
FAO/PL:1967/M/11/1
WHO/Food Add./68.30
1967 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Rome, 4 - 11 December,
1967. (FAO/WHO, 1968)
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1968
CARBARYL
This pesticide was evaluated by the 1966 Joint Meeting of the FAO
Working Party and the WHO Expert Committee on Pesticide Residues
(FAO/WHO, 1967). Since the previous evaluation the results of
additional experiment work have been reported. This work is summarized
and discussed in the following monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Carbaryl is metabolized and excreted by the rhesus monkey and pig as
conjugates of intact 4-hydroxycarbaryl and carbaryl and is metabolized
in the rat, guinea pig, ewe and man, to conjugates of 1-naphthol,
4-hydroxycarbaryl and carbaryl. In all these species, carbaryl is
metabolized by a similar route: the major difference between the
species studied is the extent to which carbaryl is hydrolyzed to yield
1-naphthol. Little or no hydrolysis of carbaryl occurs in the monkey
and pig, as opposed to the man, ewe, rat and guinea-pig (Knaak et al,
1965; 1967b).
Several metabolites of carbaryl were detected in the urine of female
dogs; only one anionic metabolite having the C-O-C-(O)-N-C structure
intact was found, while the remaining metabolites possessed only the
naphthyl label. The nature of these metabolites is unknown. The major
rat metabolites were not found in dog urines. Excretion of the
14C-naphthyl label in the faeces accounts for almost half of the
total carbaryl equivalents excreted. This is an important elimination
route for the dog and a minor pathway for the rat (10 per cent of the
dose). The N-methyl-14C label is excreted in faeces to about the same
extent in both the dog and rat (10 per cent of dose), while in the
urine, the rat excretes up to 68 per cent of the dose and the dog, 23
per cent (Knaak: and Sullivan, 1967).
In two human volunteers given single oral doses of 2 mg/kg
body-weight, urinary recoveries over the first 2 days amounted to 26
and 28 per cent of the doses. No metabolites were found in the urine
after the second day. The metabolites identified were
4-(methylcarbamoyloxy)-1-naphthyl glucuronide (4 per cent), 1-naphthyl
glucuronide (15 per cent) and 1-naphthyl sulfate (8 per cent), with
some evidence for 1-naphthyl methylimido-carbonate 0-glucuronide
(Knaak et al, 1967a; 1967b).
The following table shows the urinary metabolites, excreted after oral
administration of N-methyl-14C-carbonyl or 14C-naphthyl carbaryl to
different mammals, and nonlabelled carbaryl ingested by man (Knaak and
Sullivan, 1967; Knaak et al, 1967b).
Metabolite expressed as percentage of dose in 24-hour urines (arranged in descending
order for the rat)
Guinea
Metabolite Rat Pig Ewe Pig Dogb Man
aryl methylimidocarbonate O-glucuronidec 18 9 34 36 5 presentd
naphthyl sulfate 16 13 12 a a 8
naphthyl glucuronide 10 19 10 5 4c 13
neutrals 9 6 1 8 4 f
4-(methyl carbamoyloxy)-1-naphthylglucuronide 7 10 11 12 a 5
4-(methyl carbamoyloxy)-1-naphthylsulfate 5 6 4 a a a
a) Below the sensitivity limits of the analytical method used.
b) 17 per cent of the metabolites were unidentified and were not found in the rat.
c) This represents a maximum: the absolute presence of this metabolite has not been established.
d) Present but as yet cannot be quantitated.
e) Believed to represent a class of compounds conjugated through the enol form of the carbamate.
f) Indeterminate by the analytical method used.
In a lactating cow given single doses of carbaryl-naphthyl-14C, 0.25
and 3.05 mg/kg body-weight in feed, recoveries of the doses were,
respectively, from faeces, 11 and 15 per cent; from urine, 70 and 58
per cent, and from milk, about 0.35 per cent in both cases. Most of
the material in urine and faeces was eliminated in the first 24 hours.
In the milk, the residue 6 hours after the high dose was slightly less
than 1 ppm; after both doses, milk levels declined rapidly after 12
hours, and none was detectable after 60 hours. The major metabolite in
milk, accounting for 30 per cent of the residue, was tentatively
identified as 5,6-dihydro-5,6-dihydroxy-1-naphthyl N-methyl carbamate
(Dorough, 1967).
Acute toxicity
LD50
Animal Route Solvent (mg/kg body-weight) Reference
Mouse Oral Sunflower 437.5 Rybakova, 1966
Oil
Rat Oral Sunflower 515 " "
Oil
Special studies
Mouse. In a teratogenicity study, groups of 20 mated female mice
were fed 0, 66.7 and 200 ppm from day 6 of gestation through term. On
the 18th day, half the animals were delivered by section and the
foetuses and uteri were examined grossly. The remaining animals were
allowed to bear and rear their pups for 4 days. In 2 of the litters of
high-level animals a total of 7 cases of skeletal malalignment,
non-fusion and incomplete ossification, and one case each of cleft
palate and gross facial malformation were recorded, versus no
malformations in the low-level group and 2 cases of cleft palate in
the controls. However, the authors declined to attribute these
findings to the test material. No effect was seen on numbers or
viability of offspring or on mortality or behaviour of the dams
(Benson et al., 1967).
Rat. Groups of 12 mated female rats were fed 0, 20, 100 and 500
mg/kg bodyweight/day according to three schedules: 1. throughout
pregnancy; 2. through gestational day 7; 3. from gestational day 5
through day 15. Half the animals were delivered by section on the 20th
day, and the foetuses and uteri were examined grossly. The remaining
animals were allowed to bear and rear their pups to weaning. At the
high level, body-weight gain of the dams was seriously affected. In
the high-level group fed carbaryl throughout pregnancy, the postpartum
viability of the pups, lactation indices and weaning weights of
surviving pups were adversely affected. No effect on fertility,
viability, lactation or body-weight gain of dams or pups was seen at
the two lower levels. No abnormalities ware found in the pups of any
group (Weil and Carpenter, 1967).
Groups of 32 rats ware given peroral doses of 0 and 50 mg/kg
body-weight/day of carbaryl in oil for 50 days. Progressive blood
cholinesterase depression and decrease of the ascorbic acid level in
the adrenals were observed. The weights of the adrenals and livers
increased in the experimental animals. In the experimental males,
diminished sperm motility me found; and lengthening of the oestrus
cycle was noted in the females (Rybakova, 1966).
In a 1-year experiment, groups of 24 albino rats of each sex were
given carbaryl at levels of 0, 7, 14, and 70 mg/kg body-weight/day.
After 12 months all the doses produced disturbance of the morphologic
structure of the liver cells, kidneys, adrenals and testes. All the
test groups also showed a highly significant decrease in the motility
of spermatozoa (P <0.001). In the female rats, the duration of the
oestrus cycle increased 10, 20 and 70 per cent under the influence of
the 7, 14 and 70 mg/kg doses, respectively. An increase in
gonadotropic hormones was also observed (Rybakova, 1966).
Hen. Carbaryl in doses of 180 mg and 540 mg/kg of body-weight per
day were administered to laying White Leghorn hens for 60 consecutive
days. No noticeable adverse effects and no detectable residues of the
insecticide or its naphthol metabolite were found in the meat when the
low dose was administered. Residues in meat and histopathological
changes in various organs were found when high dose was given.
Residues were present in the fat tissue with both the high and the low
doses (Nir et al., 1966).
Carbaryl and malathion were fed to laying hens singly and in
combination at 0, 75, 150, 300 and 600 ppm for 3 weeks. Hatchability
decreased and percentage of deformities increased with increasing
concentration. Storage in the eggs was greater in the yolk than in the
white and increased as the dietary level increased (Ghadiri et al.,
1967).
Observations in man
Groups of men ware given peroral doses of 0, 0.06 and 0.12 mg/kg
body-weight/day of carbaryl in gelatin capsules for 6 weeks without
effect on several biochemical, physical and histological parameters of
body function, including erythrocytic and plasma cholinesterase
activities (Wills et al., 1967).
Comments
Several carbaryl metabolites have been identified in mammals after
administration of 14C-labelled carbaryl. This pesticide is
metabolized by a similar route in the man, rat, guinea-pig, sheep, pig
and monkey. However, it is metabolized in a different manner in the
dog.
In plants, several carbaryl metabolites (See section on EVALUATION FOR
TOLERANCES) are essentially similar to metabolites that have been
identified in mammalian metabolism studies. These metabolites occur as
glycosides in plants whereas they occur as glucuronides and sulphates
in animals.
Teratogenicity studies were negative in the rat and in the mouse.
A recent study has shown effects of relatively low doses of carbaryl
on the oestrus cycle and the motility of spermatozoa in rats and it
was felt important that the significance of such findings be further
explored.
The human data are considered as contributory; however, as only small
doses were tested and an effect level was not obtained, these data
were not employed in the calculation of the ADI.
TOXICOLOGICAL EVALUATION
It was not considered for the time being that the new data justified a
change in the previously recommended ADI which is given below.
Estimate of acceptable daily intake for man
0 - 0.02 mg/kg body weight.
Further work desirable
Investigations aimed at clarifying the changes of oestrus cycle and
the motility of the spermatozoa.
EVALUATION FOR TOLERANCES
RESIDUES RESULTING FROM SUPERVISED TRIALS
Crop Type Pre-harvest Usage lbs/A Resulting
period - days (unless otherwise residue ppm
indicated)
Tree fruits
Apples 1 1 lb/100 gal 6-10
Pears 1 1 lb/100 gal 6-7
Peaches 3 8 5-7
Apricots and nectarines 3 8 3-6
Cherries 1 1 lb/100 gal 4-9
Plums 1 8 10
Bananas 0 1 3-6
RESIDUES RESULTING FROM SUPERVISED TRIALS (cont'd)
Crop Type Pre-harvest Usage lbs/A Resulting
period - days (unless otherwise residue ppm
indicated)
Citrus
Oranges 5 1 lb/100 gal 4-5
Lemons and limes 5 1 lb/100 gal 6-8
Grapefruit 5 1 lb/100 gal 2
Melons 1 1 6
Olives 0 8 10
Cranberries and small fruits
Raspberries, Loganberries,
Blackberries, Dewberries 7 2 2-6
Blueberries 1 1 2-3
Strawberries 1 2 1-7
Cranberries 1 3 3-7
Grapes 0 6 may go
over 10
Nuts
Almonds 0 8 10
Filberts 0 5 5
Peanuts 0 1.5 5
Walnuts 0 5 10
Meats
Beef cattle 7 1 qt/animal(w.p. 0.5% S) 0
Swine 7 1 qt/animal(w.p. 0.5% S) 0
Sheep 7 1 qt/animal(w.p. 0.5% S) 0
Poultry 7 5% Dust (dust thoroughly) ( 5 meat
( and fat
( 0 eggs
Leafy vegetables
Leaf lettuce 14 2 10
Endive 14 2 10
Spinach 14 2 10
Swiss chard, parsley,
kale 14 2 1-10
RESIDUES RESULTING FROM SUPERVISED TRIALS (cont'd)
Crop Type Pre-harvest Usage lbs/A Resulting
period - days (unless otherwise residue ppm
indicated)
Cole crops
Cabbage 3 2 2-6
Brussels sprouts 3 2 4-10
Cauliflower 3 2 4-10
Broccoli 3 2 3-7
Root vegetables
Carrots 1 1-2 2
Beets 3 1.5 2
Radishes, turnips,
rutabagas 3 2 2
Sugar beats (roots) 14 1-2 0
Potatoes 0 1 0
Other vegetables
Asparagus 1 2 2-7
Beans 1 .5-2 3
Peas 1 2 3
Corn 0 1.5 1
Peppers 1 1-1.5 2-3
Tomatoes 1 4 2-4
Eggplant 1 2 2.3
Squash 1 1 2
Cucumber 1 1 2-6
Head lettuce 3 2 3-6
Cereal grains 0 1 0
(No application after
boot stage)
Rice 14 1 2.5
Miscellaneous
Cotton seed 0 2.5 2-3
FATE OF RESIDUES
In plants
The rate of dissipation of external pesticide residues from plants is
determined by many independent factors. These include physical
abrasion (wind), washing (rain) and volatility (heat, air movement).
In addition, chemical changes occur on the surface due to oxidation
(air), photooxidation (sunlight), and hydrolysis (humidity). The
half-life of carbaryl on a glass surface was reported to be about
one-third that found on the leaf surface, indicating a differential
rate of dissipation dependent upon the texture of the surface. From
0.5 to 1 per cent of the initial dose underwent some chemical change
prior to dissipation. Studies involving incorporation of carbaryl into
plants suggests that root uptake is influenced by soil composition,
water content, and microbial degradation (Fukuda and Masuda, 1966).
Plants are more efficient in absorbing carbaryl through the root
system than through the leaf surface. The bean plant removed 13 per
cent of the carbaryl available from solution culture (Herrett and
Bagley, 1965), cotton removed 40 - 47 per cent (Mostafa, et al. 1966),
and corn removed 63 per cent (Herrett, et al, 1966). Measurements of
the entrance of carbaryl into the plant resulting from foliar
application indicated that this was not an efficient means of
introduction. In corn, less than 2 per cent of the carbaryl applied
locally to aerial portions penetrated the leaf surface (Herrett, et
al, 1966). Similar tests on the bean plant showed a foliar uptake of
2.6 per cent of the applied dose (Herrett and Bagley, 1965). An early
report demonstrating the passage of carbaryl into the rice plant
(Fukuda and Masuda, 1966; Masuda and Fukuda, 1961) showed that a more
efficient movement of the pesticide occurred in the direction of roots
to leaves rather than the reverse direction. Carbaryl may be
considered to be a relatively immobile compound under conditions of
foliar application. In cocoa, following root uptake, the maximum
accumulation of carbaryl occurred in the apical regions of the leaves
and in areas of active growth (Sundarum and Sundarum, 1963). Since
carbaryl does not readily penetrate plants when applied under normal
agricultural procedures, abnormal conditions have been utilized to
develop data on metabolism. Much of the data presented have been
obtained when carbaryl has been artificially introduced in order to
collect sufficient quantities of the by-products to facilitate
experimentation and the characterization of metabolite.
Recent investigations in which 14C-carbaryl labelled at three
different sites (in the ring, the carbonyl carbon and the N-methyl
group) was stem-injected into growing bean plants yielded the first
insight into the fate of carbaryl in plant tissues (Kuhr and Casida,
1967). Serial harvests followed by homogenization, partitioning, etc.
have shown that carbaryl is readily altered metabolically through both
hydrolysis and hydroxylation. The 1-naphthol, the N-methylol carbaryl,
the 4-hydroxy carbaryl, the 5-hydroxy carbaryl and the 5,6-dihydro-5,
6-dihydroxy carbaryl (tentative identification) metabolites are each
conjugated with one of a series of sugars and are present in the plant
as water-soluble beta-glycosides. The aglycones, liberated through in
vitro enzymatic action with glucosidase, are identical with the
metabolites that have been identified previously from mammalian
metabolism studies. The rate-limiting step in the plant appears to be
the hydroxylation as the free aglycones were not detected in these
experiments. Preliminary studies on bioassay of certain metabolites
have indicated a reduced biological activity when compared with the
parent compound. Studies involving the liberated methylamine moiety in
cotton suggest the formation of a water-soluble compound, minor
evolution of a volatile basic substance (probably methylamine), and a
small quantity of carbon dioxide (Sundarum and Sundarum, 1963). By
injecting carbaryl 14-C labelled in the naphthyl ring, in the
carbaryl carbon or in the N-methyl carbon into the stem of growing
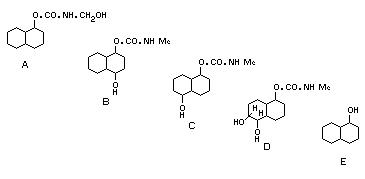
bean plants, aglycones (A) to (E) (see p. 22 ) have been identified
after hydrolysis with beta-glucosidases. The eventual plant
metabolites are glycosides with mixed sugar moieties. These
non-hydrolytic metabolites in many instances do not respond to the
usual methods of analysis for carbaryl. This may be due to the fact
that the metabolites yield a phenolic material different than
1-naphthol and do not show the same color reaction or the solubilities
are significantly different from carbaryl. These findings along with
the lack of information on the toxicity of the metabolites impair the
validity of the usual analytical procedures. Since a relatively small
proportion of the applied chemical appears as radiolabeled
metabolites, it is considered that no serious problem exists.
 In storage and processing
Raw unwashed tomatoes, harvested from field treated applications, were
stored at 55°F for approximately two weeks with samples taken at
varying intervals. Data showed that no loss of carbaryl was realized
under these storage conditions (Farrow et al, 1966; Lamb et al, 1967).
They further studied the effect of home and commercial preparation on
the level of residues of carbaryl in green beans, spinach, tomatoes
and broccoli. Rates of application were somewhat higher than
recommended rates. Water washing was highly effective in removing
residues of carbaryl and the thoroughness of the wash was a definite
factor in the efficiency of removal. Tomatoes with 5.2 ppm, green
beans with 7.6 ppm, spinach with 15.2 to 25.0 ppm and broccoli with
12.4 ppm lost 82 to 99 per cent, 68 to 71 per cent, 66 to 88 per cent,
and 75 per cent, respectively, during commercial washing or blanching
operations. Canning and processing produced further loss. Home washing
and canning procedures produced slightly less, yet highly significant,
losses in residues.
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances
Considering the additional data, summarized above, the Joint Meeting
withdraws the previously published Recommendations for tolerances on
pgs. 44-45 of the 1966 monographs (FAO/WHO, 1967) and substitutes the
following there for :
When carbaryl is utilized in accordance with good agricultural
practice, to protect food products, when necessary, against insect
infestation, the treated product may have residues as high as those
shown below:
ppm
Tree fruits including citrus 10
Small fruits and berries 10
Leafy vegetables and brassica 10
Olives and nuts 10
Cucurbits and melons 10
Other vegetables 5
Poultry* 5
Cotton seed 5
Rice 2.5
*The residue on poultry is largely in the skin
Other meat animals show no residue
By no means will all samples of these products contain this amount of
residue; in fact, only a small, yet unknown, portion of each product
in these categories to likely to be treated. Also an extensive study
of the effect of both home and commercial washing and processing of
green beans, tomatoes, and spinach has become available (Lamb et al.
1967) and has been briefly reviewed above. These data are supported by
data from total diet studies which show that essentially none appears
in food (Duggan and Dawson, 1967).
The above considerations give the meeting assurance that the above
figures as temporary tolerances will not result in an intake above the
ADI. It is recommended that the above figures be adopted as temporary
tolerances.
FURTHER WORK
Further work required by 30 June 1970
1. Further assurance from foliar application experiments that the
terminal residues obtained by stem injection studies give similar
metabolic products.
2. Further information from total diet studies.
Further work desirable
1. More sensitive method of analysis, especially for total diet
studies.
2. Toxicological investigation of carbaryl residues as they occur in
plants as the result of foliar applications. (If these
metabolites are not identical to those found in mammalian
metabolism of carbaryl, it may be necessary to revise this to
"further information required").
3. Investigations aimed at clarifying the changes reported in the
endocrine system and particularly in the sexual glands.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Benson, B.W., Scott, W.J. and Beliles, E.P. (1967) unpublished report
submitted by Union Carbide Corp.
Dorough, H.W. (1967) J. Agric. Food Chem., 15, 261
FAO/WHO. (1967) FAO, PL:CP/15; WHO Food Add./67.32
Ghadiri, M., Greenwood, D.A. and Binns, W. (1967) Toxicol. appl.
Pharmacol., 10, 392.
Knaak, J.B. and Sullivan, L.J. (1967). Unpublished report submitted by
Union Carbide Corp.
Knaak, J.B. Tallant, M.J., Bartley, W.J. and Sullivan, L.J. (1965).
J. Agric. Food Chem., 13, 537
Knaak, J.B., Sullivan, L.J. and Wills, J.H. (1967a). Toxicol. appl.
Pharmacol., 10, 390 (Abstract No. 34).
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.T. (1967b).
Unpublished report submitted by Union Carbide Corp.
Nir, I., Weisenberg, E., Hadani, A. and Egyed, M. Poultry Sci., 45,
720.
Rybakova, M.N. (1966). Gigiena i Sanitariya, 31, 42.
Weil, C.S. and Carpenter, C.P. (1967). Unpublished report submitted by
Union Carbide Corp.
Whitehurst, W.E., Bishop, E.T., Critchfield, F.E. Gyrisco, G.G.,
Huddleston, E.W., Arnold, H. and Lisk, D.J. (1963). J. Agric. Food
Chem., 11, 167-169
Wills, J.H., Jameson, E., Stein, A., Serrone, D. and Coulston, F.
(1967). Toxicol. appl. Pharmacol., 10, 390 (Abstract No. 33).
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Duggan, R. and Dawson, K. (1967) Pesticides, A report on residues in
food. FDA Papers 1 (5), 4 - 8.
FAO/WHO. (1967) Evaluation of some pesticide residues in food. FAO,
PL:CP/15; WHO Food Add./67.32
Farrow, R.P., Lamb, F., Cook, R.W., Bergmans, B., Kimball, J. and
Elkins, E.R. (1966) Removal of Sevin residues from tomatoes during
commercial and home preparative procedures. Paper 36, Pesticides
Subdivision of Agr. and Food Div., Amer. Chem. Soc. 152nd Meeting,
September 12-16, 1966.
Fukuda, H. and Masuda, T. (1966) Kyushu Agr. Expt. Stat., Entomol.
Res. Lab. (1962) Report submitted in abstract by Union Carbide.
Herrett, R.A. and Bagley, W.P. (1965) Uptake and metabolism of Sevin
insecticide in plants. Union Carbide Corp. Internal Status Report.
Herrett, R.A., Bagley, W.P. and Kramer, J.A. (1966) Uptake and
distribution of Sevin insecticide in corn. Union Carbide Corp.
Internal Status Report.
Kuhr, R. and Casida, J.E. (1967) Persistent glycosides of metabolites
of methyl carbamate insecticide chemicals formed by hydroxylation in
bean plants. J. Agr. and Food Chem. in press.
Lamb, F.C., Farrow, R.P., Mercer, W.A. and Smith, K.R. (1967)
Investigation on the effect of preparation and cooking on the
pesticide residue content of selected vegetables. National Canners
Assn. Research Foundation, Washington, D.C. Final Report November 13,
1967.
Masuda, T. and Fukuda, H. (1961) Proc. Assoc. Plant Prot. Kyushu
(Japan) 7: 76 - 78.
Mostafa, I.Y., Hassan, A. and Zayed, M.A.D. (1966) Zeit. Naturforsch.
21b : 1060.
Sundarum, A. and Sundarum, K.M.S. (1963) Penetration, translocation
and persistence of the insecticide 1-naphthyl N-methyl-carbamate
(Sevin) in cocoa seedlings of Theobroma cocoa L. Work conducted at
the University of Ghana, Legon in 1963 and reported in abstract by
Union Carbide Corp.
In storage and processing
Raw unwashed tomatoes, harvested from field treated applications, were
stored at 55°F for approximately two weeks with samples taken at
varying intervals. Data showed that no loss of carbaryl was realized
under these storage conditions (Farrow et al, 1966; Lamb et al, 1967).
They further studied the effect of home and commercial preparation on
the level of residues of carbaryl in green beans, spinach, tomatoes
and broccoli. Rates of application were somewhat higher than
recommended rates. Water washing was highly effective in removing
residues of carbaryl and the thoroughness of the wash was a definite
factor in the efficiency of removal. Tomatoes with 5.2 ppm, green
beans with 7.6 ppm, spinach with 15.2 to 25.0 ppm and broccoli with
12.4 ppm lost 82 to 99 per cent, 68 to 71 per cent, 66 to 88 per cent,
and 75 per cent, respectively, during commercial washing or blanching
operations. Canning and processing produced further loss. Home washing
and canning procedures produced slightly less, yet highly significant,
losses in residues.
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances
Considering the additional data, summarized above, the Joint Meeting
withdraws the previously published Recommendations for tolerances on
pgs. 44-45 of the 1966 monographs (FAO/WHO, 1967) and substitutes the
following there for :
When carbaryl is utilized in accordance with good agricultural
practice, to protect food products, when necessary, against insect
infestation, the treated product may have residues as high as those
shown below:
ppm
Tree fruits including citrus 10
Small fruits and berries 10
Leafy vegetables and brassica 10
Olives and nuts 10
Cucurbits and melons 10
Other vegetables 5
Poultry* 5
Cotton seed 5
Rice 2.5
*The residue on poultry is largely in the skin
Other meat animals show no residue
By no means will all samples of these products contain this amount of
residue; in fact, only a small, yet unknown, portion of each product
in these categories to likely to be treated. Also an extensive study
of the effect of both home and commercial washing and processing of
green beans, tomatoes, and spinach has become available (Lamb et al.
1967) and has been briefly reviewed above. These data are supported by
data from total diet studies which show that essentially none appears
in food (Duggan and Dawson, 1967).
The above considerations give the meeting assurance that the above
figures as temporary tolerances will not result in an intake above the
ADI. It is recommended that the above figures be adopted as temporary
tolerances.
FURTHER WORK
Further work required by 30 June 1970
1. Further assurance from foliar application experiments that the
terminal residues obtained by stem injection studies give similar
metabolic products.
2. Further information from total diet studies.
Further work desirable
1. More sensitive method of analysis, especially for total diet
studies.
2. Toxicological investigation of carbaryl residues as they occur in
plants as the result of foliar applications. (If these
metabolites are not identical to those found in mammalian
metabolism of carbaryl, it may be necessary to revise this to
"further information required").
3. Investigations aimed at clarifying the changes reported in the
endocrine system and particularly in the sexual glands.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Benson, B.W., Scott, W.J. and Beliles, E.P. (1967) unpublished report
submitted by Union Carbide Corp.
Dorough, H.W. (1967) J. Agric. Food Chem., 15, 261
FAO/WHO. (1967) FAO, PL:CP/15; WHO Food Add./67.32
Ghadiri, M., Greenwood, D.A. and Binns, W. (1967) Toxicol. appl.
Pharmacol., 10, 392.
Knaak, J.B. and Sullivan, L.J. (1967). Unpublished report submitted by
Union Carbide Corp.
Knaak, J.B. Tallant, M.J., Bartley, W.J. and Sullivan, L.J. (1965).
J. Agric. Food Chem., 13, 537
Knaak, J.B., Sullivan, L.J. and Wills, J.H. (1967a). Toxicol. appl.
Pharmacol., 10, 390 (Abstract No. 34).
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.T. (1967b).
Unpublished report submitted by Union Carbide Corp.
Nir, I., Weisenberg, E., Hadani, A. and Egyed, M. Poultry Sci., 45,
720.
Rybakova, M.N. (1966). Gigiena i Sanitariya, 31, 42.
Weil, C.S. and Carpenter, C.P. (1967). Unpublished report submitted by
Union Carbide Corp.
Whitehurst, W.E., Bishop, E.T., Critchfield, F.E. Gyrisco, G.G.,
Huddleston, E.W., Arnold, H. and Lisk, D.J. (1963). J. Agric. Food
Chem., 11, 167-169
Wills, J.H., Jameson, E., Stein, A., Serrone, D. and Coulston, F.
(1967). Toxicol. appl. Pharmacol., 10, 390 (Abstract No. 33).
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Duggan, R. and Dawson, K. (1967) Pesticides, A report on residues in
food. FDA Papers 1 (5), 4 - 8.
FAO/WHO. (1967) Evaluation of some pesticide residues in food. FAO,
PL:CP/15; WHO Food Add./67.32
Farrow, R.P., Lamb, F., Cook, R.W., Bergmans, B., Kimball, J. and
Elkins, E.R. (1966) Removal of Sevin residues from tomatoes during
commercial and home preparative procedures. Paper 36, Pesticides
Subdivision of Agr. and Food Div., Amer. Chem. Soc. 152nd Meeting,
September 12-16, 1966.
Fukuda, H. and Masuda, T. (1966) Kyushu Agr. Expt. Stat., Entomol.
Res. Lab. (1962) Report submitted in abstract by Union Carbide.
Herrett, R.A. and Bagley, W.P. (1965) Uptake and metabolism of Sevin
insecticide in plants. Union Carbide Corp. Internal Status Report.
Herrett, R.A., Bagley, W.P. and Kramer, J.A. (1966) Uptake and
distribution of Sevin insecticide in corn. Union Carbide Corp.
Internal Status Report.
Kuhr, R. and Casida, J.E. (1967) Persistent glycosides of metabolites
of methyl carbamate insecticide chemicals formed by hydroxylation in
bean plants. J. Agr. and Food Chem. in press.
Lamb, F.C., Farrow, R.P., Mercer, W.A. and Smith, K.R. (1967)
Investigation on the effect of preparation and cooking on the
pesticide residue content of selected vegetables. National Canners
Assn. Research Foundation, Washington, D.C. Final Report November 13,
1967.
Masuda, T. and Fukuda, H. (1961) Proc. Assoc. Plant Prot. Kyushu
(Japan) 7: 76 - 78.
Mostafa, I.Y., Hassan, A. and Zayed, M.A.D. (1966) Zeit. Naturforsch.
21b : 1060.
Sundarum, A. and Sundarum, K.M.S. (1963) Penetration, translocation
and persistence of the insecticide 1-naphthyl N-methyl-carbamate
(Sevin) in cocoa seedlings of Theobroma cocoa L. Work conducted at
the University of Ghana, Legon in 1963 and reported in abstract by
Union Carbide Corp.
 In storage and processing
Raw unwashed tomatoes, harvested from field treated applications, were
stored at 55°F for approximately two weeks with samples taken at
varying intervals. Data showed that no loss of carbaryl was realized
under these storage conditions (Farrow et al, 1966; Lamb et al, 1967).
They further studied the effect of home and commercial preparation on
the level of residues of carbaryl in green beans, spinach, tomatoes
and broccoli. Rates of application were somewhat higher than
recommended rates. Water washing was highly effective in removing
residues of carbaryl and the thoroughness of the wash was a definite
factor in the efficiency of removal. Tomatoes with 5.2 ppm, green
beans with 7.6 ppm, spinach with 15.2 to 25.0 ppm and broccoli with
12.4 ppm lost 82 to 99 per cent, 68 to 71 per cent, 66 to 88 per cent,
and 75 per cent, respectively, during commercial washing or blanching
operations. Canning and processing produced further loss. Home washing
and canning procedures produced slightly less, yet highly significant,
losses in residues.
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances
Considering the additional data, summarized above, the Joint Meeting
withdraws the previously published Recommendations for tolerances on
pgs. 44-45 of the 1966 monographs (FAO/WHO, 1967) and substitutes the
following there for :
When carbaryl is utilized in accordance with good agricultural
practice, to protect food products, when necessary, against insect
infestation, the treated product may have residues as high as those
shown below:
ppm
Tree fruits including citrus 10
Small fruits and berries 10
Leafy vegetables and brassica 10
Olives and nuts 10
Cucurbits and melons 10
Other vegetables 5
Poultry* 5
Cotton seed 5
Rice 2.5
*The residue on poultry is largely in the skin
Other meat animals show no residue
By no means will all samples of these products contain this amount of
residue; in fact, only a small, yet unknown, portion of each product
in these categories to likely to be treated. Also an extensive study
of the effect of both home and commercial washing and processing of
green beans, tomatoes, and spinach has become available (Lamb et al.
1967) and has been briefly reviewed above. These data are supported by
data from total diet studies which show that essentially none appears
in food (Duggan and Dawson, 1967).
The above considerations give the meeting assurance that the above
figures as temporary tolerances will not result in an intake above the
ADI. It is recommended that the above figures be adopted as temporary
tolerances.
FURTHER WORK
Further work required by 30 June 1970
1. Further assurance from foliar application experiments that the
terminal residues obtained by stem injection studies give similar
metabolic products.
2. Further information from total diet studies.
Further work desirable
1. More sensitive method of analysis, especially for total diet
studies.
2. Toxicological investigation of carbaryl residues as they occur in
plants as the result of foliar applications. (If these
metabolites are not identical to those found in mammalian
metabolism of carbaryl, it may be necessary to revise this to
"further information required").
3. Investigations aimed at clarifying the changes reported in the
endocrine system and particularly in the sexual glands.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Benson, B.W., Scott, W.J. and Beliles, E.P. (1967) unpublished report
submitted by Union Carbide Corp.
Dorough, H.W. (1967) J. Agric. Food Chem., 15, 261
FAO/WHO. (1967) FAO, PL:CP/15; WHO Food Add./67.32
Ghadiri, M., Greenwood, D.A. and Binns, W. (1967) Toxicol. appl.
Pharmacol., 10, 392.
Knaak, J.B. and Sullivan, L.J. (1967). Unpublished report submitted by
Union Carbide Corp.
Knaak, J.B. Tallant, M.J., Bartley, W.J. and Sullivan, L.J. (1965).
J. Agric. Food Chem., 13, 537
Knaak, J.B., Sullivan, L.J. and Wills, J.H. (1967a). Toxicol. appl.
Pharmacol., 10, 390 (Abstract No. 34).
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.T. (1967b).
Unpublished report submitted by Union Carbide Corp.
Nir, I., Weisenberg, E., Hadani, A. and Egyed, M. Poultry Sci., 45,
720.
Rybakova, M.N. (1966). Gigiena i Sanitariya, 31, 42.
Weil, C.S. and Carpenter, C.P. (1967). Unpublished report submitted by
Union Carbide Corp.
Whitehurst, W.E., Bishop, E.T., Critchfield, F.E. Gyrisco, G.G.,
Huddleston, E.W., Arnold, H. and Lisk, D.J. (1963). J. Agric. Food
Chem., 11, 167-169
Wills, J.H., Jameson, E., Stein, A., Serrone, D. and Coulston, F.
(1967). Toxicol. appl. Pharmacol., 10, 390 (Abstract No. 33).
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Duggan, R. and Dawson, K. (1967) Pesticides, A report on residues in
food. FDA Papers 1 (5), 4 - 8.
FAO/WHO. (1967) Evaluation of some pesticide residues in food. FAO,
PL:CP/15; WHO Food Add./67.32
Farrow, R.P., Lamb, F., Cook, R.W., Bergmans, B., Kimball, J. and
Elkins, E.R. (1966) Removal of Sevin residues from tomatoes during
commercial and home preparative procedures. Paper 36, Pesticides
Subdivision of Agr. and Food Div., Amer. Chem. Soc. 152nd Meeting,
September 12-16, 1966.
Fukuda, H. and Masuda, T. (1966) Kyushu Agr. Expt. Stat., Entomol.
Res. Lab. (1962) Report submitted in abstract by Union Carbide.
Herrett, R.A. and Bagley, W.P. (1965) Uptake and metabolism of Sevin
insecticide in plants. Union Carbide Corp. Internal Status Report.
Herrett, R.A., Bagley, W.P. and Kramer, J.A. (1966) Uptake and
distribution of Sevin insecticide in corn. Union Carbide Corp.
Internal Status Report.
Kuhr, R. and Casida, J.E. (1967) Persistent glycosides of metabolites
of methyl carbamate insecticide chemicals formed by hydroxylation in
bean plants. J. Agr. and Food Chem. in press.
Lamb, F.C., Farrow, R.P., Mercer, W.A. and Smith, K.R. (1967)
Investigation on the effect of preparation and cooking on the
pesticide residue content of selected vegetables. National Canners
Assn. Research Foundation, Washington, D.C. Final Report November 13,
1967.
Masuda, T. and Fukuda, H. (1961) Proc. Assoc. Plant Prot. Kyushu
(Japan) 7: 76 - 78.
Mostafa, I.Y., Hassan, A. and Zayed, M.A.D. (1966) Zeit. Naturforsch.
21b : 1060.
Sundarum, A. and Sundarum, K.M.S. (1963) Penetration, translocation
and persistence of the insecticide 1-naphthyl N-methyl-carbamate
(Sevin) in cocoa seedlings of Theobroma cocoa L. Work conducted at
the University of Ghana, Legon in 1963 and reported in abstract by
Union Carbide Corp.
In storage and processing
Raw unwashed tomatoes, harvested from field treated applications, were
stored at 55°F for approximately two weeks with samples taken at
varying intervals. Data showed that no loss of carbaryl was realized
under these storage conditions (Farrow et al, 1966; Lamb et al, 1967).
They further studied the effect of home and commercial preparation on
the level of residues of carbaryl in green beans, spinach, tomatoes
and broccoli. Rates of application were somewhat higher than
recommended rates. Water washing was highly effective in removing
residues of carbaryl and the thoroughness of the wash was a definite
factor in the efficiency of removal. Tomatoes with 5.2 ppm, green
beans with 7.6 ppm, spinach with 15.2 to 25.0 ppm and broccoli with
12.4 ppm lost 82 to 99 per cent, 68 to 71 per cent, 66 to 88 per cent,
and 75 per cent, respectively, during commercial washing or blanching
operations. Canning and processing produced further loss. Home washing
and canning procedures produced slightly less, yet highly significant,
losses in residues.
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances
Considering the additional data, summarized above, the Joint Meeting
withdraws the previously published Recommendations for tolerances on
pgs. 44-45 of the 1966 monographs (FAO/WHO, 1967) and substitutes the
following there for :
When carbaryl is utilized in accordance with good agricultural
practice, to protect food products, when necessary, against insect
infestation, the treated product may have residues as high as those
shown below:
ppm
Tree fruits including citrus 10
Small fruits and berries 10
Leafy vegetables and brassica 10
Olives and nuts 10
Cucurbits and melons 10
Other vegetables 5
Poultry* 5
Cotton seed 5
Rice 2.5
*The residue on poultry is largely in the skin
Other meat animals show no residue
By no means will all samples of these products contain this amount of
residue; in fact, only a small, yet unknown, portion of each product
in these categories to likely to be treated. Also an extensive study
of the effect of both home and commercial washing and processing of
green beans, tomatoes, and spinach has become available (Lamb et al.
1967) and has been briefly reviewed above. These data are supported by
data from total diet studies which show that essentially none appears
in food (Duggan and Dawson, 1967).
The above considerations give the meeting assurance that the above
figures as temporary tolerances will not result in an intake above the
ADI. It is recommended that the above figures be adopted as temporary
tolerances.
FURTHER WORK
Further work required by 30 June 1970
1. Further assurance from foliar application experiments that the
terminal residues obtained by stem injection studies give similar
metabolic products.
2. Further information from total diet studies.
Further work desirable
1. More sensitive method of analysis, especially for total diet
studies.
2. Toxicological investigation of carbaryl residues as they occur in
plants as the result of foliar applications. (If these
metabolites are not identical to those found in mammalian
metabolism of carbaryl, it may be necessary to revise this to
"further information required").
3. Investigations aimed at clarifying the changes reported in the
endocrine system and particularly in the sexual glands.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Benson, B.W., Scott, W.J. and Beliles, E.P. (1967) unpublished report
submitted by Union Carbide Corp.
Dorough, H.W. (1967) J. Agric. Food Chem., 15, 261
FAO/WHO. (1967) FAO, PL:CP/15; WHO Food Add./67.32
Ghadiri, M., Greenwood, D.A. and Binns, W. (1967) Toxicol. appl.
Pharmacol., 10, 392.
Knaak, J.B. and Sullivan, L.J. (1967). Unpublished report submitted by
Union Carbide Corp.
Knaak, J.B. Tallant, M.J., Bartley, W.J. and Sullivan, L.J. (1965).
J. Agric. Food Chem., 13, 537
Knaak, J.B., Sullivan, L.J. and Wills, J.H. (1967a). Toxicol. appl.
Pharmacol., 10, 390 (Abstract No. 34).
Knaak, J.B., Tallant, M.J., Kozbelt, S.J. and Sullivan, L.T. (1967b).
Unpublished report submitted by Union Carbide Corp.
Nir, I., Weisenberg, E., Hadani, A. and Egyed, M. Poultry Sci., 45,
720.
Rybakova, M.N. (1966). Gigiena i Sanitariya, 31, 42.
Weil, C.S. and Carpenter, C.P. (1967). Unpublished report submitted by
Union Carbide Corp.
Whitehurst, W.E., Bishop, E.T., Critchfield, F.E. Gyrisco, G.G.,
Huddleston, E.W., Arnold, H. and Lisk, D.J. (1963). J. Agric. Food
Chem., 11, 167-169
Wills, J.H., Jameson, E., Stein, A., Serrone, D. and Coulston, F.
(1967). Toxicol. appl. Pharmacol., 10, 390 (Abstract No. 33).
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Duggan, R. and Dawson, K. (1967) Pesticides, A report on residues in
food. FDA Papers 1 (5), 4 - 8.
FAO/WHO. (1967) Evaluation of some pesticide residues in food. FAO,
PL:CP/15; WHO Food Add./67.32
Farrow, R.P., Lamb, F., Cook, R.W., Bergmans, B., Kimball, J. and
Elkins, E.R. (1966) Removal of Sevin residues from tomatoes during
commercial and home preparative procedures. Paper 36, Pesticides
Subdivision of Agr. and Food Div., Amer. Chem. Soc. 152nd Meeting,
September 12-16, 1966.
Fukuda, H. and Masuda, T. (1966) Kyushu Agr. Expt. Stat., Entomol.
Res. Lab. (1962) Report submitted in abstract by Union Carbide.
Herrett, R.A. and Bagley, W.P. (1965) Uptake and metabolism of Sevin
insecticide in plants. Union Carbide Corp. Internal Status Report.
Herrett, R.A., Bagley, W.P. and Kramer, J.A. (1966) Uptake and
distribution of Sevin insecticide in corn. Union Carbide Corp.
Internal Status Report.
Kuhr, R. and Casida, J.E. (1967) Persistent glycosides of metabolites
of methyl carbamate insecticide chemicals formed by hydroxylation in
bean plants. J. Agr. and Food Chem. in press.
Lamb, F.C., Farrow, R.P., Mercer, W.A. and Smith, K.R. (1967)
Investigation on the effect of preparation and cooking on the
pesticide residue content of selected vegetables. National Canners
Assn. Research Foundation, Washington, D.C. Final Report November 13,
1967.
Masuda, T. and Fukuda, H. (1961) Proc. Assoc. Plant Prot. Kyushu
(Japan) 7: 76 - 78.
Mostafa, I.Y., Hassan, A. and Zayed, M.A.D. (1966) Zeit. Naturforsch.
21b : 1060.
Sundarum, A. and Sundarum, K.M.S. (1963) Penetration, translocation
and persistence of the insecticide 1-naphthyl N-methyl-carbamate
(Sevin) in cocoa seedlings of Theobroma cocoa L. Work conducted at
the University of Ghana, Legon in 1963 and reported in abstract by
Union Carbide Corp.
