption was markedly
depressed in animals of each sex at the high dose during the first
months of the study. It remained low in females throughout the study
and was again lower in males from week 78 of the study. Erythrocyte
counts, haemoglobin, and packed cell volume were decreased in females
at the high dose at week 53 and in males at the end of the study.
The platelet count was increased in females at the high dose.
Cholinesterase activity was inhibited in animals at the middle and
high doses. Significant decreases in erythrocyte acetylcholinesterase
were seen only at week 53 in males at the middle and high doses (78
and 70% of the control value, respectively). Significant, dose-related
decreases in brain acetylcholinesterase activity were seen in animals
of each sex at the middle and high doses at interim and terminal
sacrifice. At the end of the study, brain acetylcholinesterase
activity was only 60 and 66% of the control levels in males and
females at the high dose, respectively.
Decreases in the absolute and relative weights of lungs and ovary
(at interim sacrifice only) were seen in females at the high dose. The
absolute and relative weights of the livers (with gall-bladder) and
the relative weight of the kidneys were increased in animals of each
sex at the high dose. On histopathological examination, a dose-related
increase in the incidence and severity of intracytoplasmic protein-
like droplets was found in the urinary bladder of animals of each sex
at the middle and high doses. An increased incidence of uni- and/or
bilateral cataracts was found in high-dose males and females, and a
slight increase in the severity of extramedullary haematopoiesis
and the presence of pigment in the spleen were found at terminal
sacrifice. The incidence of renal tubular-cell neoplasia was increased
in high-dose males, that of hepatocellular neoplasia in high-dose
females, and that of vascular tissue neoplasia in all treated males
and in high-dose females (Table 2). Carbaryl was thus found to be
carcinogenic in mice. The NOAEL for non-neoplastic findings was
100 ppm, equal to 15 mg/kg bw per day, on the basis of inhibition
of erythrocyte and brain acetylcholinesterase activity and
histopathological changes in the urinary bladder (Hamada, 1993a).
A re-examination of the histological slides of the liver and
kidneys from mice in the control and high-dose groups at interim
sacrifice, performed by two pathologists from the Rhône Poulenc
Company (Debruyne & Irisarri, 1996), showed no microscopic changes in
the liver or kidney. In a position paper, Klonne (1995) compared the
incidence of vascular tumours (mostly in the liver and spleen) in the
male mice in this study with those in historical controls in the
laboratory that performed the experiment and in several other
laboratories. The tumour incidences were clearly increased over
the mean values for historical controls; but, in the performing
laboratory, data were available only from 18-month studies. When the
incidences were compared with the ranges in historical controls in
studies of up to two years' duration, the incidences of vascular
tumours in the livers of animals at the low and middle doses were just
below the upper limit for the controls, and the incidences of vascular
tumours in the spleens of animals at the middle dose were outside the
range. The incidences among animals at the high dose were outside the
range for vascular tumours in the liver and within the range for
tumours in the spleen.
Table 2. Incidences of tumours in groups of 80 mice fed diets containing carbaryl
Tumour Males Females
0 ppm 100 ppm 1000 ppm 8000 ppm 0 ppm 100 ppm 1000 ppm 8000 ppm
Renal tubular-cell adenoma 0 0 0 3 0 0 0 1
Renal tubular-cell carcinoma 0 0 0 3 0 0 0 0
Hepatocellular adenoma 12 7 13 8 0 0 1 7
Hepatocellular carcinoma 6 7 3 8 1 1 1 3
Hepatoblastoma 0 0 0 0 0 0 0 1
Haemangioma 0 1 1 3 1 0 1 0
Haemangiosarcoma 2 5 9 7 2 3 3 9
No. of vascular 2 6 10 10 3 3 4 9
tumour-bearing animals
All vascular tumours 2 9 13 18 5 6 5 10
Rats
Groups of 80 male and 80 female Sprague-Dawley rats were given
diets designed to provide concentrations of carbaryl (purity, 99%) of
0, 250, 1500, or 7500 ppm, equal to 0, 10, 60, and 350 mg/kg bw per
day for males and 0, 13, 79, and 480 mg/kg bw per day for females, for
two years. Haematology, clinical chemistry, cholinesterase activity in
plasma and erythrocytes, and urinary parameters were studied in 10
rats of each sex at weeks 26, 52, 78, and 104. Brain acetylcholin-
esterase activity was determined at interim sacrifice and at the end
of study in 10 rats of each sex. Two additional groups of 10 rats of
each sex were treated with 0 or 7500 ppm for 52 weeks and were then
kept for a recovery period of four weeks, when haematology, clinical
chemistry, and cholinesterase activity were studied. There were no
treatment-related effects on mortality, and survival was increased in
females at the high dose; however, an increased incidence of alopecia
on the limbs was found in these animals, and an increased prevalence
of urine stains was seen in animals of each sex. The mean body weights
were significantly depressed in animals at the high dose at all
intervals analysed and in those at the middle dose at many intervals.
At the end of study, the body weights of males and females at the high
dose were only 5 and 55% of those of the respective controls, and
those of animals at the middle dose were 94% and 88% of the control
values, respectively. Food consumption was markedly lower in the
high-dose group. The numbers of animals with cataracts were increased
in the high-dose group, with incidences of 4, 6, 7, and 12 males and
3, 2, 4, and 10 females at the control, low, middle, and high doses,
respectively.
There were no treatment-related findings in haematological
parameters. Increased serum cholesterol and urea nitrogen were found
in high-dose females. Plasma cholinesterase activity was depressed in
animals of each sex at the high dose, to 58-73% of the control value
in males and 43-54% in females. Erythrocyte acetylcholinesterase
activity was also significantly decreased, to 63-81% of the control
value in males at the high dose and 62-75% in females; in females at
the middle dose, the activity was 74-81% of the control value, whereas
in males at this dose the depression was found only at weeks 52
(81%) and 78 (77%). Significant, dose-related decreases in brain
acetylcholinesterase activity were found at both 52 and 104 weeks in
high-dose males and middle- and high-dose females. Males at the middle
dose showed a significant depression only at the 52-week interim
sacrifice. Changes seen on urinalysis in animals at the high dose
included an increased incidence of dark urine, decreased urine volume
in females (at week 52), and erythrocytes in urine and occult blood in
males (at weeks 78 and 104). The relative weights of lungs, brain,
kidney, and liver were significantly increased in animals at the high
dose at the interim and terminal sacrifices; testicular weight was
also increased in those at the high dose. The increase in relative
liver weight was dose-related, but a significant increase at the
middle dose was found only in the females at interim sacrifice.
Gross pathological examination of animals at the high dose
revealed increased incidences of masses in the urinary bladder, pale
areas in the lungs, and dark areas in the glandular stomach (only in
males). A decreased incidence of mammary masses was noted in females
at this dose, with 65% in controls, 57% at the low dose, 64% at the
middle dose, and 41% at the high dose. Histopathological evaluation
of tissues taken from rats at interim sacrifice revealed hyaline
inclusions in the livers of males at the high dose. At the end of the
study, the following non-neoplastic findings were observed in animals
at the high dose: an increased incidence of thyroid hypertrophy (8/70
in males and 33/70 in females, in comparison with 1/70 and 4/70 in
controls, respectively); eosinophilic hepatocellular alterations and
increased pigment in females, hepatocyte hypertrophy in animals of
each sex, and intracytoplasmic hyaline inclusions in males; an
increased incidence of transitional epithelial hyperplasia in the
kidneys of males, and squamous metaplasia, a high mitotic index, and
cytological atypia in the urinary bladders of animals of each sex, all
associated with the carcinomas found; an increased incidence of
alveolar foamy macrophages and focal pneumonitis; and an increased
incidence of degeneration of the sciatic nerve and adjacent skeletal
muscles. The neoplastic findings in thyroid, liver, and urinary
bladder are summarized in Table 3. Nearly all of the animals with
transitional-cell neoplasms in the bladder also had extensive
hyperplasia, indicating the preneoplastic nature of this change. Males
at the high dose also had an increased incidence of transitional-cell
hyperplasia in the kidney; a single carcinoma was found. The incidence
of benign interstitial-cell tumours in the testis was increased in
animals at the high dose (5/70; 2/70 in controls).
After one year of exposure and the recovery period of four weeks,
the food consumption of animals at the high dose improved and the
animals gained more weight. The effects on cholinesterase activity
were reversible, as were the histological changes in the liver. The
relative weights of the lungs, brain, kidney, and liver, however,
remained increased. Carbaryl thus induced tumours at 7500 ppm, a
dose that exceeds the maximum tolerated dose (MTD). The NOAEL for
non-neoplastic findings in this study was 250 ppm, equal to 10 mg/kg
bw for males per day, on the basis of inhibition of erythrocyte and
brain acetylcholinesterase and the decrease in mean body weight
(Hamada, 1993b).
A re-examination of the histological slides of the liver, kidney,
urinary bladder, and thyroid of rats of all doses at interim sacrifice
and of the controls and those at the high dose after recovery,
performed by two pathologists from Rhône Poulenc Company (Debruyne &
Irisarri, 1996), revealed the presence of further microscopic changes
in animals at the high dose in the bladder (irreversible epithelial
hyperplasia in males and females), kidney (reversible pelvic
urothelial hyperplasia in males), thyroid (reversible follicular
hypertrophy in males), and liver (reversible hepatocellular
hypertrophy in males and females).
Table 3. Incidences of tumours in groups of 70 rats fed diets containing carbaryl
Tumour Males Females
0 ppm 250 ppm 1500 ppm 7500 ppm 0 ppm 250 ppm 1500 ppm 7500 ppm
Thyroid follicular-cell adenoma 0 2 0 8 0 0 0 1
Thyroid follicular-cell carcinoma 0 0 0 1 1 0 0 0
Hepatocellular adenoma 1 1 2 1 1 0 3 7
Hepatocellular carcinoma 0 2 2 1 0 0 0 0
Transitional-cell hyperplasia 9 8 10 54 6 6 6 56
Transitional-cell papilloma 0 à 0 13a 1 0 0 7
Transitional-cell carcinoma 0 0 0 11 0 0 0 6
a Includes one squamous-cell papilloma
In the Environmental Health Criteria monograph on carbaryl (WHO,
1994), the results of several long-term studies in mice were
summarized, one of which was a report of the data obtained at interim
sacrifice in the study of Hamada (1993a). In another study, no effects
were observed at the highest level tested (400 ppm). Seven further
studies of carcinogenicity in mice involving different strains, routes
of administration, doses, dose schedules, and lengths of exposure and
observation were considered unsuitable for evaluation of carcinogenic
potential; only one showed a marginal response.
Several short- and long-term studies in rats were available. The
most recent report presented the interim results of the long-term
study of Hamada (1993b). In two older dietary studies, lasting 96 days
and two years, the most obvious effects were on the kidney. The NOAEL
in these studies was 200 ppm, equal to 7.9 mg/kg bw per day, with
effects on the kidney at 400 ppm. In two one-year studies of gavage
(for which only summaries were available), effects were seen on the
thyroid and on male and female reproductive organs and/or function at
doses > 5 mg/kg bw per day. The NOAEL was 2 mg/kg bw per day. Two
studies of carcinogenicity in rats were available. In a study in which
carbaryl was administered orally or by a single subcutaneous
implantation, fibrosarcomas of the skin were observed; the other
(dietary) study gave negative results. Neither was considered suitable
for evaluation of carcinogenic potential.
Three one-year studies in dogs were available. In a study in
which carbaryl was given in capsules, effects on the kidney were found
at 7.2 mg/kg bw per day, but no effects were observed at 1.8 mg/kg bw
per day (approximately 100 ppm in the diet). In the two dietary
studies, the NOAEL was 125 ppm, equivalent to 3.1 mg/kg bw per day, on
the basis of effects on liver weight and inhibition of erythrocyte and
brain acetylcholinesterase activity at 400 ppm.
(d) Reproductive toxicity
No new information had become available. The summaries in the
Environmental Health Criteria monograph on carbaryl (WHO, 1994),
although extensive, could not always be used to derive NOAELs for
various aspects of the reproductive toxicity of carbaryl. Additional
information was therefore obtained for some studies, from either the
original papers or reviews.
In two five-day studies of male mice exposed orally, no effects
were found on the testis or accessory glands at a dose of 34 mg/kg bw
per day; at 68 mg/kg bw per day, androstenedione synthesis was
decreased.
Three three-generation studies of reproductive toxicity in rats
were summarized. In the first, in which 0, 2000, 5000, or 10 000 ppm
carbaryl were administered in the diet, impaired fertility, reduced
postnatal survival, and reduced postnatal growth were observed at
5000 ppm; no effects were seen at 2000 ppm, equivalent to 125 mg/kg bw
per day. There was no clear NOAEL in this study, since the parameters
were wrongly defined. In a second dietary study, at doses of 0, 7, 25,
100, and 200 mg/kg bw per day, the NOAEL was 100 mg/kg bw per day, on
the basis of decreased maternal weight at 200 mg/kg bw per day. There
were no reproductive effects. In a study in which rats were treated by
gavage at doses of 0, 3, 7, 25, or 100 mg/kg bw per day, the NOAEL was
25 mg/kg bw per day, on the basis of decreased maternal weight and
mortality and effects on litter size and viability at the high dose.
In a study in which special attention was paid to effects on
reproductive parameters, male and female rats were exposed by gavage
for one month to doses of 1-50 mg/kg bw per day. Adverse effects were
observed on male reproductive cells; furthermore, increased embryonic
and fetal deaths, decreased numbers of implantations, and a prolonged
oestrus cycle were observed. This study was also described by Cranmer
(1986), who reported that the effects on reproductive parameters were
seen only at 1, 5, 10, and 20 mg/kg bw per day. He also noted that
only the change in the number of tubules containing spermatogonia
was seen at all doses, and most of the other treatment-related
histological and functional changes in the testis occurred at doses
> 5 mg/kg bw per day. Dose-response relationships were not seen for
the other effects on litter parameters or for the prolonged oestrus
cycle, limiting interpretation of the study. This and other studies of
reproductive toxicity were considered of dubious value for risk
assessment as they suffered from various shortcomings in study design.
A three-generation study of reproductive toxicity in which
gerbils were exposed daily via the diet to concentrations of
2000-10 000 ppm was not considered suitable for establishing a NOAEL;
however, adverse effects on various reproductive parameters were seen.
(e) Developmental toxicity
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) was supplemented by
additional information from the original papers and reviews. All of
the studies suffered from small group sizes and some deficiencies in
comparison with currently acceptable scientific standards.
Two of five studies in mice were used for evaluation, in which
animals were exposed by gavage to carbaryl on days 6-15 of gestation;
in the second study, groups were also exposed only on day 8 or 12. In
the first study, with doses of 0, 100, and 150 mg/kg bw per day, the
NOAEL was 100 mg/kg bw per day on the basis of maternal toxicity
(ataxia, lethality) and litter resorption at 150 mg/kg bw. In one
group exposed via the diet to 5560 ppm, a decrease in fetal weight was
observed. In the second study, with doses of 0, 100, 150, and
200 mg/kg bw per day, the NOAEL was 150 mg/kg bw per day, on the
basis of maternal death and fetal toxicity (weight reductions and
indications of growth retardation) at 200 mg/kg bw per day.
Three of six studies in rats were used for evaluation. In the
first study, groups of six rats were fed diets containing carbaryl on
days 1-5, 5-15, or 1-21 of gestation. The NOAEL for maternal toxicity
was 100 mg/kg bw per day, on the basis of reduced weight gain in dams
at 500 mg/kg bw per day when exposed on gestation days 5-15 or 1-21.
No adverse fetal effects were seen. The original papers (Weil &
Carpenter 1965, 1966) reported that the doses used were 0, 2.5, 10,
20, 100, and 500 mg/kg bw per day and that fetuses underwent only
skeletal examination. In addition to the effects summarized above, the
authors reported reduced postnatal survival in the pups of dams
exposed to 500 mg/kg bw per day on days 1-21. In the second study,
groups of 10 rats received oral doses of 200 or 300 mg/kg bw or an
intraperitoneal dose of 40 mg/kg bw on single or multiple days of
gestation. The only effect seen was a reduction in fetal weight in
some groups. In the third study, groups of six or seven rats received
diets containing carbaryl at doses of 0, 1, 10, or 100 mg/kg bw per
day three months before and during gestation. The NOAEL for maternal
toxicity was 10 mg/kg bw per day, on the basis of reduced weight gain
at 100 mg/kg bw per day. No adverse fetal effects were seen. This
study was also described by Cranmer (1986), who reported a slight
reduction in the numbers of implantations and live fetuses at 100
mg/kg bw per day.
One of two studies in guinea-pigs was used for evaluation. Groups
of four to nine animals were exposed to 0, 100, 200, or 300 mg/kg bw
per day in the diet or 0, 50, 100, or 200 mg/kg bw per day orally.
Reduced maternal weight gain was observed at 200 mg/kg bw per day, but
there were no effects on embryos or fetuses.
One of three studies in rabbits was used for evaluation. Groups
of 15-20 animals were given oral doses of 0, 150, or 200 mg/kg bw per
day on days 6-18 of gestation. There was no NOAEL for maternal
toxicity since a reduction in weight gain was observed in both treated
groups. A significant increase in umbilical hernia was observed in
fetuses at 200 mg/kg bw per day.
In two studies in dogs, animals were fed diets containing
carbaryl at doses of 3.1-50 mg/kg bw per day or 2-12.5 mg/kg bw per
day during gestation. Various birth defects were observed in the pups
at doses > 5 mg/kg bw per day. Maternal toxicity was observed at
all doses.
In pigs fed doses of 4-32 mg/kg bw per day in the diet, the
effects noted (prenatal lethality and malformations) were not
consistent across the studies. Another study in pigs was considered
unsuitable for evaluation.
One of two studies in monkeys showed an increased rate of
abortions after oral treatment with 2 or 20 mg/kg bw per day
throughout gestation. No adverse effects were noted in the second
study in which the doses were 0.2-32 mg.kg bw per day orally on days
20-38 of gestation.
(f) Genotoxicity
The results of studies of chromosomal aberrations in rats and
micronucleus formation in mice treated with carbaryl in vivo are
summarized in Table 4. Negative results were obtained in both studies
at doses at which limited signs of toxicity were seen; however, there
was no evidence that the test compound had reached the target organ,
as no cytotoxicity was found.
In a study of DNA binding, two groups of four male CD mice were
treated by oral intubation with 14C-carbaryl (labelled in the
naphthyl group) in 0.5% aqueous carboxymethylcellulose at a dose of
75 mg/kg bw (8 mCi/kg bw). One group was pretreated with carbaryl
(purity, 99.6%) at 8000 ppm (equal to 1900 mg/kg bw per day) in the
diet for 14 days. Four mice served as controls. Urinary excretion and
exhalation of carbon dioxide were measured over 24 h in one pretreated,
one non-pretreated, and one control mouse. Animals were sacrificed 24 h
after administration of radiolabelled material, and the liver, kidneys,
and urinary bladder were removed; however, only the liver was used
for determining DNA and protein binding. There was no significant
exhalation of 14C-carbon dioxide, and about 30% of the administered
dose was excreted in the urine of both treated groups. The pretreated
group had lower body weights and increased relative liver weights at
the end of treatment. Binding of 14C-carbaryl to chromatin protein
was found in both treated groups, resulting in specific radioactivities
of 340-537 dpm/mg protein (7-11 pmol/mg protein). As there was no
significant radioactivity in purified DNA, in the presence or absence
of pretreatment, covalent binding of the 14C-naphthyl label does not
seem to have occurred (Sagelsdorff, 1994).
Table 4. Results of tests for the genotoxicity of carbaryl in vivo
End-point Test system Concentration Purity Results Reference
(mg/kg bw) (%)
Micronucleus formation Male and female 50, 100, 200 orally for 99.9 Negativea Marshall (1996)
CD-1 mice (5 per 2 daysb; killed at 24 or
dose), bone-marrow 48 h; vehicle, 0.5%
cells carboxymethylcellulose
Chromosomal aberration Male and female Single dose of 30, 60, 99.7 Negativea McEnaney (1993)
Sprague-Dawley 120c; killed at 6, 24,
rats (5 per dose), 48 h; vehicle, 0.25%
bone-marrow cells carboxymethylcellulose
a Positive controls yielded positive results
b Clinical signs of toxicity (lethargy, slight weight loss) at highest dose; no change in polychromatic:normochromatic
erythrocyte ratio. Doses based on the results of a preliminary test in which one of six animals at 300 mg/kg bw died.
c Clinical signs of toxicity (lethargy, tremors) observed at the highest dose; no depression of mitotic index. Doses based on
the results of a preliminary test in which the LD50 was 231 mg/kg bw
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that carbaryl has been evaluated for mutagenicity in a
number of tests in vitro and in vivo, in bacterial, yeast, plant,
insect, and mammalian systems, with a variety of end-points. It
concluded that carbaryl does not damage DNA. Reports of induction of
mitotic recombination, gene conversion, and unscheduled DNA synthesis
in prokaryotes (Haemophilus influenzae, Bacillus subtilis) and
eukaryotes ( Saccharomyces cerevisiae, Aspergillus nidulans, cultured
human lymphocytes, and rat hepatocytes) in vitro have not been
confirmed. Negative results were obtained in tests for gene mutations
in all but two bacterial assays. Although several studies of gene
mutation were conducted in mammalian cells in vitro, only one
equivocally positive result was obtained, in a study that had several
shortcomings and which has not been confirmed. Chromosomal damage has
been reported in human, rat, and hamster cells and in plants treated
in vitro with high doses of carbaryl. No such effects have been
observed in mammalian tests in vivo, even at doses as high as
1000 mg/kg bw. Carbaryl induces disturbances in the spindle fibre
mechanism in plant and mammalian cells in vitro, but the relevance
of the assays in plants for humans is unclear. The Environmental
Health Criteria monograph concluded that the available database did
not indicate that carbaryl induces genetic changes in somatic or
germinal tissues of humans.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
No new information had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported that carbaryl is
not or only weakly irritating to the skin and is weakly irritating to
the eye. The available studies indicated that carbaryl has little or
no sensitizing potential.
(ii) Neurotoxicity
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported that the effects
of carbaryl on the central nervous system had been studied in rats
and monkeys treated by intraperitoneal, intramuscular, or oral
administration or by inhalation. Changes in motor activity, working
memory, and behaviour were seen. Dietary exposure to doses of
10-20 mg/kg bw per day for 50 days was reported to disrupt learning
and performance in rats. In a small study on pigs, administration of
carbaryl in the diet at 150 mg/kg bw per day for 72-82 days was
reported to produce a number of neuromuscular effects. Reversible leg
weakness was noted in chickens given high doses of carbaryl, but no
evidence of demyelination was observed in the brain, sciatic nerve, or
spinal cord sections examined microscopically. Similar effects were
not observed in long-term studies in rodents. The effects of carbaryl
on the nervous system are primarily related to inhibition of
cholinesterase activity and are usually transitory.
(iii) Immunotoxicity
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) noted that several studies
in mice, rats, rabbits, and guinea-pigs, mostly treated orally, showed
that carbaryl administered at doses that do not cause overt clinical
signs has a variety of non-life-threatening effects on both cellular
and humoral immunity. Many of the effects were detected at doses close
to the LD50. A lack of consistency and sometimes overt contradiction
between the results of several of these studies precludes definition
of the immunotoxic mechanism. Life-time exposure to carbaryl did not
increase the occurrence of disease in rats or mice, and no enhancement
of viral infections was found, even at doses close to the LD50. Most
of the studies on mice and rabbits at doses that permitted survival
did not show significant effects on the immune system. Vital
enhancement has been demonstrated in vitro in a number of studies
including prior incubation with carbaryl. Inhibition of human serum
complement activity and interleukin-2-driven proliferation of large
granular lymphocytes have also been found in vitro.
(iv) Haematological effects
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) reported effects on
coagulation in rats, rabbits, and dogs in vivo and in studies in
vitro, but the direction of the effect is unclear. In glucose-
6-phosphate dehydrogenase-deficient sheep erythrocytes, carbaryl
produced a dose-dependent increase in methaemoglobin formation. Human
serum albumin reacted in vitro with the ester group of carbaryl.
Carbaryl binds free blood amino acids.
(v) Effects on the endocrine system
No new data had become available. The Environmental Health
Criteria monograph on carbaryl (WHO, 1994) concluded from several
experiments in vivo and in vitro that carbaryl increases the
gonadotropic function of the hypophysis of rats.
(vi) Studies with N-nitrosocarbaryl
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) noted that carbaryl is a secondary amine and is therefore
capable of nitrosation in the presence of nitro donor groups, such as
sodium nitrate, to give a nitrosamide. A condition of such nitrosation
is an acidic pH (< 2), such as that found in the human stomach;
however, N-nitrosocarbaryl is not stable at this pH; its maximal
stability is at pH 3-5, at which no significant amount of carbaryl can
be nitrosated. Carbaryl was nitrosated in several studies, in vitro
as well as in vivo, in guinea-pigs, in which the gastric acidity is
similar to that of humans.
N-Nitrosocarbaryl induced local tumours in rats, consisting of
sarcomas at the site of injection and forestomach squamous-cell
carcinomas, after oral administration. This local carcinogenic effect
and the lack of systemic carcinogenicity characterize the compound as
a directly acting alkylating agent. Given the human chemistry of
carbaryl, the risk of carcinogenic effects of N-nitrosocarbaryl for
humans after exposure to carbaryl can be considered negligible.
N-Nitrosocarbaryl can induce mitotic recombination and gene
conversion in prokaryotes (H. influenzae and B. subtilis) and
eukaryotes (S. cerevisiae) in vitro and gives positive results in
Escherichia coli spot tests. It also binds to DNA, causing
alkali-sensitive bonds and single-strand breakage. It is not
clastogenic in vivo in bone marrow and germ cells, even at highly
toxic doses.
3. Observations in humans
An epidemiological study of total and cause-specific mortality
among employees exposed to carbaryl at a production plant was based on
information obtained in 1988 on the vital status and cause of death of
all individuals who were first hired between the start-up of the
carbaryl unit in 1960 through 1978. Employees hired after 1978 were
not included in the study. Three categories of workers exposed to
carbaryl were defined: those involved in production, maintenance
employees, and those working in packaging and distribution. A total of
448 employees contributing 7532 person-years to the analysis were
available, representing the combined number of years in which these
employees were followed through 1988. Mortality was measured in terms
of standardized mortality ratios (SMRs), which are the ratios of the
observed numbers of death among members of a cohort to the number
expected, on a year-specific and age-adjusted basis. The 25 deaths
identified in this cohort resulted in elevated SMRs for cancer of the
pancreas, unspecified cancer, and cancers of the brain and other parts
of the nervous system. In the first two categories, the excess was
slight and based on only one death. In the last category, the SMR was
based on two deaths due to tumours of different histological origins,
reducing the possibility that the two malignancies were caused by the
same exposure. Furthermore, in all three categories the confidence
intervals were wide, indicating a relatively imprecise SMR estimate
and reflecting the small sample size on which it is based (Pastides,
1993).
The Environmental Health Criteria monograph on carbaryl (WHO,
1994) summarized several cases of poisoning. The clinical picture is
dominated by symptoms of inhibition of cholinesterase activity. Signs
of poisoning develop quickly after absorption and disappear rapidly
after exposure ends.
In controlled studies of human volunteers, single doses of
< 2 mg/kg bw were well tolerated. A single dose of 250 mg (about
2.8 mg/kg bw) produced moderate symptoms of cholinesterase inhibition
(epigastric pains and sweating) within 20 min. Complete recovery was
seen within 2 h of treatment with atropine sulfate. Two groups of
human volunteers given carbaryl at doses of 0.06 or 0.13 mg/kg bw for
six weeks underwent physical examinations, removal of bromosulphthalein
from blood, electroencephalography, routine blood and urinalysis, and
measurement of cholinesterase in plasma and erythrocytes. No inhibition
of cholinesterase activity was observed, and no changes were seen in
the group receiving the low dose. The only finding at the high dose was
an increase in the ratio of amino acid nitrogen to creatinine in the
urine, which may represent a decrease in the ability of the proximal
convoluted tubule to reabsorb amino acids; this change was reversible.
The NOAEL was 0.06 mg/kg bw.
In cases of occupational overexposure to carbaryl, mild symptoms
are observed long before a dangerous dose is absorbed. No local
irritating effect is usually seen, although skin rash after accidental
splashing with carbaryl formulations has been described.
Reports of the effects of carbaryl on sperm count and changes in
sperm morphology in plant workers are conflicting. No adverse effects
on reproduction have been described.
Comments
Carbaryl is rapidly and almost completely absorbed after oral
administration. Excretion is rapid and occurs predominantly via the
urine; enterohepatic cycling of carbaryl metabolites is also
considerable. There were no significant dose-related or sex-specific
differences in elimination patterns, and there was no evidence of
bioaccumulation. Dermal absorption in rats was slow; after 24 h,
16-34% of the administered radiolabel had been absorbed. Higher doses
were less readily absorbed. In volunteers, 45% of a dose applied to
the skin in acetone was absorbed within 8 h. Carbaryl was rapidly
absorbed in the lungs.
The metabolism of carbaryl has been studied in various mammals,
including humans. The principal metabolic pathways are ring
hydroxylation, hydrolysis, and conjugation. There were no species
differences. The main metabolite in humans is 1-naphthol. The
hydrolysis product, N-methyl carbamic acid, spontaneously decomposes
to methylamine and carbon dioxide. The methylamine is later converted
to carbon dioxide and formate, the latter being excreted mainly in the
urine. Carbaryl metabolites are also found at small percentages of the
absorbed doses in saliva and milk.
Carbaryl is moderately toxic after acute oral administration, the
LD50 in rats being 225-721 mg/kg bw. Interspecies differences in
toxicity were found, cats (LD50, 150 mg/kg bw) being the most
sensitive species. The LD50 was increased threefold when animals were
pretreated with small doses of carbaryl. The compound is slightly
toxic after acute dermal administration, with an LD50 > 2000 mg/kg
bw. No LC50 for acute exposure by inhalation was available, but the
effects observed in dogs, cats, and rats exposed to dusts or
formulations of carbaryl were typical of those resulting from
inhibition of cholinesterase activity. In cats exposed to carbaryl
dust for 6 h, a concentration of 20 mg/m3 inhibited cholinesterase
activity in plasma and erythrocytes. Carbaryl was weakly irritating to
the eye but not the skin and was not considered to be a sensitizer.
WHO has classified carbaryl as 'moderately toxic' (WHO, 1996).
After oral administration of carbaryl in capsules to dogs at a
dose of 0.45, 1.8, or 7.2 mg/kg bw per day for one year, slight
effects were observed on the kidney at 7.2 mg/kg bw per day; the NOAEL
was 1.8 mg/kg bw per day. In two studies in which dogs were fed diets
containing carbaryl at 20-125 ppm for five weeks and 125-1250 ppm for
one year, the NOAEL was 125 ppm, equivalent to 3.1 mg/kg bw per
day, on the basis of effects on liver weight and inhibition of
acetylcholinesterase activity in erythrocytes and brain at 400 ppm.
In cats exposed to carbaryl by inhalation, cholinergic signs were
observed at 30 mg/m3 after exposure for 30 days. The NOAEL was
16 mg/m3 for 120 days. In a study in rats, no effects were observed
after exposure to 10 mg/m3 for 90 days.
Several studies of long-term toxicity or carcinogenicity in mice
cited in EHC 153 were considered to be unsuitable for evaluation of
carcinogenicity by either the Environmental Health Criteria Task Force
or the present Meeting, although they were suitable for assessing
long-term toxicity. In a recent study of carcinogenicity, mice were
given diets providing 0, 100, 1000, or 8000 ppm carbaryl for
104 weeks. Tumours were observed in the liver in females and the
kidney in males, and vascular tumours were found in animals of each
sex at the highest dose, which exceeded the maximum tolerated dose
(MTD). In male mice, increases in the incidences of vascular tumours
were also seen at the two lower doses; after considering all of the
available data, the Meeting could not identify an NOAEL for this
neoplastic lesion. The NOAEL for non-neoplastic lesions was 100 ppm
(equal to 14.7 mg/kg bw per day), on the basis of inhibition
of erythrocyte and brain acetylcholinesterase activity and
histopathological changes in the urinary bladder at 1000 ppm. This
NOAEL is consistent with the results of the earlier studies. The
Meeting concluded that the compound is carcinogenic in mice.
In several studies cited in EHC 153, carbaryl was administered in
the diet of rats for 96 days to two years. The most obvious effects
were in the kidney at doses > 400 ppm. In two one-year studies in
rats treated by gavage, effects on the thyroid and on male and
female reproductive organs and/or function were observed at doses
> 5 mg/kg bw per day; the NOAEL was 2 mg/kg bw per day. None of
these studies was considered suitable for evaluating carcinogenicity.
In a recent study of long-term toxicity and carcinogenicity, rats
were fed diets containing 0, 250, 1500, or 7500 ppm carbaryl for 104
weeks. In animals at the highest dose, which exceeded the MTD, tumours
were found in the thyroid in males, in the liver in females, and in
the urinary bladder in animals of each sex. The NOAEL for non-
neoplastic findings in this study was 250 ppm, equal to 10 mg/kg bw
per day, on the basis of inhibition of erythrocyte and brain
acetylcholinesterase activity and a decrease in mean body weight at
1500 ppm. This NOAEL is consistent with the results of earlier dietary
studies. The Committee concluded that carbaryl is carcinogenic in rats
only at levels that exceed the MTD.
The available studies on reproductive toxicity were conducted
some time ago and had some deficiencies in relation to currently
acceptable scientific standards. In three-generation studies, dietary
administration of carbaryl to rats induced reproductive effects
(impaired fertility and reduced postnatal survival and growth) at
doses > 2000 ppm (equal to 125 mg/kg bw per day); a dose of 100 mg/kg
bw per day did not induce maternal toxicity. When carbaryl was
administered by gavage, maternal toxicity was not observed at 25 mg/kg
bw per day, but both maternal and reproductive toxicity (reduced
litter size and viability) were found at 100 mg/kg bw per day. The
Meeting recommended that a new two-generation study of reproductive
toxicity be carried out in rats, with special attention to the male
reproductive system since effects on this system were observed in some
long-term studies of toxicity at gavage doses significantly lower than
those evaluated in the dietary studies of reproductive toxicity.
The available studies on developmental toxicity suffered from
small group size and had some deficiencies in relation to currently
acceptable scientific standards. In two studies in mice, the NOAEL for
maternal toxicity was 100 mg/kg per day; at 150 mg/kg bw per day,
increased litter resorption was found. In rats, administration of
carbaryl in the diet for part or all of the gestation period resulted
in maternal toxicity at 100 mg/kg bw per day. No overt signs of
fetotoxicity were seen at this dose. In a study in which rats
were exposed to carbaryl by gavage and then mated, maternal and
embryotoxicity were observed at 100 mg/kg bw per day; no effects were
seen at 10 mg/kg bw per day. In guinea-pigs, administration of
carbaryl during gestation in the diet or by gavage resulted in an
NOAEL for maternal toxicity of 100 mg/kg bw per day. No embryo- or
fetotoxicity was observed at 300 mg/kg bw, the highest dose tested. In
rabbits, teratogenic effects were reported after administration of
200 mg/kg bw per day orally; maternal toxicity was also seen at this
dose. In two studies in dogs, maternal toxicity (dystocia, at
parturition only) was observed at a dose of 3.1 mg/kg bw per day.
A variety of birth defects was found after exposure to doses
> 5 mg/kg bw per day. Thus, the LOAEL for maternal toxicity was
3.1 mg/kg bw per day, and this was the NOAEL for birth defects in the
offspring.
The Meeting concluded that carbaryl induces developmental
toxicity, manifested as deaths in utero, reduced fetal weight, and
malformations, but only at doses that cause overt maternal toxicity.
The shortcomings of these studies made them inadequate for identifying
NOAELs for developmental toxicity that could be used for assessing
risk under conditions of exposure other than in the diet.
Carbaryl has been adequately tested for genotoxicity in a series
of assays in vitro and in vivo. While chromosomal aberrations have
been induced in vitro and carbaryl has been shown to disturb spindle
fibre mechanisms in vitro, there is no evidence from well-conducted
experiments that carbaryl is clastogenic in vivo. The Meeting
concluded that carbaryl is not genotoxic.
The effects of carbaryl on the nervous system are primarily
related to cholinesterase inhibition and are usually transitory.
Dietary exposure to doses of 10-20 mg/kg bw per day for 50 days was
reported to disrupt learning and performance in rats. In chickens
given high doses of carbaryl, there was no histological evidence of
neurotoxicity.
In controlled studies in volunteers, single oral doses of
< 2 mg/kg bw were well tolerated. A single oral dose of 250 mg (about
2.8 mg/kg bw) produced moderate cholinergic symptoms. In volunteers
given repeated daily oral doses over six weeks, the NOAEL was
0.06 mg/kg bw per day, on the basis of an increased ratio of amino
acid nitrogen to creatinine in the urine at a dose of 0.13 mg/kg bw
per day. This effect may represent a decrease in the ability of the
proximal convoluted tubule to reabsorb amino acids. The change was
reversible. No inhibition of plasma or erythrocyte acetylcholin-
esterase activity was observed.
An epidemiological study on carbaryl production workers employed
between 1960 and 1978 showed no increase in cancer mortality.
An ADI of 0-0.003 mg/kg bw was established on the basis of the
LOAEL of 15 mg/kg bw per day in the study of carcinogenicity in mice,
using a safety factor of 5000, which includes an extra safety factor
of 50 to account for the presence of vascular tumours in male mice at
all doses tested. The resulting ADI provides an adequate margin of
safety, taking into account the LOAEL in the study of developmental
toxicity in dogs and the uncertainties about the effects on the male
reproductive system.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: NOAEL not identified. Lowest effective dose: 100 ppm,
equal to 14.7 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Rat: 250 ppm, equal to 10 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
2 mg/kg bw per day (one-year study of toxicity)
Dog: NOAEL not identified. Lowest effective dose: 3.1 mg/kg
bw per day (study of developmental toxicity)
1.8 mg/kg bw per day (one-year study of toxicity)
Human: 0.06 mg/kg bw per day (six-week study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Study of reproductive toxicity with special attention to the
male reproductive system
2. Studies of teratogenicity in rats and rabbits
3. Completion of on-going studies to elucidate the mechanism of
tumour formation
4. Study of developmental neurotoxicity and/or screening for
acute or subchronic neurotoxicity
5. Follow-up of the epidemiological study in workers, taking
into consideration the latent period before development of
cancer
References
Cheng, T. (1994) Dermal absorption of 14C-carbaryl (80S) in male rats
(preliminary and definitive phases). Guideline No. 85-2. Unpublished
report No. HWI 6224-207, dated July 1994 from Hazleton Wisconsin,
Inc., USA. Supplied to WHO by Rhône Poulenc, Research Triangle Park,
North Carolina, USA.
Cheng, T. (1995) Dermal absorption of 14C-carbaryl (XLR Plus) in
male rats (preliminary and definitive phases). Guideline No. 85-2.
Unpublished report No. HWI 6224-206, dated January 1995, from Hazleton
Wisconsin, Inc., USA. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Cranmer, M.F. (1986) Carbaryl; a toxicological review and risk
analysis. Neurotoxicology, 7, 247-332.
Debruyne, E. & Irisarri, E. (1996) Carbaryl technical-chronic toxicity
studies in the rat (HWA Study No. 656-139) and the mouse (HWA Study
No. 656-138): evaluation of histological slides. Unpublished report
No. R&D/CRSA/TOX-HPA-4, dated March 1996 from Rhône Poulenc Agrochimie.
Supplied to WHO by Rhône Poulenc, Research Triangle Park, North
Carolina, USA.
Hamada, N.N. (1993a) Oncogenicity study with carbaryl technical in
CD-1 mice. Unpublished report No. HWA 656-138, dated May 1993 from
Hazleton Washington, Inc., USA. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Hamada, N.N. (1993b) Combined chronic toxicity and oncogenicity study
with carbaryl technical in Sprague Dawley rats. Unpublished report No.
HWA 656-139, dated August 1993 from Hazleton Washington, Inc., USA.
Supplied to WHO by Rhône Poulenc, Research Triangle Park, North
Carolina, USA.
Klonne, D.R. (1995) Carbaryl mouse historical control data. Position
paper dated November 1995. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Marshall, R. (1996) Carbaryl: induction of micronuclei in the bone
marrow of treated mice. Unpublished report No. CH 198/89-1052, dated
March 1996 from Corning Hazleton, United Kingdom. Supplied to WHO by
Rhône Poulenc, Research Triangle Park, North Carolina, USA.
McEnaney, S. (1993) Technical study to evaluate the chromosome
damaging potential of carbaryl by its effects on the bone marrow cells
of treated rats. Unpublished report No. 198/64, dated September 1993
from Hazleton Microtest/Hazleton UK. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Pastides H. (1993) Standardized mortality ratio analysis of employees
exposed to carbaryl at the Rhône Poulenc Institute, West Virginia
Plant. Report dated January 1993. Supplied to WHO by Rhône Poulenc,
Research Triangle Park, North Carolina, USA.
Sagelsdorff, P. (1994) Investigation of the potential of protein- and
DNA-binding of carbaryl. Unpublished report No. CB93/52, dated April
1994 from Ciba-Geigy Ltd. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Strubble, C.B. (1994) Metabolism of 14C-carbaryl in rats (preliminary
and definitive phases). Unpublished report No: HWI 6224-184, dated
August 1994, from Hazleton Wisconsin, Inc., USA. Supplied to WHO by
Rhône Poulenc, Research Triangle Park, North Carolina, USA.
Thomas, H. (1994) Liver cytochrome P-450 inducer phenotyping in the
male CD-1 mouse. Unpublished report No. CB94/23, dated October 1994,
from Ciba-Geigy Ltd. Supplied to WHO by Rhône Poulenc, Research
Triangle Park, North Carolina, USA.
Weil C.S. & Carpenter, C.P. (1965) Results of a three generation
reproduction study on rats fed Sevin in their diet. Unpublished report
No. 28-53, dated April 1965, from Mellon Institute, USA. Supplied to
WHO by Rhône Poulenc, Research Triangle Park, North Carolina, USA.
Weil C.S. & Carpenter, C.P. (1966) Evaluation of the teratogenic
potential of insecticide Sevin in rats. Unpublished report No. 29-49,
dated June 1966, from Mellon Institute, USA. Supplied to WHO by Rhône
Poulenc, Research Triangle Park, North Carolina, USA.
WHO (1994) Carbaryl (Environmental Health Criteria 153), International
Programme on Chemical Safety, Geneva.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
