
CARBARYL JMPR 1976
Explanation
Carbaryl has been evaluated by the Joint Meeting on a number
of occasions. An acceptable daily intake has been established and
maximum residue limits for carbaryl residues in many commodities
have been recommended (FAO/WHO 1965b, 1967b, 1968b, 1969b, 1970b,
1971b, 1974b, 1976b).
The Meeting was informed of two important applications for
carbaryl -
(a) for the control of cereal leaf beetle, armyworm and
grasshoppers affecting small grain crops, and
(b) for post-harvest application for the protection of stored
grain, particularly for the control of the lesser grain borer,
Rhyzopertha dominica, when used in conjunction with approved
organophosphorus grain protectant insecticides.
Extensive new data on these uses and on the level and fate of
residues resulting from such applications have been made available
to the Joint Meeting. These data have been evaluated and the
following monograph addendum is offered. It should be noted that
recommendations for a maximum residue limit for carbaryl in raw
grains were made in 1965 and 1966 by the Joint Meeting. These were
apparently overlooked when carbaryl was re-evaluated in 1968. In
the meantime carbaryl has been widely used on grain crops against
a wide variety of insect pests.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest
Cereal leaf beetle, armyworm, grasshoppers and locusts are
typical of many insect pests which often appear in plague numbers
and attack small grain crops such as wheat, oats, barley and rye
and inflict extensive damage. Carbaryl-based insecticides have
proved effective against all of the above pests in the larval
(nymph) and adult form. The rate of application ranges from 0.5 to
2 kg/ha depending upon the degree of infestation, density of
foliage and whether or not the pests are in advanced stages of
their life cycle.
The usual commercial formulation used for these purposes
include wettable powders and suspensions. Two or more applications
may be required but usually it is not necessary to apply carbaryl
sprays to small grain crops within 21 days of harvest.
Extensive documentation on these uses and on the performance
of carbaryl against the major pest species has been provided by
Union Carbide (1976a).
Post-harvest
Since the early 1960s malathion has been the main means of
protecting stored grain against the wide spectrum of insect pests
attacking a variety of stored grain in Australia, Argentina, South
Africa and many other countries. Recently, several strains of
insect pests have developed resistance to malathion, in particular
the lesser grain borer Rhyzopertha dominica, which is most
destructive, and the rust red flour beetle Tribolium castaneum,
which is most prevalent.
Recently, several candidate insecticides have been evaluated
as grain protectants in laboratory and field trials set up by the
Australian Wheat Board (Bengston et al, 1975, 1976a, 1976b).
However, no single chemical gave satisfactory control of all the
common pest strains screened. Among the grain protectant
insecticides found to be most valuable were pirimiphos-methyl,
fenitrothion and chlorpyrifos-methyl. Although these compounds
controlled most common strains including those resistant to
malathion, all failed to control Rhyzopertha dominica. The
synthetic pyrethroid bioresmethrin, however, was shown to be
specifically effective against Rhyzopertha dominica at economic
rates. Several mixtures of bioresmethrin with organophosphorus
insecticides were successfully evaluated, Currently, bioresmethrin
is recommended for use at a concentration of 1-2 mg/kg in admixture
with malathion, pirimiphos-methyl and fenitrothion. However, the
widespread use of bioresmethrin raises several problems including
cost, the possibility of resistance developing and limitations in
world production and supply. Consequently, there is a need for an
alternative to bioresmethrin. One alternative is carbaryl.
Extensive trials carried out in the U.S.A., South Africa,
England, the Philippines, Argentina, Brazil and Uruguay in 1963-64
showed carbaryl to be effective against a wide spectrum of stored
product pests but a number were sufficiently tolerant to require
unacceptably high concentrations for adequate control. (Union
Carbide, 1976a). It was shown by Roan (1964) and Strong and Sbur
(1961) that formulation was highly critical in obtaining the
desired effect against stored product pests.
Extensive work carried out in Australia in 1963, 1965 and 1967
(Greening, 1976) showed that carbaryl effectively controlled
Rhyzopertha dominica at concentrations as low as 1 mg/kg in
laboratory trials and at 10 mg/kg in field trials.
Carbaryl has a number of distinct advantages for use in
combination with organophosphorus grain protectant insecticides. It
is cheap; it is readily available; it has been widely used and
evaluated as a general insecticide for more than 10 years; it is
in a different class of compounds from organophosphorus or pyrethroid
grain protectants, which reduces the possibility of resistance
developing.
Extensive studies carried out in Australia have clearly
demonstrated the advantage of carbaryl for the control of
Rhyzopertha dominica (Davies, 1976a, 1976b; Desmarchelier,
1976a, 1976b, 1976c; Bengston, et al, 1976b).
Desmarchelier showed that carbaryl applied to wheat at rates
ranging from 3-6 mg/kg and held at 25°C gave 100% control of adult
Rhyzopertha dominica and prevented reproduction of this species
for more than 6 months. Although ineffective against other species
at this concentration, the addition of normal rates of other
organophosphorus insecticides produced complete control of all
species for more than 6 months. Davies (1976) reports equally
impressive results for periods of at least 31 weeks after
treatment.
Commercially acceptable control of all major stored product
pests and complete protection of stored grains can be obtained by
the use of combinations of approved organophosphorus insecticides
to which carbaryl is added at rates equivalent to 5 mg/kg of
treated grain.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest
When carbaryl tolerances were first established on crops,
knowledge of the composition of the residue in or on plants was
limited and data for the parent compound and its hydrolytic
product, 1-naphthol, were considered adequate to define the total
toxic residue for carbaryl.
Continued study of the fate of carbaryl over the succeeding
years has shown that the small amount of carbaryl residue which
enters the plant undergoes biotransformation into a variety of
products. Residue data are therefore presented for free carbaryl,
conjugated carbaryl and conjugated methylol carbaryl. These three
compounds represent the major portion of the total residue.
The residue data on small grains presented in this monograph
are of two types. Those obtained prior to 1972 are for free
carbaryl, conjugated carbaryl, conjugated methylol carbaryl and
conjugated naphthol. The latter data reflect continued study of the
nature of the residue and the development of new analytical
techniques £or the determination of additional constituents of it.
The pre-1972 studies are of value in comparing the results for the
free parent compound with those for the same compound in the later
studies since free carbaryl is determined separately in both cases.
The pre-1972 studies are additionally valuable for forage since the
recent studies have shown carbaryl to constitute all but a small
fraction of the total residue.
Table 1, taken from studies by Conterio (1969), shows
generally low residues of free carbaryl in barley and wheat grain
(1.7 mg/kg or less), even after four and five applications of an
exaggerated 2 kg/ha treatment with samples taken immediately after
treatment. The grain residues show a drop to barely detectable
levels (0.06 mg/kg or less) and 35 days after treatment.
TABLE 1. Carbaryl residues in barley and wheat grain, mill fractions and forage
(W.A. Conterio, Illinois, 1968-1969)
Interval
Treatment after last
Rate application, Free carbaryl residues, mg/kg, in
No kg a.i./ha days grain flour shorts red dog hulls
Barley
1 1.1 35 0.06 0.05 0.05 0.04 0.06
4 2.2 0 1.0 0.11 0.35 0.24 0.45
Wheat
1 2.2 30 0.03 0.15 0.11 - 0.11
5 2.2 0 1.7 0.10 0.19 - 0.50
Straw
5 1.1 0 0.98 0.41
4 1.1 9 0.10 0.19
3 1.1 16 0.10 0.11
2 1.1 23 0.53 0.15
1 1.1 30 0.03 0.10
5 2.2 0 1.7 35
4 2.2 9 0.06 2.0
3 2.2 16 0.12 0.10
2 2.2 23 0.03 0.08
1 2.2 30 0.03 0.09
Table 2, taken from studies by Phillips (1972) presents data for
barley heads and grain. The total residues (carbaryl and metabolites
on the grain at harvest range up to 2.7 mg/kg, this value being from
two applications of 1.1 kg/ha, with samples taken 48 days after the
last application.
Table 3 presents residue data from wheat and barley forage which
are of value in determining the amount of total residue which may be
present on the plant portions consumed by livestock. The data in Table
3 show that free carbaryl is by far the predominant constituent of the
forage residue. Conjugated carbaryl is usually the next most prominent
component but is only a small fraction of the free carbaryl residue at
any given sampling interval except when total residues are very low.
The maximum free carbaryl residue in wheat forage is 44 mg/kg
immediately after an application of 3.4 kg/ha, an exaggerated rate and
in barley it is 77 mg/kg immediately after the second of two 1.7 kg/ha
applications. The conjugated carbaryl residues show a maximum of 1.5
mg/kg. There is rapid attenuation of the total deposit.
Table 4 presents data for residues in wheat and barley grain and
mill fractions. They reflect both recommended and exaggerated
application rates. Total carbaryl residues in grain are lower than in
barley (0.36 - 2.7 mg/kg) in wheat (0.08 - 0.24 mg/kg).
Post-harvest
From extensive laboratory trials in Australia Davies (1976a)
measured the decline of active carbaryl residues on wheat by bio-assay
using Rhyzopertha dominica. From his data illustrated in Figure 1,
Davies calculated the approximate biological half-life of carbaryl in
wheat treated with 5 mg/kg to be 40 weeks at 35°C, 60 weeks at 30°C,
80 weeks at 25°C and much longer than 80 weeks at 20°C.
Table 5 indicates the fate of carbaryl applied alone or in
combination with pirimiphos-methyl to wheat held in the laboratory in
sealed bins at approximately 25°C and determined by GLC analysis of
samples drawn at intervals over a 39-week storage period
(Desmarchelier, 1976b).
Degradation was more rapid in field trials. Desmarchelier (1976b)
reported on the recovery of carbaryl from bulk wheat of 12.5% moisture
held in silos over a 26-week period at different temperature ranges.
In one silo the average level fell from 4.8 to 2.4 mg/kg at 30-22°C
and in another from 9.0 to 5.8 mg/kg at 27-13°C.
Desmarchelier (1976c) reports substantially similar stability for
carbaryl applied to oats (12% moisture), malting barley (13%
moisture), paddy rice (13% moisture), brown rice (12.5% moisture) and
white rice (12-7% moisture) when stored at 25°C. In other words, a
reduction to approximately half the original concentration after some
6 months of storage.
TABLE 2. Carbaryl residues in barley heads, grain and forage (I.L. Phillips and
R.O. Leininger, California, 1971-1972)
Carbaryl residues, mg/kg
Days
Treatment after Conjugated
Rate, last Free Conjugated Conjugated Methylol Total
No. kg. a.i./ha application Carbaryl Carbaryl Naphthol Carbaryl Residue
Heads and Grain
1 1.1 7 1.5 0.31 0.06 0.05 1.9
14 0.66 0.17 0.06 0.07 1.0
21 1.0 0.18 0.10 0.11 1.4
48* 0.28 0.56 0.14 0.12 1.1
2 1.1 7 3.6 0.31 0.07 0.08 4.1
14 5.6 0.34 0.20 0.35 6.5
21 3.3 0.59 0.37 0.42 4.7
48* 0.82 1.5 0.20 0.20 2.7
1 1.7 58* 0.18 0.33 0.84 0.09 1.4
*Grain at harvest.
TABLE 3. Carbaryl residues in wheat and barley forage
Carbaryl residues, mg/kg
Crop, Treatment Days Conjugated
location Rate, after last Free Conjugated Conjugated Methylol Total
(Reference) No. kg a.i./ha application Carbaryl Carbaryl Naphthol Carbaryl Residue
Wheat, 1 1.7 0 29.9 1.2 0.06 0.04 31.2
California 7 25.6 1.5 0.13 0.35 27.6
(Leininger, 1972) 14 12.1 1.2 0.07 0.17 13.5
58 5.5 1.0 0.18 0.37 7.1
Wheat, 2 1.7 0 0.44 0.02 0.04 0.05 0.6
Illinois 7 0.21 0.05 0.05 0.02 0.3
(Conterio, 1972) 14 0.09 0.03 0.04 0.05 0.2
24 0.06 0.01 0.04 0.03 0.1
2 1.7 0 0.96 0.14 0.05 0.02 1.2
3 0.18 0.04 0.04 0.03 0.3
7 1.4 0.25 0.06 0.04 1.8
14 0.16 0.05 0.09 0.02 0.3
24 0.06 0.01 0.04 0.03 0.1
2 1.7 0 20.2 0.45 0.05 0.06 20.8
3 11.8 0.29 0.07 0.07 12.2
7 11.5 0.17 0.09 0.03 11.8
14 1.5 0.27 0.08 0.09 1.9
24 0.59 0.22 0.14 0.06 1.0
Wheat 1 1.7 0 32.9 0.54 0.12 0.17 33.7
California 3 12.9 0.39 0.12 0.29 13.7
(Leininger, 1973) 7 15.2 0.47 0.13 0.43 16.2
14 10.1 0.37 0.24 0.46 11.2
21 8.8 0.32 0.17 0.46 9.8
1 3.4 0 43.6 1.5 0.10 0.20 45.4
3 34.1 1.1 0.16 0.39 35.8
7 28.0 0.82 0.18 0.48 29.5
TABLE 3. (Cont'd.)
Carbaryl residues, mg/kg
Crop, Treatment Days Conjugated
location Rate, after last Free Conjugated Conjugated Methylol Total
(Reference) No. kg a.i./ha application Carbaryl Carbaryl Naphthol Carbaryl Residue
14 18.8 0.52 0.13 0.53 20.0
21 18.4 0.63 0.16 0.67 19.9
Wheat, 1 3.4 1 12.8 0.50 0.14 0.06 13.5
Minnesota 3 5.9 0.73 0.06 0.08 6.8
(Ruppel, 1974) 14 0.76 0.31 0.08 0.14 1.3
28 0.12 0.11 0.05 0.12 0.4
South Dakota 1 3.4 1 45.3 1.2 0.05 0.05 46.4
(Kantack, 1974) 7 8.5 0.70 0.07 0.17 9.4
14 0.51 0.24 0.18 0.12 1.1
21 0.19 0.09 0.07 0.08 0.4
35 0.15 0.15 0.08 0.13 0.5
Barley
Illinois
(Conterio, 1972) 2 1.7 0 76.5 0.23 0.03 0.03 76.8
7 31.6 0.10 0.06 0.03 31.8
14 0.24 0.10 0.08 0.02 0.4
California 1 1.7 0 47.0 0.57 0.08 0.15 47.8
(Phillips and 7 33.0 0.75 0.37 1.2 35.3
Leininger, 1971, 14 15.1 0.24 0.27 0.88 16.5
1972)
TABLE 4. Carbaryl residues in wheat and barley grain and mill fractions at harvest
Application Carbaryl residues, mg/kg
rate, kg a.i./ha
Crop, (days Conjugated
location after last Mill Free Conjugated Conjugated Methylol Total
(reference) application) Fraction Carbaryl Carbaryl Naphthol Carbaryl Residue
Wheat 1.7 grain 0.08 0.03 0.03 0.03 0.17
California (58) bran 0.05 0.06 0.09 0.05 0.25
(Leininger, flour 0.03 0.07 0.03 0.01 0.14
1972)
Minnesota 3.4 grain 0.01 0.02 0.04 0.01 0.08
(Ruppel, (36) bran 0.02 0.17 0.02 0.03 0.24
1974) flour 0.04 0.04 0.04 0.01 0.17
South Dakota 3.4 grain 0.12 0.05 0.04 0.03 0.24
(Kantack, (35) bran 0.08 0.10 0.11 0.06 0.35
1974) flour 0.06 0.05 0.04 0.02 0.17
Illinois 2 x 1.7 bran 0.02 0.06 0.04 0.03 0.15
(Conterio, flour 0.02 0.02 0.01 0.02 0.07
1972)
Barley, 1.1 grain 0.28 0.56 0.14 0.12 1.1
California (48) hulls 0.18 2.2 0.51 0.36 3.3
(Phillips, flour 0.26 0.28 0.11 0.15 0.8
1971)
2 x 1.1 grain 0.82 1.5 0.20 0.20 2.7
(48) hulls 0.31 12.0 1.4 0.43 14.1
flour 0.17 0.75 0.22 0.24 1.4
California 1.7 grain 0.27 0.32 0.08 0.07 0.74
(Leininger, (58) hulls 0.12 1.9 0.17 0.19 2.4
1972) flour 0.06 0.06 0.04 0.02 0.18
Illinois 2 x 1.7 grain 0.18 0.06 0.08 0.04 0.36
(Conterio, (24) hulls 0.07 0.26 0.14 0.04 0.51
1972) flour 0.04 0.07 0.05 0.03 0.19
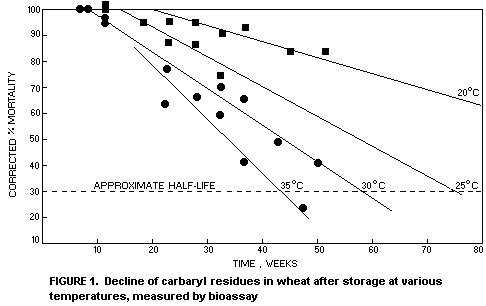 TABLE 5. Residues in wheat after laboratory application of carbaryl and/or
pirimiphos-methyl
Residues recovered by GLC analysis,
Calculated mg/kg
rate
Treatment mg/kg Day 1 Week 9 Week 18 Week 26 Week 39
Carbaryl 5 3.1 2.8 - - 1.9
Carbaryl 10 6.5 9.8 5.0 4.3 4.2
Pirimiphos-
methyl 6 5.1 - - - 3.1
Pirimiphos-
methyl 6 4.2 - - - 2.3
+ +
Carbaryl 5 3.4 3.1 - - 2.5
Pirimiphos-
methyl 6 1.8 - - - 1.1
+
Carbaryl 10 5.7 5.4 5.0 4.5 4.6
FATE OF RESIDUES
In animals
Ingested carbaryl is rapidly metabolised in cows and other
animals, 70-80% being excreted in urine within 24 hours. In a
continuous feeding study in cows, equilibration of total radioactive
residues in milk, urine and faeces occurred by the second day. Within
18 hours after the last of 14 days continuous feeding, the highest
total of residues were found in the kidneys. These data are summarised
in Table 6. The lowest residues were found in fat, indicating that
metabolites are not stored in body tissues. The major components of
the residue in tissue were carbaryl, naphthol, naphthyl sulphate,
5,6-dihydrodihydroxycarbaryl and 5,6-dihydrodihydroxynaphthol. Methods
are available to determine these compounds in beef kidney and liver.
Of the radioactivity appearing in the milk, carbaryl per se comprised
less than 10%. The two principal residues were
5,6-dihydrodihydroxycarbaryl and conjugated
5-methoxy-6-hydroxycarbaryl. An analytical method is available to
measure the major metabolites in milk. The concentration of the total
radioactive residue in the milk was only about 1/300, and in the
tissues 1/100 or less of the carbaryl concentration in the feed. These
ratios and the composition of the metabolites in the milk did not vary
significantly over the three feeding levels studied (Dorough, 1974;
Union Carbide Corporation, 1974).
TABLE 6. Residues in meat and milk from carbaryl in feed.
Total 14C expressed as carbaryl (mg/kg) at
indicated feeding levels (ppm)
Tissue,
etc. 10 ppm 30 ppm 100 ppm
Kidney 0.095 0.531 1.003
Liver 0.033 0.100 0.411
Milk 0.024 0.071 0.278
fat 0.000 0.015 0.025
Ingested carbaryl is also rapidly metabolised in poultry by
pathways similar to those in mammals. In continuous feeding studies
with radio-labelled carbaryl (Andrawes et al, 1972), residues reached
maximum levels within one day in the excrement, 2 days in egg white
and 6-8 days in egg yolk. The residues in the whole egg (yolk plus
white) were directly proportional to the amount of carbaryl fed. An
intake of 7 mg/kg of carbaryl in the feed resulted in a residue of
0.04 mg/kg carbaryl equivalents in the whole egg. Radio-labelled
residues in the excrement 15 hours after the initial treatment reached
80-100% of the dose. Within one day after the discontinuation of
dosing, the highest tissue residues were found in the excretory organs
while very low residues were found in the fat indicating that carbaryl
residues are not stored in body tissues. This work shows that carbaryl
is metabolised in laying hens by pathways similar to those in mammals.
In plants
Of the carbaryl deposited on plant surfaces, only the relatively
small proportion which penetrates the plant tissues is metabolised.
Once in the plant, the insecticide is largely transformed by
hydrolysis or oxidation to several hydroxylated metabolites. These
compounds in turn are rapidly conjugated to form watersoluble
glycosides. The metabolic transformations which take place are similar
whether the compound is applied by surface application, root
absorption or artificially by stem injections. In general, all plant
and animal metabolites of carbaryl are considered to be less toxic
than the parent compound, some substantially so.
The metabolites found in greatest abundance in plants and those
judged of most toxicological significance are conjugated 1-naphthol,
conjugated methylol carbaryl, and carbaryl per se. The latter is found
in both the free state and/or conjugated with plant constituents. The
residue methods available are capable of releasing the metabolites
from their conjugates and determining their concentration either
individually or collectively. The remaining metabolites are of either
no toxicological or no quantitative significance.
TABLE 7. Effect of milling and baking on carbaryl residues in wheat
Location Location
N B
Carbaryl applied, mg/kg 5 10
Carbaryl recovered, day 1, mg/kg 3.4 6.5
Wheat withdrawn after 19 weeks 13 weeks
Residues in wheat, mg/kg 3.1 6.0
Residues in mill fractions, mg/kg
wholemeal flour 1.2 2.6
wholemeal bread 0.7 1.5
bran 7.0 14
shorts 1.5 3
white flour 0.07 0.15
white bread <0.05 0.08
Reduction in residues, %
wheat/wholemeal bread 77 75
wholemeal flour/wholemeal bread 42 42
wheat/white flour 97 97
white flour/white bread 50 50
wheat/white bread 98 99
In processing and cooking
A number of studies are available to show the distribution and
fate of carbaryl residues on various grains, milling fractions of
grains and prepared cereals subjected to cooking.
Table 1 indicates the distribution of free carbaryl residues and
Table 4 the distribution of carbaryl and its metabolites, in grain and
milling fractions of barley and wheat following the pre-harvest
application of carbaryl. Again these results show that free carbaryl
and conjugated carbaryl together represent the major proportion of the
residues. These data indicate that residues resulting from pre-harvest
application are substantially confined to the outer portion of the
grain and therefore do not find their way into flour. There is a
significant partitioning of the residues in the milling offals.
Desmarchelier (1976b) showed that wheat treated with carbaryl 3-5
months previously but still containing residues of 3-6 mg/kg carbaryl
when converted into wholemeal flour, yielded a flour containing 1-3
mg/kg of carbaryl. When the same grain was milled for the preparation
of white flour (approximately 70% extraction), the residue level in
flour was reduced to about 0.1 mg/kg. The baking of wholemeal or white
bread resulted in a further loss of about half the carbaryl content,
bringing the final residue in wholemeal bread into the range of 1
mg/kg, and in white bread to below 0.1 mg/kg. Thus the reduction in
residues between raw grain and white bread was 99%, and between grain
and wholemeal bread about 75% (Table 7). Using other wheat containing
carbaryl, residues in the range 2.5-3 mg/kg, Desmarchelier (1976b)
showed that the final residue in white bread was less than 0.05 mg/kg
and in wholemeal bread 0.5 mg/kg.
In a series of experiments representing the most primitive type
of processing to which husked rice, polished rice, oats and barley
would be subjected, Desmarchelier (1976c) showed that simple boiling
in minimal amounts of water for 15 minutes reduced carbaryl residues
in husked rice, polished rice and oats by 84%. A primitive malting
procedure reduced residues in barley by 77% (Table 8).
METHODS OF RESIDUE ANALYSIS
Methods of residue analysis have been dealt with at length in
previous monographs. A number of modifications were developed to
obtain the data on grain, forage and milling offals as well as beef
tissues and milk. References to these methods were provided by Union
Carbide Ltd. The method of Holden (1973) is widely used for the
determination of carbaryl residues on a number of commodities.
Desmarchelier (1976e) using the principles discovered by Pschorr
and Sumuleanu (1899) and Lumiere et al (1906) and further developed by
Chattaway (1931) has developed a method for the esterification of
phenols, at residue levels, in dilute aqueous base. The method is
based on a procedure for the acetylation of amines by reaction with
anhydrides in aqueous solution which is successful because amines
react with certain anhydrides more quickly than the anhydrides are
hydrolyzed. Chattaway's work extended this reaction to phenols, by
reacting them at 0°C in dilute sodium hydroxide with acetic anhydride.
Acetylation was quantitative and instantaneous. By using Chattaway's
procedure and either acetic or propionic anhydride as reagents,
TABLE 8. Effect of storage at 25°C, processing and cooking on carbaryl residues in treated grains
Residue, mg/kg,
Application after storage for Processed Residue, mg/kg
Moisture rate 3 6 after Processed after cooking for
Grain % mg/kg months months (months) into - 15 min 25 min
Barley 13 10.6 6.5 3.5 3 Primitive 1.5
malt
6 Commercial 0.2
malt
Oats 12 10.0 7.5 3.5 3 Rolled - 1.2
oats
Rice in husk 13 10.0 7.5 3.5 6 Husked 0.4 0.2
6 Milled 0.07 <0.05
Husked rice 12.5 10.0 7.5 3.4 3 Cooked 1.2
6 Cooked 0.7
Polished rice 12.7 10.0 7.5 3.5 3 Cooked 1.2
Wheat 12 10.0 7.2 6.3 5 wholemeal 2.5 1.5
(4.2 after
storage for
9 months)
Desmarchelier (1976d) esterified the phenols from parathion,
fenitrothion, chlorpyrifos, fenchlorphos, cyanophos and carbaryl.
To test the procedure for determining carbaryl in grain,
Desmarchelier extracted 10 g of wheat with 25 ml of acetone for 24
hours. An aliquot of 1 ml was hydrolysed with 1 ml of 1 M sodium
hydroxide in 90% ethanol (one hour at ambient temperature) and mixture
diluted and washed with ether. The aqueous solution was adjusted to pH
10 and stirred at 0°C with 2 x 20 ml of 1% chloracetic anhydride in
ether. The organic extracts were diluted, dried and analysed by gas
chromatography on 5% SE-30 Chromosorb W at 175°C with electron capture
detection. Recoveries were better than 90% at residue levels in the
range 0.1-5 mg/kg. This procedure was used for developing much of the
data on the level of carbaryl residues on grain and milling fractions
following post-harvest use of carbaryl as a grain protectant.
Desmarchelier (1976d) evaluated two TLC procedures with
fluorescent layers suitable for estimating carbaryl residues on grain.
One used aluminium oxide developed with 15% ethyl acetate/85% hexane,
and the other polyamide developed with 50% methanol/50% water.
Carbaryl was detected by hydrolysing with 1 M sodium hydroxide in 90%
ethanol. 1-naphthol shows as a blue colour at 254 nm with a detection
limit of 0.1 µg.
The method outlined in "Official Methods of Analysis", of the
Association of Official Analytical Chemists, 11th Edition (1970),
Section 29.071. for the use of chromogenic reagent is both more
precise and more sensitive when using the polyamide system. 0.05-0.1
microgram can be readily detected. Desmarchelier (1976d) indicated
that similar results were obtained on aged residues of carbaryl on
wheat. Acetone proved the most suitable solvent, giving good
resolution free of interference from water. Although the GLC procedure
described above is more precise and more sensitive, the TLC procedure
is simpler and requires less expensive equipment.
A paper on the significance of pesticide residues which discussed
the importance and variability of analytical procedures applied for
the determination of residues on raw grains, was presented at the
International Working Conference on Stored Product Entomology (Snelson
and Desmarchelier, 1975).
NATIONAL TOLERANCES
References to the following national tolerances for carbaryl
residues in raw grain were reported to the Meeting.
TABLE 9. National tolerances for carbaryl on grains reported to the
Meeting
Tolerance
Country Grain mg/kg
Argentina barley grains, oats, rye, wheat 0
rice 2
Australia rice 3
Belgium raw rice 0.8
Canada barley, oats, rye, wheat 2
Germany rice 0.8
India rice 1.25
Israel rice 2.5
Japan rice (unpolished) 0.1
Netherlands rice (coarse) 0.8
South Africa all food products 10
Switzerland rice 2.5
U.S.A. grains of barley, oats, rye, wheat 0
rice 1
U.S.S.R. maize not permitted
APPRAISAL
Following the evaluation of carbaryl by the Joint Meeting on a
number of occasions (FAO/WHO, 1965b, 1967b, 1968b, 1969b, 1970b,
1971b, 1974b, 1976b) the Meeting was informed of two important
applications for carbaryl:
(a) the control of pests of small grain crops previously
controlled with organochlorine or organophosphorus compounds, and
(b) the protection of stored grain, particularly for the control
of lesser grain borer, Rhyzopertha dominica, when used in
conjunction with approved organophosphorus insecticides.
Extensive new data on these uses and the level and fate of
residues resulting from such applications, have been made available to
the Joint Meeting.
For pre-harvest use the rate of application ranges from 0.5 to 2
kg/ha depending upon the degree of infestation, density of foliage and
whether or not the pests are in advanced stages of their life cycle.
For post-harvest use the rate of application depends upon the humidity
and temperature of the grain, whether high temperatures will be
maintained during storage, whether the grain will be aerated, and the
expected period in storage. Treatment is usually at the rate of 5
mg/kg but hotter grain kept in unaerated storage for long periods will
require a higher rate of application or repeated treatment.
Extensive studies in the U.S.A. indicate that when carbaryl is
used for the pre-harvest control of major pests of small grain crops,
residues of carbaryl and its more important metabolites in the grain
will usually range between 1 and 3 mg/kg, though some data indicate
the likelihood of residues reaching the vicinity of 4 mg/kg, when the
pre-harvest interval is 21 days. In the case of treatments made 14 to
7 days prior to harvest, the residues may be significantly higher but
it is generally considered that such treatments would seldom be
necessary.
Such spray treatments give rise to residues on the forage of
grain crops ranging up to 50 mg/kg on the day following application.
However, the loss of residues from the forage is rapid so that 21 days
later they seldom exceed 1 mg/kg.
Extensive studies on stored wheat and confirmatory data from
trials on barley, oats and rice indicate that carbaryl residues on
grain are relatively stable having a half-life between 26 and 80
weeks, depending upon the temperature.
Studies with radio-labelled carbaryl indicate that only a
relatively small proportion of the amount applied penetrates the plant
tissue where it may be metabolised. The nature and extent of the
metabolism is well documented.
A number of studies are available to show the distribution and
fate of carbaryl residues on various grains and milling fractions of
grains and prepared cereals subjected to cooking. Carbaryl residues
are not completely destroyed when prepared cereals are cooked but the
loss ranges from 75 to 99%, depending upon the cereal, the processing
and the cooking. The least loss occurs in the preparation of wholemeal
bread where a residue of the order of 1-1.5 mg/kg could remain in the
finished bread.
No residue data are presented for rye but, because of the close
similarity in physical composition and use of the two crops, the
residue data for wheat and its milled fractions can be translated to
rye and its milled fractions.
A number of national governments have already established maximum
residue limits for carbaryl on raw grains. The Joint Meeting has
previously recommended a limit of 3 mg/kg for rice (FAO/WHO 1968b) and
10 mg/kg for sorghum (FAO/WHO 1974b).
Extensive data are available to indicate the fate of such
residues on grain or forage when fed to livestock or poultry. These
indicate that the feeding of forage from treated small grain crops or
of mill offals from such grain or grain treated post-harvest, could
give rise to small but significant residues in animal tissues and
milk. The magnitude of such residues is not likely to be higher than
that arising from the feeding of other forage for which a limit of 100
mg/kg has been recommended. For this reason the limits for carbaryl
residues in meat, milk and eggs expressed as the parent compound are
confirmed.
In 1973 the Meeting advised that the AOAC colorimetric method
(Holden 1973) remained the method of choice for regulatory purposes.
This method only determines the carbaryl parent and the free 1-
naphthol.
The Meeting studied the recommendations made in 1973 and all data
available and came to the conclusion that the residues determined in
the supervised trials represented the carbaryl parent and that the
maximum residue limits were intended to represent only the sum of
carbaryl and 1-naphthol not withstanding the fact that the heading of
the list of recommendations for maximum residue limits indicates that
the limits are expressed in terms of carbaryl and metabolites.
RECOMMENDATIONS
The following maximum residue limits are recommended to cover
residues resulting from either pre-harvest or post-harvest use of
carbaryl. They refer to carbaryl only. The limit for rice is raised to
provide for postharvest application.
Commodity Limit (mg/kg)
Bran 20
Barley, oats, rice (in husk and hulled)
rye, wheat 5
wholemeal flour 2
Wheat flour (white) 0.2
(The level and fate of carbaryl residues in processed cereal products
is given in the monograph but, in keeping with accepted practice,
separate limits have not been proposed for such processed foods.)
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the effects of carbaryl on renal
function.
2. Further studies to resolve the differences in observations of
different investigators on reproductive physiology, especially
with regard to neuroendocrine and behavioural changes.
3. Details of analytical methods for use in the determination of
carbaryl and metabolite residues in raw grain, milled cereal
fractions, bread, meat and milk.
REFERENCES
Andrawes, N.R., Chancey, E.L., Crabtree, R.J., Herrett, R. A.
1972 and Weiden, M.H.J. Fate of Maphthyl-1-14C
Carbaryl in laying chickens. J. agr. Food Chem.,
20:608.
Argauer, R.J. J.agr. Food Chem., 17: 889
1969
Association of Official Analytical Chemists, "Official Methods of
1970 Analysis", 11th Ed., p.
Bengston, M., Cooper, L.M., and Grant-Taylor, F.J. A comparison
1975 of bioresmethrin, chlorpyrifosmethyl and
pirimiphos-methyl as grain protectants against
malathion resistant insects in wheat. Queensland
J. agr. Animal Sci., 32,(1): 51-78.
Bengston, M., Connell, M., Crook, I.D., Desmarchelier, J., Hart,
1976a R.J., Phillips, M., Snelson, J.T. and Sticka, R.
Field trials to compare CGA-20168 (methacriphos),
chlorpyrifos-methyl, fenitrothion,
pirimiphos-methyl and malathion for control of
malathion resistant insects infesting wheat in
Australia. Submitted for publication in J. Stored
Prod. Res. 1976.
Bengston, M., Connell, M., Desmarchelier, J., Phillips, Snelson,
1976b J.T., and Sticka, R. Report of study of new grain
protectant insecticides. Report to Australian
Wheat Board. June 1976.
Chattaway, F.D. J. Chem. Sox. 2495
1931
Conterio, W.A. Carbaryl residues in barley and wheat grain,
1969 mill fractions and forage. Report to Union Carbide
Corporation from Crop Chemical Testing Service,
Humbolt, Illinois.
Davies, R.A.H. Carbaryl plus pirimiphos-methyl for stored grain
1976a protection. 1 Laboratory bioassay and residue
analysis: Social Report for J.T. Snelson -
Pesticides Co-ordinator. ICI Australia Ltd., Rural
Division, August 1976.
Davies, R.A.H. Carbaryl plus pirimiphos-methyl for stored grain
1976b protection. 2 Silo pilot trials 1975-76. Special
Report for J.T. Snelson - Pesticides Co-Ordinator.
ICI Australia Ltd., Rural Division, August 1976.
Desmarchelier, J.M. Field trials with fenitrothion plus fenoxythrin
1976a and pirimiphos-methyl plus carbaryl. Part 1.
Biological efficiency. Preliminary report of
ongoing study. CSIRO Division of Entomology,
Canberra, Australia, August 1976.
Desmarchelier, J.M. Field trials with fenitrothion plus fenoxythrin
1976b and pirimiphos-methyl plus carbaryl. Part 2.
Residue studies - Preliminary report of ongoing
study. CSIRO Division of Entomology, Canberra,
Australia, August 1976.
Desmarchelier, J.M. Degradation of carbaryl, CGA 20168,
1976c fenitrothion, pirimiphos-methyl and carbaryl on
rice, oats and barley. Preliminary report of
ongoing study. CSIRO Division of Entomology,
Canberra, Australia. August 1976.
Desmarchelier, J.M. Analytical procedures for carbaryl. Personal
1976d Communication. CSIRC Division of Entomology,
Canberra, Australia. August 1976.
Desmarchelier, J.M. Esterification of phenols, at residue levels,
1976e in dilute base. (In preparation) CSIRO Division of
Entomology, Canberra, Australia.
Dorough, H.W. Animal feeding studies with carbaryl. Details to be
1974 provided by Union Carbide.
FAO/WHO 1973 evaluation of some pesticide residues in food.
1974b FAO/AGP/1973/9/1; WHO Pesticide Residues Series,
No. 3.
Greening, H.G. Wheat-protectant dust trials 1965-67. N.S.W.
1976 Department of Agriculture. Biological and Chemical
Research Institute, Rydalmere, N.S.W. Report
prepared for publication September 1976.
Holden, E.R. Gas chromatographic determination of residues
1973 of methylcarbamate insecticides in crops as their
2,4-dinitrophenyl ether derivatives. J.A.O.A.C.,
56-713.
Leininger, R.O. Carbaryl residues in wheat forage, Report to Union
1973 Carbide Corporation from R.O. Leininger,
Greenfield. California,
Lumière, A., Lumière, L., and Barbier, H. Bull. Soc.Chim.
1906 Fr., 35: 625.
Phillips, I.L. Carbaryl residues in barley heads, grain and
1972 forage. Report to Union Carbide Corporation from
Soilserv. Inc., Salinas, California.
Pschorr, R., and Sumuleanu, P. Ber. der Deutsch. Chem. Ges.,
1899 22: 3407.
Roan, C.L. Comparison of performance of various carbaryl formulations
1964 against rice weevil and confused flour beetle.
Report from Entomology Department, Kansas State
University to Union Carbide Corporation.
Snelson, J.T. and Desmarchelier, J.M. The significance of pesticide
1975 residues. Proc. 1st int. Wking Conf.
Stored-product Entomology. Savannah, Georgia,
U.S.A., 1974, p. 465-477.
Strong, R.G., and Sbur, D.E. Evaluation of insecticides as
1961 protectants against pests of stored grains and
seeds. J. Econ. Entom., 54: 235-238.
Tilden, R.L., and van Middelem, C.H. Determination of carbaryl
1970 as an amide derivative by electron capture GLC. J.
Agric. Food Chem., 18(1): 154-6.
Union Carbide. Summary of studies on the nature of residues of
1974 carbaryl in the meat of dairy cattle. Union
Carbide Corporation Report, May 24, 1974.
Union Carbide. Submission to FAO by Union Carbide Corporation,
1976a September 1976.
Union Carbide Corporation. Results of tests investigating
1976b Sevin as a stored grain insecticide (1958-1962).
Extract of petition 2859.
TABLE 5. Residues in wheat after laboratory application of carbaryl and/or
pirimiphos-methyl
Residues recovered by GLC analysis,
Calculated mg/kg
rate
Treatment mg/kg Day 1 Week 9 Week 18 Week 26 Week 39
Carbaryl 5 3.1 2.8 - - 1.9
Carbaryl 10 6.5 9.8 5.0 4.3 4.2
Pirimiphos-
methyl 6 5.1 - - - 3.1
Pirimiphos-
methyl 6 4.2 - - - 2.3
+ +
Carbaryl 5 3.4 3.1 - - 2.5
Pirimiphos-
methyl 6 1.8 - - - 1.1
+
Carbaryl 10 5.7 5.4 5.0 4.5 4.6
FATE OF RESIDUES
In animals
Ingested carbaryl is rapidly metabolised in cows and other
animals, 70-80% being excreted in urine within 24 hours. In a
continuous feeding study in cows, equilibration of total radioactive
residues in milk, urine and faeces occurred by the second day. Within
18 hours after the last of 14 days continuous feeding, the highest
total of residues were found in the kidneys. These data are summarised
in Table 6. The lowest residues were found in fat, indicating that
metabolites are not stored in body tissues. The major components of
the residue in tissue were carbaryl, naphthol, naphthyl sulphate,
5,6-dihydrodihydroxycarbaryl and 5,6-dihydrodihydroxynaphthol. Methods
are available to determine these compounds in beef kidney and liver.
Of the radioactivity appearing in the milk, carbaryl per se comprised
less than 10%. The two principal residues were
5,6-dihydrodihydroxycarbaryl and conjugated
5-methoxy-6-hydroxycarbaryl. An analytical method is available to
measure the major metabolites in milk. The concentration of the total
radioactive residue in the milk was only about 1/300, and in the
tissues 1/100 or less of the carbaryl concentration in the feed. These
ratios and the composition of the metabolites in the milk did not vary
significantly over the three feeding levels studied (Dorough, 1974;
Union Carbide Corporation, 1974).
TABLE 6. Residues in meat and milk from carbaryl in feed.
Total 14C expressed as carbaryl (mg/kg) at
indicated feeding levels (ppm)
Tissue,
etc. 10 ppm 30 ppm 100 ppm
Kidney 0.095 0.531 1.003
Liver 0.033 0.100 0.411
Milk 0.024 0.071 0.278
fat 0.000 0.015 0.025
Ingested carbaryl is also rapidly metabolised in poultry by
pathways similar to those in mammals. In continuous feeding studies
with radio-labelled carbaryl (Andrawes et al, 1972), residues reached
maximum levels within one day in the excrement, 2 days in egg white
and 6-8 days in egg yolk. The residues in the whole egg (yolk plus
white) were directly proportional to the amount of carbaryl fed. An
intake of 7 mg/kg of carbaryl in the feed resulted in a residue of
0.04 mg/kg carbaryl equivalents in the whole egg. Radio-labelled
residues in the excrement 15 hours after the initial treatment reached
80-100% of the dose. Within one day after the discontinuation of
dosing, the highest tissue residues were found in the excretory organs
while very low residues were found in the fat indicating that carbaryl
residues are not stored in body tissues. This work shows that carbaryl
is metabolised in laying hens by pathways similar to those in mammals.
In plants
Of the carbaryl deposited on plant surfaces, only the relatively
small proportion which penetrates the plant tissues is metabolised.
Once in the plant, the insecticide is largely transformed by
hydrolysis or oxidation to several hydroxylated metabolites. These
compounds in turn are rapidly conjugated to form watersoluble
glycosides. The metabolic transformations which take place are similar
whether the compound is applied by surface application, root
absorption or artificially by stem injections. In general, all plant
and animal metabolites of carbaryl are considered to be less toxic
than the parent compound, some substantially so.
The metabolites found in greatest abundance in plants and those
judged of most toxicological significance are conjugated 1-naphthol,
conjugated methylol carbaryl, and carbaryl per se. The latter is found
in both the free state and/or conjugated with plant constituents. The
residue methods available are capable of releasing the metabolites
from their conjugates and determining their concentration either
individually or collectively. The remaining metabolites are of either
no toxicological or no quantitative significance.
TABLE 7. Effect of milling and baking on carbaryl residues in wheat
Location Location
N B
Carbaryl applied, mg/kg 5 10
Carbaryl recovered, day 1, mg/kg 3.4 6.5
Wheat withdrawn after 19 weeks 13 weeks
Residues in wheat, mg/kg 3.1 6.0
Residues in mill fractions, mg/kg
wholemeal flour 1.2 2.6
wholemeal bread 0.7 1.5
bran 7.0 14
shorts 1.5 3
white flour 0.07 0.15
white bread <0.05 0.08
Reduction in residues, %
wheat/wholemeal bread 77 75
wholemeal flour/wholemeal bread 42 42
wheat/white flour 97 97
white flour/white bread 50 50
wheat/white bread 98 99
In processing and cooking
A number of studies are available to show the distribution and
fate of carbaryl residues on various grains, milling fractions of
grains and prepared cereals subjected to cooking.
Table 1 indicates the distribution of free carbaryl residues and
Table 4 the distribution of carbaryl and its metabolites, in grain and
milling fractions of barley and wheat following the pre-harvest
application of carbaryl. Again these results show that free carbaryl
and conjugated carbaryl together represent the major proportion of the
residues. These data indicate that residues resulting from pre-harvest
application are substantially confined to the outer portion of the
grain and therefore do not find their way into flour. There is a
significant partitioning of the residues in the milling offals.
Desmarchelier (1976b) showed that wheat treated with carbaryl 3-5
months previously but still containing residues of 3-6 mg/kg carbaryl
when converted into wholemeal flour, yielded a flour containing 1-3
mg/kg of carbaryl. When the same grain was milled for the preparation
of white flour (approximately 70% extraction), the residue level in
flour was reduced to about 0.1 mg/kg. The baking of wholemeal or white
bread resulted in a further loss of about half the carbaryl content,
bringing the final residue in wholemeal bread into the range of 1
mg/kg, and in white bread to below 0.1 mg/kg. Thus the reduction in
residues between raw grain and white bread was 99%, and between grain
and wholemeal bread about 75% (Table 7). Using other wheat containing
carbaryl, residues in the range 2.5-3 mg/kg, Desmarchelier (1976b)
showed that the final residue in white bread was less than 0.05 mg/kg
and in wholemeal bread 0.5 mg/kg.
In a series of experiments representing the most primitive type
of processing to which husked rice, polished rice, oats and barley
would be subjected, Desmarchelier (1976c) showed that simple boiling
in minimal amounts of water for 15 minutes reduced carbaryl residues
in husked rice, polished rice and oats by 84%. A primitive malting
procedure reduced residues in barley by 77% (Table 8).
METHODS OF RESIDUE ANALYSIS
Methods of residue analysis have been dealt with at length in
previous monographs. A number of modifications were developed to
obtain the data on grain, forage and milling offals as well as beef
tissues and milk. References to these methods were provided by Union
Carbide Ltd. The method of Holden (1973) is widely used for the
determination of carbaryl residues on a number of commodities.
Desmarchelier (1976e) using the principles discovered by Pschorr
and Sumuleanu (1899) and Lumiere et al (1906) and further developed by
Chattaway (1931) has developed a method for the esterification of
phenols, at residue levels, in dilute aqueous base. The method is
based on a procedure for the acetylation of amines by reaction with
anhydrides in aqueous solution which is successful because amines
react with certain anhydrides more quickly than the anhydrides are
hydrolyzed. Chattaway's work extended this reaction to phenols, by
reacting them at 0°C in dilute sodium hydroxide with acetic anhydride.
Acetylation was quantitative and instantaneous. By using Chattaway's
procedure and either acetic or propionic anhydride as reagents,
TABLE 8. Effect of storage at 25°C, processing and cooking on carbaryl residues in treated grains
Residue, mg/kg,
Application after storage for Processed Residue, mg/kg
Moisture rate 3 6 after Processed after cooking for
Grain % mg/kg months months (months) into - 15 min 25 min
Barley 13 10.6 6.5 3.5 3 Primitive 1.5
malt
6 Commercial 0.2
malt
Oats 12 10.0 7.5 3.5 3 Rolled - 1.2
oats
Rice in husk 13 10.0 7.5 3.5 6 Husked 0.4 0.2
6 Milled 0.07 <0.05
Husked rice 12.5 10.0 7.5 3.4 3 Cooked 1.2
6 Cooked 0.7
Polished rice 12.7 10.0 7.5 3.5 3 Cooked 1.2
Wheat 12 10.0 7.2 6.3 5 wholemeal 2.5 1.5
(4.2 after
storage for
9 months)
Desmarchelier (1976d) esterified the phenols from parathion,
fenitrothion, chlorpyrifos, fenchlorphos, cyanophos and carbaryl.
To test the procedure for determining carbaryl in grain,
Desmarchelier extracted 10 g of wheat with 25 ml of acetone for 24
hours. An aliquot of 1 ml was hydrolysed with 1 ml of 1 M sodium
hydroxide in 90% ethanol (one hour at ambient temperature) and mixture
diluted and washed with ether. The aqueous solution was adjusted to pH
10 and stirred at 0°C with 2 x 20 ml of 1% chloracetic anhydride in
ether. The organic extracts were diluted, dried and analysed by gas
chromatography on 5% SE-30 Chromosorb W at 175°C with electron capture
detection. Recoveries were better than 90% at residue levels in the
range 0.1-5 mg/kg. This procedure was used for developing much of the
data on the level of carbaryl residues on grain and milling fractions
following post-harvest use of carbaryl as a grain protectant.
Desmarchelier (1976d) evaluated two TLC procedures with
fluorescent layers suitable for estimating carbaryl residues on grain.
One used aluminium oxide developed with 15% ethyl acetate/85% hexane,
and the other polyamide developed with 50% methanol/50% water.
Carbaryl was detected by hydrolysing with 1 M sodium hydroxide in 90%
ethanol. 1-naphthol shows as a blue colour at 254 nm with a detection
limit of 0.1 µg.
The method outlined in "Official Methods of Analysis", of the
Association of Official Analytical Chemists, 11th Edition (1970),
Section 29.071. for the use of chromogenic reagent is both more
precise and more sensitive when using the polyamide system. 0.05-0.1
microgram can be readily detected. Desmarchelier (1976d) indicated
that similar results were obtained on aged residues of carbaryl on
wheat. Acetone proved the most suitable solvent, giving good
resolution free of interference from water. Although the GLC procedure
described above is more precise and more sensitive, the TLC procedure
is simpler and requires less expensive equipment.
A paper on the significance of pesticide residues which discussed
the importance and variability of analytical procedures applied for
the determination of residues on raw grains, was presented at the
International Working Conference on Stored Product Entomology (Snelson
and Desmarchelier, 1975).
NATIONAL TOLERANCES
References to the following national tolerances for carbaryl
residues in raw grain were reported to the Meeting.
TABLE 9. National tolerances for carbaryl on grains reported to the
Meeting
Tolerance
Country Grain mg/kg
Argentina barley grains, oats, rye, wheat 0
rice 2
Australia rice 3
Belgium raw rice 0.8
Canada barley, oats, rye, wheat 2
Germany rice 0.8
India rice 1.25
Israel rice 2.5
Japan rice (unpolished) 0.1
Netherlands rice (coarse) 0.8
South Africa all food products 10
Switzerland rice 2.5
U.S.A. grains of barley, oats, rye, wheat 0
rice 1
U.S.S.R. maize not permitted
APPRAISAL
Following the evaluation of carbaryl by the Joint Meeting on a
number of occasions (FAO/WHO, 1965b, 1967b, 1968b, 1969b, 1970b,
1971b, 1974b, 1976b) the Meeting was informed of two important
applications for carbaryl:
(a) the control of pests of small grain crops previously
controlled with organochlorine or organophosphorus compounds, and
(b) the protection of stored grain, particularly for the control
of lesser grain borer, Rhyzopertha dominica, when used in
conjunction with approved organophosphorus insecticides.
Extensive new data on these uses and the level and fate of
residues resulting from such applications, have been made available to
the Joint Meeting.
For pre-harvest use the rate of application ranges from 0.5 to 2
kg/ha depending upon the degree of infestation, density of foliage and
whether or not the pests are in advanced stages of their life cycle.
For post-harvest use the rate of application depends upon the humidity
and temperature of the grain, whether high temperatures will be
maintained during storage, whether the grain will be aerated, and the
expected period in storage. Treatment is usually at the rate of 5
mg/kg but hotter grain kept in unaerated storage for long periods will
require a higher rate of application or repeated treatment.
Extensive studies in the U.S.A. indicate that when carbaryl is
used for the pre-harvest control of major pests of small grain crops,
residues of carbaryl and its more important metabolites in the grain
will usually range between 1 and 3 mg/kg, though some data indicate
the likelihood of residues reaching the vicinity of 4 mg/kg, when the
pre-harvest interval is 21 days. In the case of treatments made 14 to
7 days prior to harvest, the residues may be significantly higher but
it is generally considered that such treatments would seldom be
necessary.
Such spray treatments give rise to residues on the forage of
grain crops ranging up to 50 mg/kg on the day following application.
However, the loss of residues from the forage is rapid so that 21 days
later they seldom exceed 1 mg/kg.
Extensive studies on stored wheat and confirmatory data from
trials on barley, oats and rice indicate that carbaryl residues on
grain are relatively stable having a half-life between 26 and 80
weeks, depending upon the temperature.
Studies with radio-labelled carbaryl indicate that only a
relatively small proportion of the amount applied penetrates the plant
tissue where it may be metabolised. The nature and extent of the
metabolism is well documented.
A number of studies are available to show the distribution and
fate of carbaryl residues on various grains and milling fractions of
grains and prepared cereals subjected to cooking. Carbaryl residues
are not completely destroyed when prepared cereals are cooked but the
loss ranges from 75 to 99%, depending upon the cereal, the processing
and the cooking. The least loss occurs in the preparation of wholemeal
bread where a residue of the order of 1-1.5 mg/kg could remain in the
finished bread.
No residue data are presented for rye but, because of the close
similarity in physical composition and use of the two crops, the
residue data for wheat and its milled fractions can be translated to
rye and its milled fractions.
A number of national governments have already established maximum
residue limits for carbaryl on raw grains. The Joint Meeting has
previously recommended a limit of 3 mg/kg for rice (FAO/WHO 1968b) and
10 mg/kg for sorghum (FAO/WHO 1974b).
Extensive data are available to indicate the fate of such
residues on grain or forage when fed to livestock or poultry. These
indicate that the feeding of forage from treated small grain crops or
of mill offals from such grain or grain treated post-harvest, could
give rise to small but significant residues in animal tissues and
milk. The magnitude of such residues is not likely to be higher than
that arising from the feeding of other forage for which a limit of 100
mg/kg has been recommended. For this reason the limits for carbaryl
residues in meat, milk and eggs expressed as the parent compound are
confirmed.
In 1973 the Meeting advised that the AOAC colorimetric method
(Holden 1973) remained the method of choice for regulatory purposes.
This method only determines the carbaryl parent and the free 1-
naphthol.
The Meeting studied the recommendations made in 1973 and all data
available and came to the conclusion that the residues determined in
the supervised trials represented the carbaryl parent and that the
maximum residue limits were intended to represent only the sum of
carbaryl and 1-naphthol not withstanding the fact that the heading of
the list of recommendations for maximum residue limits indicates that
the limits are expressed in terms of carbaryl and metabolites.
RECOMMENDATIONS
The following maximum residue limits are recommended to cover
residues resulting from either pre-harvest or post-harvest use of
carbaryl. They refer to carbaryl only. The limit for rice is raised to
provide for postharvest application.
Commodity Limit (mg/kg)
Bran 20
Barley, oats, rice (in husk and hulled)
rye, wheat 5
wholemeal flour 2
Wheat flour (white) 0.2
(The level and fate of carbaryl residues in processed cereal products
is given in the monograph but, in keeping with accepted practice,
separate limits have not been proposed for such processed foods.)
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the effects of carbaryl on renal
function.
2. Further studies to resolve the differences in observations of
different investigators on reproductive physiology, especially
with regard to neuroendocrine and behavioural changes.
3. Details of analytical methods for use in the determination of
carbaryl and metabolite residues in raw grain, milled cereal
fractions, bread, meat and milk.
REFERENCES
Andrawes, N.R., Chancey, E.L., Crabtree, R.J., Herrett, R. A.
1972 and Weiden, M.H.J. Fate of Maphthyl-1-14C
Carbaryl in laying chickens. J. agr. Food Chem.,
20:608.
Argauer, R.J. J.agr. Food Chem., 17: 889
1969
Association of Official Analytical Chemists, "Official Methods of
1970 Analysis", 11th Ed., p.
Bengston, M., Cooper, L.M., and Grant-Taylor, F.J. A comparison
1975 of bioresmethrin, chlorpyrifosmethyl and
pirimiphos-methyl as grain protectants against
malathion resistant insects in wheat. Queensland
J. agr. Animal Sci., 32,(1): 51-78.
Bengston, M., Connell, M., Crook, I.D., Desmarchelier, J., Hart,
1976a R.J., Phillips, M., Snelson, J.T. and Sticka, R.
Field trials to compare CGA-20168 (methacriphos),
chlorpyrifos-methyl, fenitrothion,
pirimiphos-methyl and malathion for control of
malathion resistant insects infesting wheat in
Australia. Submitted for publication in J. Stored
Prod. Res. 1976.
Bengston, M., Connell, M., Desmarchelier, J., Phillips, Snelson,
1976b J.T., and Sticka, R. Report of study of new grain
protectant insecticides. Report to Australian
Wheat Board. June 1976.
Chattaway, F.D. J. Chem. Sox. 2495
1931
Conterio, W.A. Carbaryl residues in barley and wheat grain,
1969 mill fractions and forage. Report to Union Carbide
Corporation from Crop Chemical Testing Service,
Humbolt, Illinois.
Davies, R.A.H. Carbaryl plus pirimiphos-methyl for stored grain
1976a protection. 1 Laboratory bioassay and residue
analysis: Social Report for J.T. Snelson -
Pesticides Co-ordinator. ICI Australia Ltd., Rural
Division, August 1976.
Davies, R.A.H. Carbaryl plus pirimiphos-methyl for stored grain
1976b protection. 2 Silo pilot trials 1975-76. Special
Report for J.T. Snelson - Pesticides Co-Ordinator.
ICI Australia Ltd., Rural Division, August 1976.
Desmarchelier, J.M. Field trials with fenitrothion plus fenoxythrin
1976a and pirimiphos-methyl plus carbaryl. Part 1.
Biological efficiency. Preliminary report of
ongoing study. CSIRO Division of Entomology,
Canberra, Australia, August 1976.
Desmarchelier, J.M. Field trials with fenitrothion plus fenoxythrin
1976b and pirimiphos-methyl plus carbaryl. Part 2.
Residue studies - Preliminary report of ongoing
study. CSIRO Division of Entomology, Canberra,
Australia, August 1976.
Desmarchelier, J.M. Degradation of carbaryl, CGA 20168,
1976c fenitrothion, pirimiphos-methyl and carbaryl on
rice, oats and barley. Preliminary report of
ongoing study. CSIRO Division of Entomology,
Canberra, Australia. August 1976.
Desmarchelier, J.M. Analytical procedures for carbaryl. Personal
1976d Communication. CSIRC Division of Entomology,
Canberra, Australia. August 1976.
Desmarchelier, J.M. Esterification of phenols, at residue levels,
1976e in dilute base. (In preparation) CSIRO Division of
Entomology, Canberra, Australia.
Dorough, H.W. Animal feeding studies with carbaryl. Details to be
1974 provided by Union Carbide.
FAO/WHO 1973 evaluation of some pesticide residues in food.
1974b FAO/AGP/1973/9/1; WHO Pesticide Residues Series,
No. 3.
Greening, H.G. Wheat-protectant dust trials 1965-67. N.S.W.
1976 Department of Agriculture. Biological and Chemical
Research Institute, Rydalmere, N.S.W. Report
prepared for publication September 1976.
Holden, E.R. Gas chromatographic determination of residues
1973 of methylcarbamate insecticides in crops as their
2,4-dinitrophenyl ether derivatives. J.A.O.A.C.,
56-713.
Leininger, R.O. Carbaryl residues in wheat forage, Report to Union
1973 Carbide Corporation from R.O. Leininger,
Greenfield. California,
Lumière, A., Lumière, L., and Barbier, H. Bull. Soc.Chim.
1906 Fr., 35: 625.
Phillips, I.L. Carbaryl residues in barley heads, grain and
1972 forage. Report to Union Carbide Corporation from
Soilserv. Inc., Salinas, California.
Pschorr, R., and Sumuleanu, P. Ber. der Deutsch. Chem. Ges.,
1899 22: 3407.
Roan, C.L. Comparison of performance of various carbaryl formulations
1964 against rice weevil and confused flour beetle.
Report from Entomology Department, Kansas State
University to Union Carbide Corporation.
Snelson, J.T. and Desmarchelier, J.M. The significance of pesticide
1975 residues. Proc. 1st int. Wking Conf.
Stored-product Entomology. Savannah, Georgia,
U.S.A., 1974, p. 465-477.
Strong, R.G., and Sbur, D.E. Evaluation of insecticides as
1961 protectants against pests of stored grains and
seeds. J. Econ. Entom., 54: 235-238.
Tilden, R.L., and van Middelem, C.H. Determination of carbaryl
1970 as an amide derivative by electron capture GLC. J.
Agric. Food Chem., 18(1): 154-6.
Union Carbide. Summary of studies on the nature of residues of
1974 carbaryl in the meat of dairy cattle. Union
Carbide Corporation Report, May 24, 1974.
Union Carbide. Submission to FAO by Union Carbide Corporation,
1976a September 1976.
Union Carbide Corporation. Results of tests investigating
1976b Sevin as a stored grain insecticide (1958-1962).
Extract of petition 2859.
 TABLE 5. Residues in wheat after laboratory application of carbaryl and/or
pirimiphos-methyl
Residues recovered by GLC analysis,
Calculated mg/kg
rate
Treatment mg/kg Day 1 Week 9 Week 18 Week 26 Week 39
Carbaryl 5 3.1 2.8 - - 1.9
Carbaryl 10 6.5 9.8 5.0 4.3 4.2
Pirimiphos-
methyl 6 5.1 - - - 3.1
Pirimiphos-
methyl 6 4.2 - - - 2.3
+ +
Carbaryl 5 3.4 3.1 - - 2.5
Pirimiphos-
methyl 6 1.8 - - - 1.1
+
Carbaryl 10 5.7 5.4 5.0 4.5 4.6
FATE OF RESIDUES
In animals
Ingested carbaryl is rapidly metabolised in cows and other
animals, 70-80% being excreted in urine within 24 hours. In a
continuous feeding study in cows, equilibration of total radioactive
residues in milk, urine and faeces occurred by the second day. Within
18 hours after the last of 14 days continuous feeding, the highest
total of residues were found in the kidneys. These data are summarised
in Table 6. The lowest residues were found in fat, indicating that
metabolites are not stored in body tissues. The major components of
the residue in tissue were carbaryl, naphthol, naphthyl sulphate,
5,6-dihydrodihydroxycarbaryl and 5,6-dihydrodihydroxynaphthol. Methods
are available to determine these compounds in beef kidney and liver.
Of the radioactivity appearing in the milk, carbaryl per se comprised
less than 10%. The two principal residues were
5,6-dihydrodihydroxycarbaryl and conjugated
5-methoxy-6-hydroxycarbaryl. An analytical method is available to
measure the major metabolites in milk. The concentration of the total
radioactive residue in the milk was only about 1/300, and in the
tissues 1/100 or less of the carbaryl concentration in the feed. These
ratios and the composition of the metabolites in the milk did not vary
significantly over the three feeding levels studied (Dorough, 1974;
Union Carbide Corporation, 1974).
TABLE 6. Residues in meat and milk from carbaryl in feed.
Total 14C expressed as carbaryl (mg/kg) at
indicated feeding levels (ppm)
Tissue,
etc. 10 ppm 30 ppm 100 ppm
Kidney 0.095 0.531 1.003
Liver 0.033 0.100 0.411
Milk 0.024 0.071 0.278
fat 0.000 0.015 0.025
Ingested carbaryl is also rapidly metabolised in poultry by
pathways similar to those in mammals. In continuous feeding studies
with radio-labelled carbaryl (Andrawes et al, 1972), residues reached
maximum levels within one day in the excrement, 2 days in egg white
and 6-8 days in egg yolk. The residues in the whole egg (yolk plus
white) were directly proportional to the amount of carbaryl fed. An
intake of 7 mg/kg of carbaryl in the feed resulted in a residue of
0.04 mg/kg carbaryl equivalents in the whole egg. Radio-labelled
residues in the excrement 15 hours after the initial treatment reached
80-100% of the dose. Within one day after the discontinuation of
dosing, the highest tissue residues were found in the excretory organs
while very low residues were found in the fat indicating that carbaryl
residues are not stored in body tissues. This work shows that carbaryl
is metabolised in laying hens by pathways similar to those in mammals.
In plants
Of the carbaryl deposited on plant surfaces, only the relatively
small proportion which penetrates the plant tissues is metabolised.
Once in the plant, the insecticide is largely transformed by
hydrolysis or oxidation to several hydroxylated metabolites. These
compounds in turn are rapidly conjugated to form watersoluble
glycosides. The metabolic transformations which take place are similar
whether the compound is applied by surface application, root
absorption or artificially by stem injections. In general, all plant
and animal metabolites of carbaryl are considered to be less toxic
than the parent compound, some substantially so.
The metabolites found in greatest abundance in plants and those
judged of most toxicological significance are conjugated 1-naphthol,
conjugated methylol carbaryl, and carbaryl per se. The latter is found
in both the free state and/or conjugated with plant constituents. The
residue methods available are capable of releasing the metabolites
from their conjugates and determining their concentration either
individually or collectively. The remaining metabolites are of either
no toxicological or no quantitative significance.
TABLE 7. Effect of milling and baking on carbaryl residues in wheat
Location Location
N B
Carbaryl applied, mg/kg 5 10
Carbaryl recovered, day 1, mg/kg 3.4 6.5
Wheat withdrawn after 19 weeks 13 weeks
Residues in wheat, mg/kg 3.1 6.0
Residues in mill fractions, mg/kg
wholemeal flour 1.2 2.6
wholemeal bread 0.7 1.5
bran 7.0 14
shorts 1.5 3
white flour 0.07 0.15
white bread <0.05 0.08
Reduction in residues, %
wheat/wholemeal bread 77 75
wholemeal flour/wholemeal bread 42 42
wheat/white flour 97 97
white flour/white bread 50 50
wheat/white bread 98 99
In processing and cooking
A number of studies are available to show the distribution and
fate of carbaryl residues on various grains, milling fractions of
grains and prepared cereals subjected to cooking.
Table 1 indicates the distribution of free carbaryl residues and
Table 4 the distribution of carbaryl and its metabolites, in grain and
milling fractions of barley and wheat following the pre-harvest
application of carbaryl. Again these results show that free carbaryl
and conjugated carbaryl together represent the major proportion of the
residues. These data indicate that residues resulting from pre-harvest
application are substantially confined to the outer portion of the
grain and therefore do not find their way into flour. There is a
significant partitioning of the residues in the milling offals.
Desmarchelier (1976b) showed that wheat treated with carbaryl 3-5
months previously but still containing residues of 3-6 mg/kg carbaryl
when converted into wholemeal flour, yielded a flour containing 1-3
mg/kg of carbaryl. When the same grain was milled for the preparation
of white flour (approximately 70% extraction), the residue level in
flour was reduced to about 0.1 mg/kg. The baking of wholemeal or white
bread resulted in a further loss of about half the carbaryl content,
bringing the final residue in wholemeal bread into the range of 1
mg/kg, and in white bread to below 0.1 mg/kg. Thus the reduction in
residues between raw grain and white bread was 99%, and between grain
and wholemeal bread about 75% (Table 7). Using other wheat containing
carbaryl, residues in the range 2.5-3 mg/kg, Desmarchelier (1976b)
showed that the final residue in white bread was less than 0.05 mg/kg
and in wholemeal bread 0.5 mg/kg.
In a series of experiments representing the most primitive type
of processing to which husked rice, polished rice, oats and barley
would be subjected, Desmarchelier (1976c) showed that simple boiling
in minimal amounts of water for 15 minutes reduced carbaryl residues
in husked rice, polished rice and oats by 84%. A primitive malting
procedure reduced residues in barley by 77% (Table 8).
METHODS OF RESIDUE ANALYSIS
Methods of residue analysis have been dealt with at length in
previous monographs. A number of modifications were developed to
obtain the data on grain, forage and milling offals as well as beef
tissues and milk. References to these methods were provided by Union
Carbide Ltd. The method of Holden (1973) is widely used for the
determination of carbaryl residues on a number of commodities.
Desmarchelier (1976e) using the principles discovered by Pschorr
and Sumuleanu (1899) and Lumiere et al (1906) and further developed by
Chattaway (1931) has developed a method for the esterification of
phenols, at residue levels, in dilute aqueous base. The method is
based on a procedure for the acetylation of amines by reaction with
anhydrides in aqueous solution which is successful because amines
react with certain anhydrides more quickly than the anhydrides are
hydrolyzed. Chattaway's work extended this reaction to phenols, by
reacting them at 0°C in dilute sodium hydroxide with acetic anhydride.
Acetylation was quantitative and instantaneous. By using Chattaway's
procedure and either acetic or propionic anhydride as reagents,
TABLE 8. Effect of storage at 25°C, processing and cooking on carbaryl residues in treated grains
Residue, mg/kg,
Application after storage for Processed Residue, mg/kg
Moisture rate 3 6 after Processed after cooking for
Grain % mg/kg months months (months) into - 15 min 25 min
Barley 13 10.6 6.5 3.5 3 Primitive 1.5
malt
6 Commercial 0.2
malt
Oats 12 10.0 7.5 3.5 3 Rolled - 1.2
oats
Rice in husk 13 10.0 7.5 3.5 6 Husked 0.4 0.2
6 Milled 0.07 <0.05
Husked rice 12.5 10.0 7.5 3.4 3 Cooked 1.2
6 Cooked 0.7
Polished rice 12.7 10.0 7.5 3.5 3 Cooked 1.2
Wheat 12 10.0 7.2 6.3 5 wholemeal 2.5 1.5
(4.2 after
storage for
9 months)
Desmarchelier (1976d) esterified the phenols from parathion,
fenitrothion, chlorpyrifos, fenchlorphos, cyanophos and carbaryl.
To test the procedure for determining carbaryl in grain,
Desmarchelier extracted 10 g of wheat with 25 ml of acetone for 24
hours. An aliquot of 1 ml was hydrolysed with 1 ml of 1 M sodium
hydroxide in 90% ethanol (one hour at ambient temperature) and mixture
diluted and washed with ether. The aqueous solution was adjusted to pH
10 and stirred at 0°C with 2 x 20 ml of 1% chloracetic anhydride in
ether. The organic extracts were diluted, dried and analysed by gas
chromatography on 5% SE-30 Chromosorb W at 175°C with electron capture
detection. Recoveries were better than 90% at residue levels in the
range 0.1-5 mg/kg. This procedure was used for developing much of the
data on the level of carbaryl residues on grain and milling fractions
following post-harvest use of carbaryl as a grain protectant.
Desmarchelier (1976d) evaluated two TLC procedures with
fluorescent layers suitable for estimating carbaryl residues on grain.
One used aluminium oxide developed with 15% ethyl acetate/85% hexane,
and the other polyamide developed with 50% methanol/50% water.
Carbaryl was detected by hydrolysing with 1 M sodium hydroxide in 90%
ethanol. 1-naphthol shows as a blue colour at 254 nm with a detection
limit of 0.1 µg.
The method outlined in "Official Methods of Analysis", of the
Association of Official Analytical Chemists, 11th Edition (1970),
Section 29.071. for the use of chromogenic reagent is both more
precise and more sensitive when using the polyamide system. 0.05-0.1
microgram can be readily detected. Desmarchelier (1976d) indicated
that similar results were obtained on aged residues of carbaryl on
wheat. Acetone proved the most suitable solvent, giving good
resolution free of interference from water. Although the GLC procedure
described above is more precise and more sensitive, the TLC procedure
is simpler and requires less expensive equipment.
A paper on the significance of pesticide residues which discussed
the importance and variability of analytical procedures applied for
the determination of residues on raw grains, was presented at the
International Working Conference on Stored Product Entomology (Snelson
and Desmarchelier, 1975).
NATIONAL TOLERANCES
References to the following national tolerances for carbaryl
residues in raw grain were reported to the Meeting.
TABLE 9. National tolerances for carbaryl on grains reported to the
Meeting
Tolerance
Country Grain mg/kg
Argentina barley grains, oats, rye, wheat 0
rice 2
Australia rice 3
Belgium raw rice 0.8
Canada barley, oats, rye, wheat 2
Germany rice 0.8
India rice 1.25
Israel rice 2.5
Japan rice (unpolished) 0.1
Netherlands rice (coarse) 0.8
South Africa all food products 10
Switzerland rice 2.5
U.S.A. grains of barley, oats, rye, wheat 0
rice 1
U.S.S.R. maize not permitted
APPRAISAL
Following the evaluation of carbaryl by the Joint Meeting on a
number of occasions (FAO/WHO, 1965b, 1967b, 1968b, 1969b, 1970b,
1971b, 1974b, 1976b) the Meeting was informed of two important
applications for carbaryl:
(a) the control of pests of small grain crops previously
controlled with organochlorine or organophosphorus compounds, and
(b) the protection of stored grain, particularly for the control
of lesser grain borer, Rhyzopertha dominica, when used in
conjunction with approved organophosphorus insecticides.
Extensive new data on these uses and the level and fate of
residues resulting from such applications, have been made available to
the Joint Meeting.
For pre-harvest use the rate of application ranges from 0.5 to 2
kg/ha depending upon the degree of infestation, density of foliage and
whether or not the pests are in advanced stages of their life cycle.
For post-harvest use the rate of application depends upon the humidity
and temperature of the grain, whether high temperatures will be
maintained during storage, whether the grain will be aerated, and the
expected period in storage. Treatment is usually at the rate of 5
mg/kg but hotter grain kept in unaerated storage for long periods will
require a higher rate of application or repeated treatment.
Extensive studies in the U.S.A. indicate that when carbaryl is
used for the pre-harvest control of major pests of small grain crops,
residues of carbaryl and its more important metabolites in the grain
will usually range between 1 and 3 mg/kg, though some data indicate
the likelihood of residues reaching the vicinity of 4 mg/kg, when the
pre-harvest interval is 21 days. In the case of treatments made 14 to
7 days prior to harvest, the residues may be significantly higher but
it is generally considered that such treatments would seldom be
necessary.
Such spray treatments give rise to residues on the forage of
grain crops ranging up to 50 mg/kg on the day following application.
However, the loss of residues from the forage is rapid so that 21 days
later they seldom exceed 1 mg/kg.
Extensive studies on stored wheat and confirmatory data from
trials on barley, oats and rice indicate that carbaryl residues on
grain are relatively stable having a half-life between 26 and 80
weeks, depending upon the temperature.
Studies with radio-labelled carbaryl indicate that only a
relatively small proportion of the amount applied penetrates the plant
tissue where it may be metabolised. The nature and extent of the
metabolism is well documented.
A number of studies are available to show the distribution and
fate of carbaryl residues on various grains and milling fractions of
grains and prepared cereals subjected to cooking. Carbaryl residues
are not completely destroyed when prepared cereals are cooked but the
loss ranges from 75 to 99%, depending upon the cereal, the processing
and the cooking. The least loss occurs in the preparation of wholemeal
bread where a residue of the order of 1-1.5 mg/kg could remain in the
finished bread.
No residue data are presented for rye but, because of the close
similarity in physical composition and use of the two crops, the
residue data for wheat and its milled fractions can be translated to
rye and its milled fractions.
A number of national governments have already established maximum
residue limits for carbaryl on raw grains. The Joint Meeting has
previously recommended a limit of 3 mg/kg for rice (FAO/WHO 1968b) and
10 mg/kg for sorghum (FAO/WHO 1974b).
Extensive data are available to indicate the fate of such
residues on grain or forage when fed to livestock or poultry. These
indicate that the feeding of forage from treated small grain crops or
of mill offals from such grain or grain treated post-harvest, could
give rise to small but significant residues in animal tissues and
milk. The magnitude of such residues is not likely to be higher than
that arising from the feeding of other forage for which a limit of 100
mg/kg has been recommended. For this reason the limits for carbaryl
residues in meat, milk and eggs expressed as the parent compound are
confirmed.
In 1973 the Meeting advised that the AOAC colorimetric method
(Holden 1973) remained the method of choice for regulatory purposes.
This method only determines the carbaryl parent and the free 1-
naphthol.
The Meeting studied the recommendations made in 1973 and all data
available and came to the conclusion that the residues determined in
the supervised trials represented the carbaryl parent and that the
maximum residue limits were intended to represent only the sum of
carbaryl and 1-naphthol not withstanding the fact that the heading of
the list of recommendations for maximum residue limits indicates that
the limits are expressed in terms of carbaryl and metabolites.
RECOMMENDATIONS
The following maximum residue limits are recommended to cover
residues resulting from either pre-harvest or post-harvest use of
carbaryl. They refer to carbaryl only. The limit for rice is raised to
provide for postharvest application.
Commodity Limit (mg/kg)
Bran 20
Barley, oats, rice (in husk and hulled)
rye, wheat 5
wholemeal flour 2
Wheat flour (white) 0.2
(The level and fate of carbaryl residues in processed cereal products
is given in the monograph but, in keeping with accepted practice,
separate limits have not been proposed for such processed foods.)
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the effects of carbaryl on renal
function.
2. Further studies to resolve the differences in observations of
different investigators on reproductive physiology, especially
with regard to neuroendocrine and behavioural changes.
3. Details of analytical methods for use in the determination of
carbaryl and metabolite residues in raw grain, milled cereal
fractions, bread, meat and milk.
REFERENCES
Andrawes, N.R., Chancey, E.L., Crabtree, R.J., Herrett, R. A.
1972 and Weiden, M.H.J. Fate of Maphthyl-1-14C
Carbaryl in laying chickens. J. agr. Food Chem.,
20:608.
Argauer, R.J. J.agr. Food Chem., 17: 889
1969
Association of Official Analytical Chemists, "Official Methods of
1970 Analysis", 11th Ed., p.
Bengston, M., Cooper, L.M., and Grant-Taylor, F.J. A comparison
1975 of bioresmethrin, chlorpyrifosmethyl and
pirimiphos-methyl as grain protectants against
malathion resistant insects in wheat. Queensland
J. agr. Animal Sci., 32,(1): 51-78.
Bengston, M., Connell, M., Crook, I.D., Desmarchelier, J., Hart,
1976a R.J., Phillips, M., Snelson, J.T. and Sticka, R.
Field trials to compare CGA-20168 (methacriphos),
chlorpyrifos-methyl, fenitrothion,
pirimiphos-methyl and malathion for control of
malathion resistant insects infesting wheat in
Australia. Submitted for publication in J. Stored
Prod. Res. 1976.
Bengston, M., Connell, M., Desmarchelier, J., Phillips, Snelson,
1976b J.T., and Sticka, R. Report of study of new grain
protectant insecticides. Report to Australian
Wheat Board. June 1976.
Chattaway, F.D. J. Chem. Sox. 2495
1931
Conterio, W.A. Carbaryl residues in barley and wheat grain,
1969 mill fractions and forage. Report to Union Carbide
Corporation from Crop Chemical Testing Service,
Humbolt, Illinois.
Davies, R.A.H. Carbaryl plus pirimiphos-methyl for stored grain
1976a protection. 1 Laboratory bioassay and residue
analysis: Social Report for J.T. Snelson -
Pesticides Co-ordinator. ICI Australia Ltd., Rural
Division, August 1976.
Davies, R.A.H. Carbaryl plus pirimiphos-methyl for stored grain
1976b protection. 2 Silo pilot trials 1975-76. Special
Report for J.T. Snelson - Pesticides Co-Ordinator.
ICI Australia Ltd., Rural Division, August 1976.
Desmarchelier, J.M. Field trials with fenitrothion plus fenoxythrin
1976a and pirimiphos-methyl plus carbaryl. Part 1.
Biological efficiency. Preliminary report of
ongoing study. CSIRO Division of Entomology,
Canberra, Australia, August 1976.
Desmarchelier, J.M. Field trials with fenitrothion plus fenoxythrin
1976b and pirimiphos-methyl plus carbaryl. Part 2.
Residue studies - Preliminary report of ongoing
study. CSIRO Division of Entomology, Canberra,
Australia, August 1976.
Desmarchelier, J.M. Degradation of carbaryl, CGA 20168,
1976c fenitrothion, pirimiphos-methyl and carbaryl on
rice, oats and barley. Preliminary report of
ongoing study. CSIRO Division of Entomology,
Canberra, Australia. August 1976.
Desmarchelier, J.M. Analytical procedures for carbaryl. Personal
1976d Communication. CSIRC Division of Entomology,
Canberra, Australia. August 1976.
Desmarchelier, J.M. Esterification of phenols, at residue levels,
1976e in dilute base. (In preparation) CSIRO Division of
Entomology, Canberra, Australia.
Dorough, H.W. Animal feeding studies with carbaryl. Details to be
1974 provided by Union Carbide.
FAO/WHO 1973 evaluation of some pesticide residues in food.
1974b FAO/AGP/1973/9/1; WHO Pesticide Residues Series,
No. 3.
Greening, H.G. Wheat-protectant dust trials 1965-67. N.S.W.
1976 Department of Agriculture. Biological and Chemical
Research Institute, Rydalmere, N.S.W. Report
prepared for publication September 1976.
Holden, E.R. Gas chromatographic determination of residues
1973 of methylcarbamate insecticides in crops as their
2,4-dinitrophenyl ether derivatives. J.A.O.A.C.,
56-713.
Leininger, R.O. Carbaryl residues in wheat forage, Report to Union
1973 Carbide Corporation from R.O. Leininger,
Greenfield. California,
Lumière, A., Lumière, L., and Barbier, H. Bull. Soc.Chim.
1906 Fr., 35: 625.
Phillips, I.L. Carbaryl residues in barley heads, grain and
1972 forage. Report to Union Carbide Corporation from
Soilserv. Inc., Salinas, California.
Pschorr, R., and Sumuleanu, P. Ber. der Deutsch. Chem. Ges.,
1899 22: 3407.
Roan, C.L. Comparison of performance of various carbaryl formulations
1964 against rice weevil and confused flour beetle.
Report from Entomology Department, Kansas State
University to Union Carbide Corporation.
Snelson, J.T. and Desmarchelier, J.M. The significance of pesticide
1975 residues. Proc. 1st int. Wking Conf.
Stored-product Entomology. Savannah, Georgia,
U.S.A., 1974, p. 465-477.
Strong, R.G., and Sbur, D.E. Evaluation of insecticides as
1961 protectants against pests of stored grains and
seeds. J. Econ. Entom., 54: 235-238.
Tilden, R.L., and van Middelem, C.H. Determination of carbaryl
1970 as an amide derivative by electron capture GLC. J.
Agric. Food Chem., 18(1): 154-6.
Union Carbide. Summary of studies on the nature of residues of
1974 carbaryl in the meat of dairy cattle. Union
Carbide Corporation Report, May 24, 1974.
Union Carbide. Submission to FAO by Union Carbide Corporation,
1976a September 1976.
Union Carbide Corporation. Results of tests investigating
1976b Sevin as a stored grain insecticide (1958-1962).
Extract of petition 2859.
TABLE 5. Residues in wheat after laboratory application of carbaryl and/or
pirimiphos-methyl
Residues recovered by GLC analysis,
Calculated mg/kg
rate
Treatment mg/kg Day 1 Week 9 Week 18 Week 26 Week 39
Carbaryl 5 3.1 2.8 - - 1.9
Carbaryl 10 6.5 9.8 5.0 4.3 4.2
Pirimiphos-
methyl 6 5.1 - - - 3.1
Pirimiphos-
methyl 6 4.2 - - - 2.3
+ +
Carbaryl 5 3.4 3.1 - - 2.5
Pirimiphos-
methyl 6 1.8 - - - 1.1
+
Carbaryl 10 5.7 5.4 5.0 4.5 4.6
FATE OF RESIDUES
In animals
Ingested carbaryl is rapidly metabolised in cows and other
animals, 70-80% being excreted in urine within 24 hours. In a
continuous feeding study in cows, equilibration of total radioactive
residues in milk, urine and faeces occurred by the second day. Within
18 hours after the last of 14 days continuous feeding, the highest
total of residues were found in the kidneys. These data are summarised
in Table 6. The lowest residues were found in fat, indicating that
metabolites are not stored in body tissues. The major components of
the residue in tissue were carbaryl, naphthol, naphthyl sulphate,
5,6-dihydrodihydroxycarbaryl and 5,6-dihydrodihydroxynaphthol. Methods
are available to determine these compounds in beef kidney and liver.
Of the radioactivity appearing in the milk, carbaryl per se comprised
less than 10%. The two principal residues were
5,6-dihydrodihydroxycarbaryl and conjugated
5-methoxy-6-hydroxycarbaryl. An analytical method is available to
measure the major metabolites in milk. The concentration of the total
radioactive residue in the milk was only about 1/300, and in the
tissues 1/100 or less of the carbaryl concentration in the feed. These
ratios and the composition of the metabolites in the milk did not vary
significantly over the three feeding levels studied (Dorough, 1974;
Union Carbide Corporation, 1974).
TABLE 6. Residues in meat and milk from carbaryl in feed.
Total 14C expressed as carbaryl (mg/kg) at
indicated feeding levels (ppm)
Tissue,
etc. 10 ppm 30 ppm 100 ppm
Kidney 0.095 0.531 1.003
Liver 0.033 0.100 0.411
Milk 0.024 0.071 0.278
fat 0.000 0.015 0.025
Ingested carbaryl is also rapidly metabolised in poultry by
pathways similar to those in mammals. In continuous feeding studies
with radio-labelled carbaryl (Andrawes et al, 1972), residues reached
maximum levels within one day in the excrement, 2 days in egg white
and 6-8 days in egg yolk. The residues in the whole egg (yolk plus
white) were directly proportional to the amount of carbaryl fed. An
intake of 7 mg/kg of carbaryl in the feed resulted in a residue of
0.04 mg/kg carbaryl equivalents in the whole egg. Radio-labelled
residues in the excrement 15 hours after the initial treatment reached
80-100% of the dose. Within one day after the discontinuation of
dosing, the highest tissue residues were found in the excretory organs
while very low residues were found in the fat indicating that carbaryl
residues are not stored in body tissues. This work shows that carbaryl
is metabolised in laying hens by pathways similar to those in mammals.
In plants
Of the carbaryl deposited on plant surfaces, only the relatively
small proportion which penetrates the plant tissues is metabolised.
Once in the plant, the insecticide is largely transformed by
hydrolysis or oxidation to several hydroxylated metabolites. These
compounds in turn are rapidly conjugated to form watersoluble
glycosides. The metabolic transformations which take place are similar
whether the compound is applied by surface application, root
absorption or artificially by stem injections. In general, all plant
and animal metabolites of carbaryl are considered to be less toxic
than the parent compound, some substantially so.
The metabolites found in greatest abundance in plants and those
judged of most toxicological significance are conjugated 1-naphthol,
conjugated methylol carbaryl, and carbaryl per se. The latter is found
in both the free state and/or conjugated with plant constituents. The
residue methods available are capable of releasing the metabolites
from their conjugates and determining their concentration either
individually or collectively. The remaining metabolites are of either
no toxicological or no quantitative significance.
TABLE 7. Effect of milling and baking on carbaryl residues in wheat
Location Location
N B
Carbaryl applied, mg/kg 5 10
Carbaryl recovered, day 1, mg/kg 3.4 6.5
Wheat withdrawn after 19 weeks 13 weeks
Residues in wheat, mg/kg 3.1 6.0
Residues in mill fractions, mg/kg
wholemeal flour 1.2 2.6
wholemeal bread 0.7 1.5
bran 7.0 14
shorts 1.5 3
white flour 0.07 0.15
white bread <0.05 0.08
Reduction in residues, %
wheat/wholemeal bread 77 75
wholemeal flour/wholemeal bread 42 42
wheat/white flour 97 97
white flour/white bread 50 50
wheat/white bread 98 99
In processing and cooking
A number of studies are available to show the distribution and
fate of carbaryl residues on various grains, milling fractions of
grains and prepared cereals subjected to cooking.
Table 1 indicates the distribution of free carbaryl residues and
Table 4 the distribution of carbaryl and its metabolites, in grain and
milling fractions of barley and wheat following the pre-harvest
application of carbaryl. Again these results show that free carbaryl
and conjugated carbaryl together represent the major proportion of the
residues. These data indicate that residues resulting from pre-harvest
application are substantially confined to the outer portion of the
grain and therefore do not find their way into flour. There is a
significant partitioning of the residues in the milling offals.
Desmarchelier (1976b) showed that wheat treated with carbaryl 3-5
months previously but still containing residues of 3-6 mg/kg carbaryl
when converted into wholemeal flour, yielded a flour containing 1-3
mg/kg of carbaryl. When the same grain was milled for the preparation
of white flour (approximately 70% extraction), the residue level in
flour was reduced to about 0.1 mg/kg. The baking of wholemeal or white
bread resulted in a further loss of about half the carbaryl content,
bringing the final residue in wholemeal bread into the range of 1
mg/kg, and in white bread to below 0.1 mg/kg. Thus the reduction in
residues between raw grain and white bread was 99%, and between grain
and wholemeal bread about 75% (Table 7). Using other wheat containing
carbaryl, residues in the range 2.5-3 mg/kg, Desmarchelier (1976b)
showed that the final residue in white bread was less than 0.05 mg/kg
and in wholemeal bread 0.5 mg/kg.
In a series of experiments representing the most primitive type
of processing to which husked rice, polished rice, oats and barley
would be subjected, Desmarchelier (1976c) showed that simple boiling
in minimal amounts of water for 15 minutes reduced carbaryl residues
in husked rice, polished rice and oats by 84%. A primitive malting
procedure reduced residues in barley by 77% (Table 8).
METHODS OF RESIDUE ANALYSIS
Methods of residue analysis have been dealt with at length in
previous monographs. A number of modifications were developed to
obtain the data on grain, forage and milling offals as well as beef
tissues and milk. References to these methods were provided by Union
Carbide Ltd. The method of Holden (1973) is widely used for the
determination of carbaryl residues on a number of commodities.
Desmarchelier (1976e) using the principles discovered by Pschorr
and Sumuleanu (1899) and Lumiere et al (1906) and further developed by
Chattaway (1931) has developed a method for the esterification of
phenols, at residue levels, in dilute aqueous base. The method is
based on a procedure for the acetylation of amines by reaction with
anhydrides in aqueous solution which is successful because amines
react with certain anhydrides more quickly than the anhydrides are
hydrolyzed. Chattaway's work extended this reaction to phenols, by
reacting them at 0°C in dilute sodium hydroxide with acetic anhydride.
Acetylation was quantitative and instantaneous. By using Chattaway's
procedure and either acetic or propionic anhydride as reagents,
TABLE 8. Effect of storage at 25°C, processing and cooking on carbaryl residues in treated grains
Residue, mg/kg,
Application after storage for Processed Residue, mg/kg
Moisture rate 3 6 after Processed after cooking for
Grain % mg/kg months months (months) into - 15 min 25 min
Barley 13 10.6 6.5 3.5 3 Primitive 1.5
malt
6 Commercial 0.2
malt
Oats 12 10.0 7.5 3.5 3 Rolled - 1.2
oats
Rice in husk 13 10.0 7.5 3.5 6 Husked 0.4 0.2
6 Milled 0.07 <0.05
Husked rice 12.5 10.0 7.5 3.4 3 Cooked 1.2
6 Cooked 0.7
Polished rice 12.7 10.0 7.5 3.5 3 Cooked 1.2
Wheat 12 10.0 7.2 6.3 5 wholemeal 2.5 1.5
(4.2 after
storage for
9 months)
Desmarchelier (1976d) esterified the phenols from parathion,
fenitrothion, chlorpyrifos, fenchlorphos, cyanophos and carbaryl.
To test the procedure for determining carbaryl in grain,
Desmarchelier extracted 10 g of wheat with 25 ml of acetone for 24
hours. An aliquot of 1 ml was hydrolysed with 1 ml of 1 M sodium
hydroxide in 90% ethanol (one hour at ambient temperature) and mixture
diluted and washed with ether. The aqueous solution was adjusted to pH
10 and stirred at 0°C with 2 x 20 ml of 1% chloracetic anhydride in
ether. The organic extracts were diluted, dried and analysed by gas
chromatography on 5% SE-30 Chromosorb W at 175°C with electron capture
detection. Recoveries were better than 90% at residue levels in the
range 0.1-5 mg/kg. This procedure was used for developing much of the
data on the level of carbaryl residues on grain and milling fractions
following post-harvest use of carbaryl as a grain protectant.
Desmarchelier (1976d) evaluated two TLC procedures with
fluorescent layers suitable for estimating carbaryl residues on grain.
One used aluminium oxide developed with 15% ethyl acetate/85% hexane,
and the other polyamide developed with 50% methanol/50% water.
Carbaryl was detected by hydrolysing with 1 M sodium hydroxide in 90%
ethanol. 1-naphthol shows as a blue colour at 254 nm with a detection
limit of 0.1 µg.
The method outlined in "Official Methods of Analysis", of the
Association of Official Analytical Chemists, 11th Edition (1970),
Section 29.071. for the use of chromogenic reagent is both more
precise and more sensitive when using the polyamide system. 0.05-0.1
microgram can be readily detected. Desmarchelier (1976d) indicated
that similar results were obtained on aged residues of carbaryl on
wheat. Acetone proved the most suitable solvent, giving good
resolution free of interference from water. Although the GLC procedure
described above is more precise and more sensitive, the TLC procedure
is simpler and requires less expensive equipment.
A paper on the significance of pesticide residues which discussed
the importance and variability of analytical procedures applied for
the determination of residues on raw grains, was presented at the
International Working Conference on Stored Product Entomology (Snelson
and Desmarchelier, 1975).
NATIONAL TOLERANCES
References to the following national tolerances for carbaryl
residues in raw grain were reported to the Meeting.
TABLE 9. National tolerances for carbaryl on grains reported to the
Meeting
Tolerance
Country Grain mg/kg
Argentina barley grains, oats, rye, wheat 0
rice 2
Australia rice 3
Belgium raw rice 0.8
Canada barley, oats, rye, wheat 2
Germany rice 0.8
India rice 1.25
Israel rice 2.5
Japan rice (unpolished) 0.1
Netherlands rice (coarse) 0.8
South Africa all food products 10
Switzerland rice 2.5
U.S.A. grains of barley, oats, rye, wheat 0
rice 1
U.S.S.R. maize not permitted
APPRAISAL
Following the evaluation of carbaryl by the Joint Meeting on a
number of occasions (FAO/WHO, 1965b, 1967b, 1968b, 1969b, 1970b,
1971b, 1974b, 1976b) the Meeting was informed of two important
applications for carbaryl:
(a) the control of pests of small grain crops previously
controlled with organochlorine or organophosphorus compounds, and
(b) the protection of stored grain, particularly for the control
of lesser grain borer, Rhyzopertha dominica, when used in
conjunction with approved organophosphorus insecticides.
Extensive new data on these uses and the level and fate of
residues resulting from such applications, have been made available to
the Joint Meeting.
For pre-harvest use the rate of application ranges from 0.5 to 2
kg/ha depending upon the degree of infestation, density of foliage and
whether or not the pests are in advanced stages of their life cycle.
For post-harvest use the rate of application depends upon the humidity
and temperature of the grain, whether high temperatures will be
maintained during storage, whether the grain will be aerated, and the
expected period in storage. Treatment is usually at the rate of 5
mg/kg but hotter grain kept in unaerated storage for long periods will
require a higher rate of application or repeated treatment.
Extensive studies in the U.S.A. indicate that when carbaryl is
used for the pre-harvest control of major pests of small grain crops,
residues of carbaryl and its more important metabolites in the grain
will usually range between 1 and 3 mg/kg, though some data indicate
the likelihood of residues reaching the vicinity of 4 mg/kg, when the
pre-harvest interval is 21 days. In the case of treatments made 14 to
7 days prior to harvest, the residues may be significantly higher but
it is generally considered that such treatments would seldom be
necessary.
Such spray treatments give rise to residues on the forage of
grain crops ranging up to 50 mg/kg on the day following application.
However, the loss of residues from the forage is rapid so that 21 days
later they seldom exceed 1 mg/kg.
Extensive studies on stored wheat and confirmatory data from
trials on barley, oats and rice indicate that carbaryl residues on
grain are relatively stable having a half-life between 26 and 80
weeks, depending upon the temperature.
Studies with radio-labelled carbaryl indicate that only a
relatively small proportion of the amount applied penetrates the plant
tissue where it may be metabolised. The nature and extent of the
metabolism is well documented.
A number of studies are available to show the distribution and
fate of carbaryl residues on various grains and milling fractions of
grains and prepared cereals subjected to cooking. Carbaryl residues
are not completely destroyed when prepared cereals are cooked but the
loss ranges from 75 to 99%, depending upon the cereal, the processing
and the cooking. The least loss occurs in the preparation of wholemeal
bread where a residue of the order of 1-1.5 mg/kg could remain in the
finished bread.
No residue data are presented for rye but, because of the close
similarity in physical composition and use of the two crops, the
residue data for wheat and its milled fractions can be translated to
rye and its milled fractions.
A number of national governments have already established maximum
residue limits for carbaryl on raw grains. The Joint Meeting has
previously recommended a limit of 3 mg/kg for rice (FAO/WHO 1968b) and
10 mg/kg for sorghum (FAO/WHO 1974b).
Extensive data are available to indicate the fate of such
residues on grain or forage when fed to livestock or poultry. These
indicate that the feeding of forage from treated small grain crops or
of mill offals from such grain or grain treated post-harvest, could
give rise to small but significant residues in animal tissues and
milk. The magnitude of such residues is not likely to be higher than
that arising from the feeding of other forage for which a limit of 100
mg/kg has been recommended. For this reason the limits for carbaryl
residues in meat, milk and eggs expressed as the parent compound are
confirmed.
In 1973 the Meeting advised that the AOAC colorimetric method
(Holden 1973) remained the method of choice for regulatory purposes.
This method only determines the carbaryl parent and the free 1-
naphthol.
The Meeting studied the recommendations made in 1973 and all data
available and came to the conclusion that the residues determined in
the supervised trials represented the carbaryl parent and that the
maximum residue limits were intended to represent only the sum of
carbaryl and 1-naphthol not withstanding the fact that the heading of
the list of recommendations for maximum residue limits indicates that
the limits are expressed in terms of carbaryl and metabolites.
RECOMMENDATIONS
The following maximum residue limits are recommended to cover
residues resulting from either pre-harvest or post-harvest use of
carbaryl. They refer to carbaryl only. The limit for rice is raised to
provide for postharvest application.
Commodity Limit (mg/kg)
Bran 20
Barley, oats, rice (in husk and hulled)
rye, wheat 5
wholemeal flour 2
Wheat flour (white) 0.2
(The level and fate of carbaryl residues in processed cereal products
is given in the monograph but, in keeping with accepted practice,
separate limits have not been proposed for such processed foods.)
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the effects of carbaryl on renal
function.
2. Further studies to resolve the differences in observations of
different investigators on reproductive physiology, especially
with regard to neuroendocrine and behavioural changes.
3. Details of analytical methods for use in the determination of
carbaryl and metabolite residues in raw grain, milled cereal
fractions, bread, meat and milk.
REFERENCES
Andrawes, N.R., Chancey, E.L., Crabtree, R.J., Herrett, R. A.
1972 and Weiden, M.H.J. Fate of Maphthyl-1-14C
Carbaryl in laying chickens. J. agr. Food Chem.,
20:608.
Argauer, R.J. J.agr. Food Chem., 17: 889
1969
Association of Official Analytical Chemists, "Official Methods of
1970 Analysis", 11th Ed., p.
Bengston, M., Cooper, L.M., and Grant-Taylor, F.J. A comparison
1975 of bioresmethrin, chlorpyrifosmethyl and
pirimiphos-methyl as grain protectants against
malathion resistant insects in wheat. Queensland
J. agr. Animal Sci., 32,(1): 51-78.
Bengston, M., Connell, M., Crook, I.D., Desmarchelier, J., Hart,
1976a R.J., Phillips, M., Snelson, J.T. and Sticka, R.
Field trials to compare CGA-20168 (methacriphos),
chlorpyrifos-methyl, fenitrothion,
pirimiphos-methyl and malathion for control of
malathion resistant insects infesting wheat in
Australia. Submitted for publication in J. Stored
Prod. Res. 1976.
Bengston, M., Connell, M., Desmarchelier, J., Phillips, Snelson,
1976b J.T., and Sticka, R. Report of study of new grain
protectant insecticides. Report to Australian
Wheat Board. June 1976.
Chattaway, F.D. J. Chem. Sox. 2495
1931
Conterio, W.A. Carbaryl residues in barley and wheat grain,
1969 mill fractions and forage. Report to Union Carbide
Corporation from Crop Chemical Testing Service,
Humbolt, Illinois.
Davies, R.A.H. Carbaryl plus pirimiphos-methyl for stored grain
1976a protection. 1 Laboratory bioassay and residue
analysis: Social Report for J.T. Snelson -
Pesticides Co-ordinator. ICI Australia Ltd., Rural
Division, August 1976.
Davies, R.A.H. Carbaryl plus pirimiphos-methyl for stored grain
1976b protection. 2 Silo pilot trials 1975-76. Special
Report for J.T. Snelson - Pesticides Co-Ordinator.
ICI Australia Ltd., Rural Division, August 1976.
Desmarchelier, J.M. Field trials with fenitrothion plus fenoxythrin
1976a and pirimiphos-methyl plus carbaryl. Part 1.
Biological efficiency. Preliminary report of
ongoing study. CSIRO Division of Entomology,
Canberra, Australia, August 1976.
Desmarchelier, J.M. Field trials with fenitrothion plus fenoxythrin
1976b and pirimiphos-methyl plus carbaryl. Part 2.
Residue studies - Preliminary report of ongoing
study. CSIRO Division of Entomology, Canberra,
Australia, August 1976.
Desmarchelier, J.M. Degradation of carbaryl, CGA 20168,
1976c fenitrothion, pirimiphos-methyl and carbaryl on
rice, oats and barley. Preliminary report of
ongoing study. CSIRO Division of Entomology,
Canberra, Australia. August 1976.
Desmarchelier, J.M. Analytical procedures for carbaryl. Personal
1976d Communication. CSIRC Division of Entomology,
Canberra, Australia. August 1976.
Desmarchelier, J.M. Esterification of phenols, at residue levels,
1976e in dilute base. (In preparation) CSIRO Division of
Entomology, Canberra, Australia.
Dorough, H.W. Animal feeding studies with carbaryl. Details to be
1974 provided by Union Carbide.
FAO/WHO 1973 evaluation of some pesticide residues in food.
1974b FAO/AGP/1973/9/1; WHO Pesticide Residues Series,
No. 3.
Greening, H.G. Wheat-protectant dust trials 1965-67. N.S.W.
1976 Department of Agriculture. Biological and Chemical
Research Institute, Rydalmere, N.S.W. Report
prepared for publication September 1976.
Holden, E.R. Gas chromatographic determination of residues
1973 of methylcarbamate insecticides in crops as their
2,4-dinitrophenyl ether derivatives. J.A.O.A.C.,
56-713.
Leininger, R.O. Carbaryl residues in wheat forage, Report to Union
1973 Carbide Corporation from R.O. Leininger,
Greenfield. California,
Lumière, A., Lumière, L., and Barbier, H. Bull. Soc.Chim.
1906 Fr., 35: 625.
Phillips, I.L. Carbaryl residues in barley heads, grain and
1972 forage. Report to Union Carbide Corporation from
Soilserv. Inc., Salinas, California.
Pschorr, R., and Sumuleanu, P. Ber. der Deutsch. Chem. Ges.,
1899 22: 3407.
Roan, C.L. Comparison of performance of various carbaryl formulations
1964 against rice weevil and confused flour beetle.
Report from Entomology Department, Kansas State
University to Union Carbide Corporation.
Snelson, J.T. and Desmarchelier, J.M. The significance of pesticide
1975 residues. Proc. 1st int. Wking Conf.
Stored-product Entomology. Savannah, Georgia,
U.S.A., 1974, p. 465-477.
Strong, R.G., and Sbur, D.E. Evaluation of insecticides as
1961 protectants against pests of stored grains and
seeds. J. Econ. Entom., 54: 235-238.
Tilden, R.L., and van Middelem, C.H. Determination of carbaryl
1970 as an amide derivative by electron capture GLC. J.
Agric. Food Chem., 18(1): 154-6.
Union Carbide. Summary of studies on the nature of residues of
1974 carbaryl in the meat of dairy cattle. Union
Carbide Corporation Report, May 24, 1974.
Union Carbide. Submission to FAO by Union Carbide Corporation,
1976a September 1976.
Union Carbide Corporation. Results of tests investigating
1976b Sevin as a stored grain insecticide (1958-1962).
Extract of petition 2859.
